Abstract
Background
Single nucleotide polymorphisms (SNPs) in the promoter region of the FAS and FASLG may alter the transcriptional activity of these genes. We, therefore, investigated the association between the FAS and FASLG polymorphisms and risk of second primary tumor (SPM) after index squamous cell carcinoma of the head and neck (SCCHN).
Methods
We used Log-rank test and Cox proportional hazard models to assess the association of the four SNPs (FAS -1377G>A, FAS -670A>G, FASLG -844C>T and FASLG -124 A>G) with the SPM-free survival and SPM risk among 1,286 incident SCCHN patients.
Results
Compared to patients having the FAS -670 AA or the FASLG -844CC genotypes, the patients having variant genotypes of FAS -670 AG/GG or FASLG -844 CT/TT genotypes had a significantly increased risk of SPM, respectively. A trend for significantly increased SPM risk with increasing number of risk genotypes of the four polymorphisms was observed in a dose-response manner. Moreover, the patients with three or four combined risk genotypes had an appropriately 1.8- or 2.5-fold increased risk for developing SPM compared with patients with zero or one risk genotypes, respectively.
Conclusions
Our results suggest a modestly increased risk of SPM after index SCCHN with FAS -670 A>G and FASLG -844 C>T polymorphisms and an even greater risk of SPM with multiple combined FAS and FASLG risk genotypes.
Impact
The FAS and FASLG polymorphisms may serve as a susceptible marker for SCCHN patients at high SPM risk.
Keywords: FAS/FASLG, Squamous cell carcinoma of head and neck, Second primary malignancy, Genetic susceptibility, Polymorphism
Translational relevance
The high frequency of second primary malignancies (SPM) occurs in approximately 15% of squamous cell carcinoma of the head and neck (SCCHN) patients. Although the incidence of SCCHN in the U.S. has been in decline over past two decades and the diagnostic and therapeutic approaches for the patients have been improved, the poor prognosis for SCCHN patients has not significantly improved. Therefore, FAS and FASLG polymorphisms may serve as a marker for genetic susceptibility to SPMs after index SCCHN, and for identifying high-risk subgroups of SCCHN patients who might benefit from management of alternative treatment and predictable patient outcome for an improved survival and a better quality of life. Moreover, identifying markers of risk for SPM among cancer survivors would greatly enhance secondary prevention, which is currently limited to rather simplistic clinical post-treatment screenings.
Introduction
The incidence of squamous cell carcinoma of the head and neck (SCCHN) in the U.S. has been in decline over past two decades, largely due to a decline in the prevalence of smoking (1). The poor prognosis for SCCHN patients has not significantly improved, partly because of the high frequency of second primary malignancies (SPM), which occurs in approximately 15% of SCCHN patients (2–4), although the diagnostic and therapeutic approaches for SCCHN patients have been improved.
Although previous and continued exposures to smoking and alcohol use are associated with risk of developing SPMs (5–7), only a small proportion of exposed individuals develops SPM, suggesting that genetic factors may contribute to the inter-individual variation in susceptibility to SPMs (8–10). We and others have reported that genetic predisposition involved in several molecular pathways, such as carcinogen metabolism, DNA repair, and cell cycle control, is associated with the risk of SPM after primary SCCHN (11–16).
Apoptosis is the physiological mechanism of programmed cell death that plays an important role in diverse biological processes such as development, homeostasis of tissues, and elimination of cancer cells (17, 18). The acquired ability to resist apoptotic stimuli is one of the primary characteristics of a malignant cell, and abnormal regulation of apoptosis is a key mechanism in the development of cancer (19). FAS is a cell surface receptor that can interact with the FAS ligand (FASLG) to trigger apoptosis (20–22). Therefore, the FAS/FASLG pathway plays an important role in regulation of apoptosis and maintenance of cellular homeostasis, and genetic alteration of the FAS/FASLG signaling pathway may result in immune escape, and thus tumorigenesis including SPM.
Existing data suggest that polymorphisms of FAS/FASLG have been associated with increased susceptibility to a variety of cancers, including SCCHN (23–30). Single nucleotide polymorphisms (SNPs) are the most common form of human genetic variation, and the functional SNPs in the promoters of FAS and FASLG genes have been identified to be related to the differential expression of these two genes (31–33), which may affect risk of SPM after index SCCHN. For example, the FAS -1377G>A and -670A>G polymorphisms have been shown to interfere with the SP1 and STAT1 transcription factor binding sites, respectively, hence decreasing promoter activity and in turn FAS gene expression (31, 32), while the C allele of the FASLG -844C>T polymorphism creates a binding site for the CAAT/enhancer binding protein β transcription factor, resulting in higher basal expression of the FASLG gene (33). However, there is no report on the functional relevance of the FASLG -124 A>G polymorphism. Our previous study showed that the FAS -670 A>G and -1377G>A polymorphisms were associated with an increased risk of SCCHN (30), but no risk of SCCHN was associated with the FASLG -844C>T and -124 A>G polymorphisms. To date, the association between the FAS and FASLG polymorphisms and risk of SPM after index SCCHN has not been reported.
Given the role of the FAS and FASLG genes in regulating cell death and abnormal expression of FAS and/or FASLG in various types of tumors, including SCCHN, we hypothesized that FAS and FASLG polymorphisms contribute to genetic susceptibility to SPMs after index SCCHN, and these polymorphisms may be genetic markers to identify high-risk subgroups of SCCHN patients who might benefit from management of alternative treatment and predictable patient outcome. To test the hypothesis, we compared the SPM-free survival and the risk of SPM between the different genotyping groups in a cohort of 1286 incident SCCHN patients.
Materials and Methods
Study subjects
Between May 1995 and January 2007, 1,667 patients with incident SCCHN were consecutively recruited at the University of Texas M. D. Anderson Cancer Center as part of a ongoing molecular epidemiologic study of SCCHN. These patients were newly diagnosed, histopathologically confirmed, and untreated squamous cell carcinomas of the oral cavity, oropharynx, hypopharynx or larynx. All patients completed an IRB-approved informed consent, without the restriction of age, sex, ethnicity, or clinical stage. Approximately 95% of contacted patients consented to enrollment in the study. The exclusion criteria included any prior cancer history excepting nonmelanoma skin cancer, distant metastases at presentation, primary sinonasal tumors, salivary gland tumors, cervical metastases of unknown origin, and tumors outside the upper aerodigestive tract. In addition, blood samples for genotyping data were not available for some patients recruited early in the study, and these patients were excluded from this analysis, as were patients without follow-up and patients who underwent only palliative treatment. Therefore, there are a total of 1,286 patients available for the final analysis of this study.
Patients were monitored through their treatment and post-treatment course with regularly scheduled clinical and radiographic examinations. SPMs were distinguished from local recurrences based on modified criteria of Warren and Gates (34). Second lesions with different histopathologic type, and/or occurring more than 5 years following treatment for the primary tumor, and/or clearly separated by normal epithelium based on clinical and radiographic assessment were considered SPM. The second lesion was classified as a local recurrence rather than a SPM if there was discrepancy or differing opinion regarding the origin of the tumor. Pulmonary lesions were considered SPM if they had a non-squamous histology; or if they were isolated squamous lesions greater than 5 years from initial SCCHN and felt to be SPM by the thoracic oncologist and thoracic surgeon. SPMs were then classified as tobacco-associated (e.g, SCCHN or cancers of the esophagus, lung, or bladder) and non tobacco-associated SPM.
At presentation all patients provided epidemiological data, including alcohol and smoking status. Those subjects who had smoked at least 100 cigarettes in their lifetime were defined as ever smokers, otherwise, they were considered never smokers. Subjects who had drunk at least one alcoholic beverage/per day for at least one year during their lifetime were defined as ever drinkers and those who never had such a pattern of drinking were defined as never drinkers. Clinical data were obtained at initial presentation and through follow-up examinations and included overall stage at presentation of index tumor, site of index tumor, and treatment. Index cancer stage was then dichotomized into the early stage (including I and II clinical stage) and late stage (III and IV). We also grouped treatment into four categories: surgery only, surgery with radiotherapy and/or chemotherapy, radiotherapy, and radiotherapy plus chemotherapy.
Genotyping of the FAS and FASLG Polymorphisms
DNA was extracted from 1 ml of blood sample with the Qiagen DNA Blood Mini Kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. We genotyped the four single nucleotide polymorphisms (SNPs) of the FAS and FASLG gene: FAS -1377G>A, FAS -670A>G, FASLG -844C>T and FASLG -124 A>G by the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay (30). The primers, polymerase chain reaction and restriction enzymes for these polymorphisms have been described previously (30). Approximately 10% of samples have been reassayed demonstrating 100% concordance.
Statistical Analysis
For all analyses in this study, statistical significance was set at P < 0.05, and all tests were two-sided. The Statistical Analysis System software (version 9.1.3; SAS Institute) was used to perform all statistical analyses. SPM occurrence was considered as the primary endpoint of the study. The Student’s t test was used to compare the mean age and follow-up time of the patients who developed a SPM and those who did not. The differences in ethnicity, sex, smoking and alcohol status, index tumor site, index tumor stage, treatment, and genotype distributions between the two groups were evaluated using the chi-squared test. Time-to-event was calculated from the date of diagnosis of the index SCCHN to the date of SPM occurrence. Patients who were not known to have an event at the date of last contact, or who died were censored. The associations between individual epidemiological risk factors, clinical characteristics including index tumor site, index tumor stage, and treatment variables, and time to the occurrence of SPMs, were initially assessed using univariate Cox proportional hazards regression models. The data were consistent with the assumptions of the Cox proportional hazards regression model from the examination of Kaplan-Meier survival curves and log-minus-log survival plots.
In the univariate analysis, we evaluated epidemiological variables, assessed at the time of diagnosis, such as age in years, ethnicity, sex, and smoking and alcohol status, and clinical characteristics, such as index tumor site, index tumor stage, and treatment. We did not incorporate any interaction terms in the first step in building a multivariable model for time to SPM occurrence. A multivariable proportional hazards model was built using the variables that had prognostic potential suggested by the univariate analysis (P < 0.25). Due to epidemiological and clinical considerations in building the model, age, sex, and ethnicity were always retained in the main-effects and final multivariable model. We used a stepwise search strategy to build the multivariable models, for which a threshold level of 0.25 for the likelihood ratio test was used as a cutoff to determine whether a variable could be entered into, or removed from, the regression model. We assessed associations using hazard ratios (HR) and their 95% confidence intervals (CI) for a SPM development. The final fully adjusted Cox regression models included age, sex, ethnicity, and smoking and alcohol status.
Results
Patient Characteristics
Table 1 showed the demographics, risk exposure, and clinical variables for the 1,286 patients, which included 1166 patients who did not develop SPM while 120 (9.3%) patients who developed SPM. The overall median follow-up time was 29.7 months (range 0 to 142.4 months). Of the 120 patients with SPM, 81 patients developed SPMs at tobacco-associated sites including 44 (36.7%) SCCHN and 37 (30.8%) other tobacco-associated cancers (34, 28.3% lung cancer, 2, 1.7% esophagus cancer, and 1, 0.83% bladder cancer); 35 (29.2%) developed SPMs at other sites (10, 8.3% prostate cancer, 8, 6.7% papillary thyroid carcinoma, 4, 3.3% colon adenocarcinoma, 3, 2.5% lymphoma, 3, 2.5% hepatic adenocarcinoma, 2, 1.7% breast cancer, and 1, 0.83% each for the remainder including sarcoma, renal cell carcinoma, endometrial carcinoma, leukemia, and maxillary sinus adenocarcinoma); and 4 (3.3%) developed SPMs at both sites (2, 1.7% patients with both SCCHN and prostate cancer and 2, 1.7% patients with both SCCHN and papillary thyroid carcinoma). Of the 44 patients with second SCCHN, 24 (55%) were synchronous SCCHN primaries. Of these 24 patients with synchronous SCCHN, two patients had bilateral oral cavity cancers, three had bilateral oropharyngeal cancers, one had bilateral hypopharyngeal cancers, and the remainder had simultaneous cancers of more than one head and neck subsite.
Table 1.
Distribution of selected characteristics of the patient cohort (n =1,286)
| Variable | Total | SPM-Free | SPM | P-valuesc | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Total patients | 1,286 | 100 | 1,166 | 90.7 | 120 | 9.3 | |
| Age | |||||||
| ≤ median (57 years) | 665 | 51.7 | 626 | 53.7 | 39 | 32.5 | <.0001 |
| > median (57 years) | 621 | 48.3 | 540 | 46.3 | 81 | 67.5 | |
| Sex | |||||||
| Male | 977 | 76.0 | 883 | 75.7 | 94 | 78.3 | 0.525 |
| Female | 309 | 24.0 | 283 | 24.3 | 26 | 21.7 | |
| Ethnicity | |||||||
| Non-Hispanic White | 1,087 | 84.5 | 993 | 85.2 | 94 | 78.3 | 0.050 |
| Other | 199 | 15.5 | 173 | 14.8 | 26 | 21.7 | |
| Smoking | |||||||
| Never | 343 | 26.7 | 318 | 27.3 | 25 | 20.8 | 0.129 |
| Ever | 943 | 73.3 | 848 | 72.7 | 95 | 79.2 | |
| Alcohol | |||||||
| Never | 335 | 26.1 | 308 | 26.4 | 27 | 22.5 | 0.352 |
| Ever | 951 | 73.9 | 858 | 73.6 | 93 | 77.5 | |
| Index Cancer Site | |||||||
| Oral cavity | 417 | 32.4 | 379 | 32.5 | 38 | 31.7 | 0.322 |
| Oropharynx | 573 | 44.6 | 525 | 45.0 | 48 | 40.0 | |
| Larynx/Hypopharynx | 296 | 23.0 | 262 | 22.5 | 34 | 28.3 | |
| Index Cancer Stage | |||||||
| I or II | 323 | 25.1 | 291 | 25.0 | 32 | 26.7 | 0.681 |
| III or IV | 963 | 74.9 | 875 | 75.0 | 88 | 73.3 | |
| Treatment | |||||||
| Surgery only | 229 | 17.8 | 208 | 17.8 | 21 | 17.5 | 0.889 |
| Surgery + Adjuvant Txa | 320 | 24.9 | 287 | 24.6 | 33 | 27.5 | |
| XRTb | 329 | 25.6 | 301 | 25.8 | 28 | 23.3 | |
| XRT + Chemotherapy | 408 | 31.7 | 370 | 31.7 | 38 | 31.7 | |
Adjuvant Treatment: adjuvant radiotherapy and/or chemotherapy
XRT: radiotherapy
P values were calculated from chi-square test
The mean age at diagnosis for the total patients was 57.5 years (range, 18–94 years, median, 57 years), and the mean age of patients at index SCCHN who developed SPM was significantly older compared with the mean age of patients who did not develop SPM (60.8 years vs. 57.1 years, respectively; P < 0.0001). Compared with the SPM-free group, patients who developed SPM were more likely older (P < 0.0001) and non-Hispanic whites (P = 0.050). However, no significant differences were observed between patients who did not develop SPM and patients who developed SPM, regarding sex (P = 0.525), smoking (P = 0.129), alcohol drinking (P = 0.352), index cancer site (P = 0.322), index cancer stage (P = 0.681), and treatment (P = 0.889).
Association between the FAS and FASLG polymorphisms and risk of SPM
As shown in Table 2, FAS -670 AG+GG genotypes were more frequent in the patients who developed SPM (83.3%) than the patients who did not develop SPM (73.2%) and were associated with a significantly increased risk of SPM compared with the FAS -670 AA genotype (OR,1.57; 95% CI, 1.00–2.54). Compared with the FASLG -844 CC genotype, the FASLG -844 CT+TT genotypes were also more frequent in the patients who developed SPM (70.8%) than the patients who did not develop SPM (59.2%) and were associated with a significantly increased risk of SPM (OR,1.71; 95% CI, 1.15–2.54). However, the differences between the variant genotypes (FAS -1377 GA+AA or FASLG -124 AG+GG) and the wild-type homozygous genotypes (FAS -1377 GG or FASLG-124 AA) for FAS -1377G>A or FASLG -124A>G polymorphism were not statistically significant (P = 0.879 and P = 0.458, respectively). For these two polymorphisms, no significant SPM risks were observed between the patients who developed SPM and who did not develop SPM (OR, 0.87; 95% CI, 0.56–1.36 for FAS -1377G>A and OR, 1.15; 95% CI, 0.75–1.77 for FASLG -124A>G, respectively).
Table 2.
SPM risk associated with FAS and FASLG polymorphisms after index SCCH
| Genotypes | Total (No. =1,286) | SPM-free (No. =1,166) | SPM (No. =120) | Pa | HR(95% CI)b | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| FAS -1377G>A | ||||||||
| GG (Ref.c) | 1,023 | 79.6 | 927 | 79.5 | 96 | 80.0 | ||
| GA+AA | 263 | 20.4 | 239 | 20.5 | 24 | 20.0 | 0.879 | 0.87 (0.56–1.36) |
| FAS -670A>G | ||||||||
| AA (Ref.c) | 333 | 25.9 | 313 | 26.8 | 20 | 16.7 | ||
| AG+GG | 953 | 74.1 | 853 | 73.2 | 100 | 83.3 | 0.014 | 1.57 (1.00–2.54) |
| FASLG -844C>T | ||||||||
| CC (Ref.c) | 511 | 39.7 | 476 | 40.8 | 35 | 29.2 | ||
| CT+TT | 775 | 60.3 | 690 | 59.2 | 85 | 70.8 | 0.038 | 1.71 (1.15–2.54) |
| FASLG -124A>G | ||||||||
| AA (Ref.c) | 981 | 76.3 | 889 | 76.2 | 92 | 76.7 | ||
| AG+GG | 305 | 23.7 | 277 | 23.8 | 28 | 23.3 | 0.458 | 1.15 (0.75–1.77) |
χ2 test for differences in the distributionof FAS and FASLG genotypes between the patients who developed SPM and the patients who did not.
Adjusted for age, sex, ethnicity, tobacco smoking and alcohol drinking in a Cox model.
Ref. = reference group.
Association between the combined genotypes of the FAS and FASLG polymorphisms and SPM risk
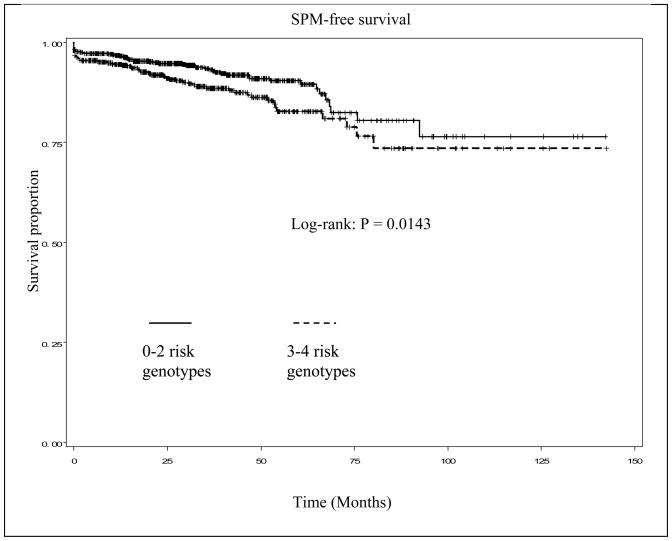
Because any of the four SNPs of the FAS and FASLG genes in the apoptotic pathway appeared to have a minor effect on risk of SPM, we then performed combined analysis of all four SNPs to focus on potentially modifying effect of the combined genotypes on risk of SPM (Table 3). In the 1,286 patients who had data available on all four SNPs, we categorized all putative risk (ORs > 1.0) genotypes of each SNP into a new variable according to the number of risk genotypes (for the protective genotype, e.g., FAS -1377G>A, we reversed the reference group). For the combined analysis, we found that the patients with 0–2 risk genotypes of the four polymorphisms experienced a significantly reduced SPM-free survival compared with patients with 3–4 risk genotypes (log-rank, P = 0.0143, Fig. 1). There was a trend for increased SPM risk with increasing number of risk genotypes, and this trend in risk was statistically significant in a dose-response manner (P = 0.004 for trend). Specifically, the patients with 3 or 4 risk genotypes had an approximately 1.8- (HR, 1.83; 95% CI, 1.00–3.36) or 2.5-fold (HR, 2.53; 95% CI, 1.26–5.06) increased risk for developing SPM, compared to patients with 0–1 risk genotypes, respectively.
Table 3.
SPM risk associated with FAS and FASLG polymorphisms after index SCCHN
| No. risk genotypes | Total (No. =1,286) | SPM-free (No. =1,166) | SPM (No. =120) | Pa | HR(95% CI)b | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| 0–1 (Ref.c) | 235 | 18.3 | 221 | 19.0 | 14 | 11.7 | 0.103 | 1.00 (Ref) |
| 2 | 480 | 37.3 | 438 | 37.6 | 42 | 35.0 | 1.43 (0.78–2.63) | |
| 3 | 420 | 32.7 | 375 | 32.1 | 45 | 37.5 | 1.83 (1.00–3.36) | |
| 4 | 151 | 11.7 | 132 | 11.3 | 19 | 15.8 | 2.53 (1.26–5.06) | |
| Trend | P=0.004 | |||||||
χ2 test for differences in the distribution of combined genotypes between the patients who developed SPM and the patients who did not.
Adjusted for age, sex, ethnicity, tobacco smoking and alcohol drinking in a Cox model.
Ref. = reference group.
Fig. 1.
Kaplan-Meier SPM-free survival curve stratified by combined FAS/FASLG risk genotypes
Discussion
In this study, we investigated the association between the FAS -1377G>A and -670A>G and the FASLG -844C>T and FASLG -124 A>G polymorphisms on the risk of SPM after index SCCHN. We found that both the FAS -670 A>G and the FASLG -844 C>T polymorphisms were associated with a significantly increased risk of SPM in patients with SCCHN. Although we did not observe any significant association of FAS -1377G>A or FASLG -124A>G polymorphism with risk of SPM, we did observe an effect of the combined risk genotypes of the four polymorphisms on SPM risk in patients with primary SCCHN, and the trend in risk was statistically significant in a dose-response manner. In addition, the patients with 3 or 4 risk genotypes had almost 1.8- or 2.5-fold increased risk for developing SPM compared with patients with 0 or1 risk genotypes. To the best of our knowledge, there have been no previous studies examining the combined effects of genetic variants in the apoptotic pathway on risk of SPM after index SCCHN.
It has been shown that downregulation of FAS may protect tumor cells from elimination by antitumor immune responses, whereas up-regulation of FASLG may increase the ability of tumor cells to counterattack the immune system by inducing apoptosis of FAS-sensitive lymphocytes (35–38). Alteration of FAS and FASLG expression decrease the apoptotic capacity of cells and many tumor cells might evade or suppress the immune system. Most studies indicated that decreasing the expression of FAS and/or increasing the expression of FASLG is a common feature of malignant transformation and an early event associated with the development of most human cancers, including SCCHN (23–27, 30, 39–41). Given the important roles of FAS and FASLG in apoptosis process, it is biologically plausible that alteration of FAS and FASLG genes, such as genetic polymorphisms, may affect risk of cancer including SPM.
The FAS -1377G>A polymorphism has been reported to be associated with increased risk of developing lung cancer (24), breast cancer (25, 39), esophageal squamous cell cancer (26), colorectal cancer (27), SCCHN (30) and acute myeloid leukemia (32). FAS -670A>G polymorphism was found to be associated with increased risk of esophageal squamous cell cancer (26), SCCHN (30) and gynecological cancer (40). In the current study, we observed the significant association of FAS -670A>G but not FAS -1377G>A polymorphism with risk of SPM after index SCCHN. Although the exact mechanism of how the polymorphism affect SPM development is unclear, Sibley et al. reported that the FAS -670G allele had a greatly reduced ability to bind transcription factor signal transducers and activators of transcription 1(STAT1) (32) and less expressed on ex vivo-stimulated T cells (41). Decreased FAS expression resulting from a FAS promoter polymorphism may help the transformed cells evade FAS-mediated cell death, subsequently affecting risk of cancer including SPM.
The FASLG -844T/C polymorphism is also located in the promoter region of the gene, and basal FASLG expression is higher in cells carrying the C allele than in cells carrying the T allele, as measured in a luciferase reporter assay and when expressed in peripheral blood fibrocytes (33). Sun et al. found that FASLG -844 C allele is associated with increased activation-induced T cell apoptosis in vitro, which is consistent with the findings in current study (25, 41). Transformed cells with the FASLG -844CC genotype that express a high level of FASLG may create an immuno-privileged site by killing cytotoxic immune cells and thus escape host immuno-surveillance. The association between the FASLG -844C>T polymorphism and increased risk of some cancers has been reported in previous studies (24–27, 33, 41). In this study, we found that the FASLG -844 variant genotypes (CT+TT) were associated with a significantly increased risk of SPM in patients after index SCCHN compared with the FASLG -844CC genotype, although our previous case-control study indicated that no risk of SCCHN was associated with any of the FASLG genotypes (30). The exact mechanism for these conflicting results remains unknown. It might be possible that effect of this FASLG -844C>T polymorphism in normal epithelium of the head and neck differs from those in SCCHN tumor tissues which have numerous somatic changes. It also might be that this FASLG -844C>T polymorphism may function differently in etiology (case–control study) and prognosis (case only study) because the normal epithelium of the head and neck and SCCHN tumor tissues have significant differences in genetic profiles such as somatic genetic changes. Moreover, this polymorphism of FASLG -844C>T may have different roles in etiology and prognosis through the interaction of this FASLG -844C>T variant with the normal genes in normal tissues, genetically altered genes in SCCHN tissues, smoking behavior, human papillomavirus (HPV), and other environmental risk factors, respectively. Several studies have also suggested that genetic factors, previous treatments, within the context of previous or continued exposure to risk factors, may affect the risk of SPM after index SCCHN (42–44). Therefore, all these factors may affect functionality of this FASLG -844C>T polymorphism in development of both SPM and SCCHN. However, these hypotheses need to be tested in future studies.
Although this was a large and well-characterized cohort in SCCHN patients by Head and Neck Center at M. D. Anderson Cancer Center, there were several inherent limitations in our study. Firstly, multiple ethnicities were included in this cohort, in which 84.5% of patients were non-Hispanic whites. Secondly, the demographics, exposure, and clinical data for the cohort were collected prospectively, while clinical outcomes including SPM were collected retrospectively without a strictly defined screening or follow-up regimen. Furthermore, the follow-up time to the development of SPM in this study may have been limited by patients with stage III and IV index cancer who were lost to follow-up. These patients may not have had as much opportunity to develop SPM because of being recruited lately or dying relatively soon after diagnosis. It is also possible that a screening bias in the detection of SPMs exists such that tobacco-associated SPMs (i.e., SCCHN, esophagus, or lung cancers) were detected more readily than non-tobacco associated cancers. However, such a bias should be non-differential (i.e., not different between groups having different genotypes). In addition, the low SPM rate may be due to our high prevalence of never smokers (26.7%) and our strict criteria in defining SPM. Finally, the absence of human papillomavirus (HPV) status did not allow us to evaluate its potential influence on the development of SPMs in patients with index SCCHN. With the information available, we will take HPV and smoking status into account as confounders in our future studies when we analyze the associations between this and/or other genetic polymorphisms and risk of SPM. Despite these limitations, the current investigation supports a significant role of FAS and FASLG polymorphisms in individual variation in susceptibility to SPM after index SCCHN.
Acknowledgments
Funded by: Research Training Award, The American Laryngological, Rhinological, and Otological Society (to E.M.S.); U.T. M.D. Anderson Cancer Center Start-up Funds (to E.M.S.); National Institute of Environmental Health Sciences Grant R01 ES-11740 (to Q.W.); N.I.H. Grant P-30 CA-16672 (to The University of Texas M.D. Anderson Cancer Center); and N.I.H. grant CA135679 (to G.L.) and CA133099 (to G.L.)
The authors wish to thank Ms. Margaret Lung, Ms. Angeli Fairley, Ms. Liliana Mugartegui, and Ms. Kathryn Tipton with assistance with patient recruitment.
Abbreviations
- SCCHN
squamous cell carcinoma of the head and neck
- SPM
second primary malignancies
- HR
hazard ratio
- 95% CI
95% confidence interval
- HPV
human papillomavirus
References
- 1.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence. Cancer. 2007;110:1429–35. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 2.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JS, Pajak TF, Rubin P, et al. Second malignancies in patients who have head and neck cancer: incidence, effect on survival and implications based on the RTOG experience. Int J Radiat Oncol Biol Phys. 1989;17:449–56. doi: 10.1016/0360-3016(89)90094-1. [DOI] [PubMed] [Google Scholar]
- 4.Kotwall C, Razack MS, Sako K, Rao U. Multiple primary cancers in squamous cell cancer of the head and neck. J Surg Oncol. 1989;40:97–9. doi: 10.1002/jso.2930400208. [DOI] [PubMed] [Google Scholar]
- 5.Day GL, Blot WJ, Shore RE, et al. Second cancers following oral and pharyngeal cancers: role of tobacco and alcohol. J Natl Cancer Inst. 1994;86:131–7. doi: 10.1093/jnci/86.2.131. [DOI] [PubMed] [Google Scholar]
- 6.Do KA, Johnson MM, Lee JJ, et al. Longitudinal study of smoking patterns in relation to the development of smoking-related secondary primary tumors in patients with upper aerodigestive tract malignancies. Cancer. 2004;101:2837–42. doi: 10.1002/cncr.20714. [DOI] [PubMed] [Google Scholar]
- 7.León X, Del PradoVenegas M, Orús C, Kolañczak K, García J, Quer M. Metachronous second primary tumours in the aerodigestive tract in patients with early stage head and neck squamous cell carcinomas. Eur Arch Otorhinolaryngol. 2005;262:905–9. doi: 10.1007/s00405-005-0922-5. [DOI] [PubMed] [Google Scholar]
- 8.Spitz MR, Hoque A, Trizna Z, et al. Mutagen sensitivity as a risk factor for second malignant tumors following malignancies of the upper aerodigestive tract. J Natl Cancer Inst. 1994;86:1681–4. doi: 10.1093/jnci/86.22.1681. [DOI] [PubMed] [Google Scholar]
- 9.Cloos J, Leemans CR, van der Sterre MLT, Kuik DJ, Snow GB, Braakhuis BJM. Mutagen sensitivity as a biomarker for second primary tumors after head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2000;9:713–7. [PubMed] [Google Scholar]
- 10.Foulkes WD, Brunet JS, Sieh W, Black MJ, Shenouda G, Narod SA. Familial risks of squamous cell carcinoma of the head and neck: retrospective case-control study. BMJ. 1996;313:716–21. doi: 10.1136/bmj.313.7059.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Spitz MR, Lee JJ, et al. Novel susceptibility loci for second primary tumors/recurrence in head and neck cancer patients: large-scale evaluation of genetic variants. Cancer Prev Res. 2009;2:617–24. doi: 10.1158/1940-6207.CAPR-09-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gal TJ, Huang WY, Chen C, Hayes RB, Schwartz SM. DNA repair gene polymorphisms and risk of second primary neoplasms and mortality in oral cancer patients. Laryngoscope. 2005;115:2221–31. doi: 10.1097/01.mlg.0000183736.96004.f7. [DOI] [PubMed] [Google Scholar]
- 13.Minard CG, Spitz MR, Wu X, Hong WK, Etzel CJ. Evaluation of glutathione S-transferase polymorphisms and mutagen sensitivity as risk factors for the development of second primary tumors in patients previously diagnosed with early-stage head and neck cancer. Cancer. 2006;106:2636–44. doi: 10.1002/cncr.21928. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Sturgis EM, Zafereo ME, et al. p73 G4C14-to-A4T14 polymorphism and risk of second primary malignancy after index squamous cell carcinoma of head and neck. Int J Cancer. 2009;125:2660–5. doi: 10.1002/ijc.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zafereo ME, Sturgis EM, Aleem S, Chaung K, Wei Q, Li G. Glutathione S-transferase polymorphisms and risk of second primary malignancy after index squamous cell carcinoma of the head and neck. Cancer Prev Res. 2009;2:432–9. doi: 10.1158/1940-6207.CAPR-08-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zafereo ME, Sturgis EM, Liu Z, Wang LE, Wei Q, Li G. Nucleotide excision repair core gene polymorphisms and risk of second primary malignancy in patients with index squamous cell carcinoma of the head and neck. Carcinogenesis. 2009;30:997–1002. doi: 10.1093/carcin/bgp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zörnig M, Hueber A, Baum W, Evan G. Apoptosis regulators and their role in tumorigenesis. Biochim Biophys Acta. 2001;1551:F1–37. doi: 10.1016/s0304-419x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 18.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 19.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 20.Krammer PH, Behrmann I, Daniel P, Dhein J, Debatin KM. Regulation of apoptosis in the immune system. Curr Opin Immunol. 1994;6:279–89. doi: 10.1016/0952-7915(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 21.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 22.Oehm A, Behrmann I, Falk W, et al. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992;267:10709–15. [PubMed] [Google Scholar]
- 23.Lai HC, Lin WY, Lin YW, et al. Genetic polymorphisms of FAS and FASL (CD95/CD95L) genes in cervical carcinogenesis: an analysis of haplotype and gene-gene interaction. Gynecol Oncol. 2005;99:113–8. doi: 10.1016/j.ygyno.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Miao X, Sun T, et al. Functional polymorphisms in cell death pathway genes FAS and FASL contribute to risk of lung cancer. J Med Genet. 2005;2:479–84. doi: 10.1136/jmg.2004.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, Sun T, Xue L, et al. Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer. Carcinogenesis. 2007;28:1067–73. doi: 10.1093/carcin/bgl250. [DOI] [PubMed] [Google Scholar]
- 26.Sun T, Miao X, Zhang X, Tan W, Xiong P, Lin D. Polymorphisms of death pathway genes FAS and FASL in esophageal squamous-cell carcinoma. J Natl Cancer Inst. 2004;96:1030–6. doi: 10.1093/jnci/djh187. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Miao XP, Zhang XM, et al. Genetic polymorphisms of apoptosis-associated genes FAS and FASL and risk of colorectal cancer. Zhonghua Yi Xue Za Zhi. 2005;85:2132–5. (in Chinese) [PubMed] [Google Scholar]
- 28.Muraki Y, Yoshioka C, Tateishi A, Fukuda J, Haneji T, Kobayashi N. Localization of Fas antigen in oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 1999;37:37–40. doi: 10.1054/bjom.1998.0298. [DOI] [PubMed] [Google Scholar]
- 29.Sundelin K, Jadner M, Norberg-Spaak L, Davidsson A, Hellquist HB. Metallothionein and Fas (CD95) are expressed in squamous cell carcinoma of the tongue. Eur J Cancer. 1997;33:1860–4. doi: 10.1016/s0959-8049(97)00216-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Wang LE, Sturgis EM, et al. Polymorphisms of FAS and FAS ligand genes involved in the death pathway and risk and progression of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2006;12:5596–602. doi: 10.1158/1078-0432.CCR-05-1739. [DOI] [PubMed] [Google Scholar]
- 31.Huang QR, Morris D, Manolios N. Identification and characterization of polymorphisms in the promoter region of the human Apo-1/Fas (CD95) gene. Mol Immunol. 1997;34:577–82. doi: 10.1016/s0161-5890(97)00081-3. [DOI] [PubMed] [Google Scholar]
- 32.Sibley K, Rollinson S, Allan JM, et al. Functional FAS promoter polymorphisms are associated with increased risk of acute myeloid leukemia. Cancer Res. 2003;63:4327–30. [PubMed] [Google Scholar]
- 33.Wu J, Metz C, Xu X, et al. A novel polymorphic CAAT/enhancer-binding protein h element in the FasL gene promoter alters Fas ligand expression: a candidate background gene in African American systemic lupus erythematosus patients. J Immunol. 2003;170:132–8. doi: 10.4049/jimmunol.170.1.132. [DOI] [PubMed] [Google Scholar]
- 34.Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J Cancer. 1932;51:1358. [Google Scholar]
- 35.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas-ligand induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 36.Strand S, Hofmann WJ, Hug H, et al. Lymphocyte apoptosis induced by CD95 (APO-1/CD95) ligandexpressing tumor cellsMa mechanism of immune evasion? Nat Med. 1996;2:1361–6. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 37.Muschen M, Warskulat U, Beckmann MW. Defining CD95 as a tumor suppressor gene. JMol Med. 2000;78:312–25. doi: 10.1007/s001090000112. [DOI] [PubMed] [Google Scholar]
- 38.Reichman E. The biological role of the Fas/FasL system during tumor formation and progression. Semin Cancer Biol. 2002;12:309–15. doi: 10.1016/s1044-579x(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 39.Kripple P, Langsenlehner U, Renner W, Koppel H, Samonigg H. Polymorphisms of death pathway genes FAS and FASL in esophageal squamous-cell carcinoma. J Natl Cancer Inst. 2004;96:1478–9. doi: 10.1093/jnci/djh289. [DOI] [PubMed] [Google Scholar]
- 40.Ueda M, Terai Y, Kanda K, et al. Fas gene promoter –670 polymorphism in gynecological cancer. Int J Gynecol Cancer. 2006;16(Suppl 1):179–82. doi: 10.1111/j.1525-1438.2006.00505.x. [DOI] [PubMed] [Google Scholar]
- 41.Sun T, Zhou Y, Li H, et al. FASL 844C polymorphism is associated with increased activation-induced T cell death and risk of cervical cancer. J Exp Med. 2005;202:967–74. doi: 10.1084/jem.20050707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Do KA, Johnson MM, Doherty DA, et al. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States) Cancer Causes Control. 2003;14:131–8. doi: 10.1023/a:1023060315781. [DOI] [PubMed] [Google Scholar]
- 43.Hashibe M, Ritz B, Le AD, Li G, Sankaranarayanan R, Zhang ZF. Radiotherapy for oral cancer as a risk factor for second primary cancers. Cancer Lett. 2005;220:185–95. doi: 10.1016/j.canlet.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Argiris A, Brockstein BE, Haraf DJ, et al. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin Cancer Res. 2004;10:1956–62. doi: 10.1158/1078-0432.ccr-03-1077. [DOI] [PubMed] [Google Scholar]