Abstract
Study Objectives:
We hypothesized that positional therapy would be equivalent to continuous positive airway pressure (CPAP) at normalizing the apnea-hypopnea index (AHI) in patients with positional obstructive sleep apnea (OSA).
Methods:
Thirty-eight patients (25 men, 49 ± 12 years of age, body mass index 31 ± 5 kg/m2) with positional OSA (nonsupine AHI < 5 events/h) identified on a baseline polysomnogram were studied. Patients were randomly assigned to a night with a positional device (PD) and a night on CPAP (10 ± 3 cm H2O).
Results:
Positional therapy was equivalent to CPAP at normalizing the AHI to less than 5 events per hour (92% and 97%, respectively [p = 0.16]). The AHI decreased from a median of 11 events per hour (interquartile range 9-15, range 6-26) to 2 (1-4, 0-8) and 0 events per hour (0-2, 0-7) with the PD and CPAP, respectively; the difference between treatments was significant (p < 0.001). The percentage of total sleep time in the supine position decreased from 40% (23%-67%, 7%-82%) to 0% (0%-0%, 0%-27%) with the PD (p < 0.001) but was unchanged with CPAP (51% [36%-69%, 0%-100%]). The lowest SaO2increased with the PD and CPAP therapy, from 85% (83%-89%, 76%-93%) to 89% (86%-9%1, 78%-95%) and 89% (87%-91%, 81%-95%), respectively (p < 0.001). The total sleep time was unchanged with the PD, but decreased with CPAP, from 338 (303-374, 159-449) minutes to 334 (287-366, 194-397) and 319 (266-343, 170-386) minutes, respectively (p = 0.02). Sleep efficiency, spontaneous arousal index, and sleep architecture were unchanged with both therapies.
Conclusion:
Positional therapy is equivalent to CPAP at normalizing the AHI in patients with positional OSA, with similar effects on sleep quality and nocturnal oxygenation.
Citation:
Permut I; Diaz-Abad M; Chatila W; Crocetti J; Gaughan JP; D'Alonzo GE; Krachman SL. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med 2010;6(3):238-243.
Keywords: Positional obstructive sleep apnea, positional therapy, CPAP, polysomnogram
Obstructive sleep apnea (OSA) syndrome is highly prevalent in the general population1 and has been identified as an independent risk factor for a number of cardiovascular diseases.2–5 Continuous positive airway pressure (CPAP) is presently the most common treatment for patients with OSA, as it has been shown to be efficacious in regard to its effect on sleep disordered breathing,6 as well as cognition and quality of life.7 However, adherence with CPAP therapy is poor, with compliance rates of less than 50%.8 Other common therapeutic options have included surgical intervention of the upper airway, weight loss, the use of oral appliances, and when indicated, positional therapy.9–13
BRIEF SUMMARY
Current Knowledge/Study Rationale: Previous studies evaluating positional therapy simply defined positional obstructive sleep apnea (OSA) as a 50% reduction in the apnea-hypopnea index (AHI) while in the nonsupine position, and as a result many patients still demonstrated mild to severe OSA with the use of positional therapy. The present study evaluates the effectiveness of positional therapy, as compared to CPAP therapy, to normalize the AHI to < 5 events/hr in patients with mild to moderate positional OSA.
Study Impact: Positional therapy was found to be effective at maintaining patients in the nonsupine position during the night, and was equivalent to CPAP therapy at normalizing the AHI to < 5 events/hr, with similar effects on nocturnal oxygenation and sleep quality. As a result, positional therapy may be an appropriate primary treatment for patients with positional OSA.
More recently, data have identified that positional OSA, where sleep-disordered breathing events occur predominantly in the supine position, is quite prevalent in patients with mild and moderate OSA.14 Using the definitions for OSA severity from the American Academy of Sleep Medicine (AASM),15 Mador et al.14 found that 50% of patients with mild OSA (apnea-hypopnea index [AHI] of 5-15 events/h) and 19% of patients with moderate OSA (AHI of 15-30 events/h) both normalized their AHI to less than 5 events per hour and reduced their AHI by more than 50% when in the nonsupine position.
Although the AASM has recommended that positional therapy be regarded as an effective secondary therapy or as a supplement to primary therapies for OSA in patients who have a low AHI in the nonsupine versus the supine position,16,17 many of the previously reported studies upon which these recommendations are based defined successful treatment as an AHI that would still be considered OSA and have been underpowered.9–13 These studies have included those utilizing training methods9–10 or devices designed to prevent the patients from rolling on their back.11–13
Because of the reported prevalence of positional OSA, and the lack of well-designed studies, we compared positional therapy with conventional therapy with CPAP in patients with positional OSA. We hypothesized that positional therapy would be equivalent to CPAP at normalizing the AHI to less than 5 events per hour in patients with positional OSA. In addition, we hypothesized that positional therapy would be effective at maintaining patients in the lateral position during sleep and would be equivalent to CPAP in regard to changes in nocturnal oxygenation and sleep quality.
MATERIALS AND METHODS
Patient Selection
Patients with diagnosed positional OSA from 3 participating sleep centers were asked to participate in the study. Positional OSA was defined as an overall AHI of at least 5 events per hour with symptoms of excessive daytime sleepiness or an AHI of at least 15 events per hour with a 50% decrease in the AHI when the patient was sleeping in the nonsupine position, as compared with in the supine position. Additionally, the AHI must have fallen to less than 5 events per hour when the patient was in the nonsupine position, and the patient must have slept in the lateral position for a minimum of 1 hour during the study. Only patients with mild (AHI = 5-15 events/h) and moderate (AHI = 15-30 events/h) OSA were asked to participate.15 Patients were excluded from participating in the study if they (1) had other conditions that might interfere with sleep (underlying chronic respiratory disorders, uncontrolled allergies, heart failure, narcolepsy, periodic leg movements); (2) currently used ventilatory stimulants or depressants (nicotine, theophylline, acetozolamide, morphine derivatives, sedatives, β-adrenergic receptor-blocking agents, salicylates); (3) had associated obesity hypoventilation syndrome; (4) had facial abnormalities that would preclude the effective use of CPAP; (5) used CPAP within the past year; (6) were pregnant; (7) were unwilling to participate in all aspects of the study; or (8) were unable to sign informed consent. The study was approved by the Institutional Review Board for Human Research (Temple University School of Medicine, Philadelphia, PA).
Positional Device
The Zzoma Positional Sleeper is 12 × 5.5 × 4 inches in size and made of lightweight semirigid synthetic foam (Sleep Specialists, LLC, Abington, PA) (Figure 1). It is contained in a backpack-type material with an associated Velcro elastic belt. The Zzoma Positional Sleeper is worn on the back, with the elastic belts brought around each side of the patient and secured anteriorly (Figure 1). The theoretical advantage of this new device comes from its particular size and wedge-shaped design on both sides, which keeps patients comfortably positioned on their side and prevents them from assuming the supine position.
Figure 1.
Photographs of the positional device (left panel) and how it was positioned on the patients during the study (right panel)
Protocol
All patients had an initial baseline polysomnogram study that identified the presence of positional OSA. Patients were then randomly assigned to a second polysomnogram study that consisted of either a CPAP therapy night or a full-night polysomnogram using the positional device (PD). A third polysomnogram study consisted of the opposite therapy for each patient.
During the CPAP-titration polysomnogram, the patients had CPAP therapy administered starting at 5 cm h2o and titrated upward in 2-cm h2o increments to a level that eliminated most obstructive apneas and hypopneas and could be tolerated. For the PD polysomnogram studies, the PD was placed on the patient and secured for fit and comfort (Figure 1). A questionnaire was completed at the end of the protocol comparing the treatment modalities, and no patients received any therapy until they completed the study protocol.
Polysomnogram
Polysomnograms were performed while the patients were breathing room air and consisted of a recording of rib cage and abdominal motion (Respitrace; Ambulatory Monitoring; White Plains, NY), with air flow measured using a pressure transducer. Snoring was monitored using a snore microphone. The patients wore a position sensor on their chests. In addition, body position was recorded by the technician who was directly observing the patient during the night. Synchronized digital video recordings were also obtained on all patients and reviewed during the scoring process to confirm body position. Any discrepancies between the sensor- and technician-recorded body position were resolved upon review of the video recordings. Other recordings included pulse oximetry (Cephalo Pro, Viasys Healthcare, Yorba Linda, CA), electrocardiogram, electrooculogram, digastric electromyogram, and electroencephalogram. All variables were continuously recorded and stored in a computerized system (Viasys Healthcare). Sleep was staged, and arousals were defined using established criteria.18 Obstructive apneas were defined by the lack of airflow for more than 10 seconds, associated with the presence of ribcage and abdominal movement.18 Obstructive hypopneas were defined by a 30% decrease in airflow for more than 10 seconds, associated with the presence of ribcage and abdominal movement, and accompanied by an oxygen desaturation of at least 4% or a 50% decrease in airflow associated with a 3% or greater decrease in oxygen saturation or an arousal.18 Apneas were defined as central if there was a lack of respiratory effort during the period of absent airflow.18 The AHI was calculated as the number of apneic and hypopneic events per hour of sleep. An arousal was defined as an abrupt shift of electroencephalographic frequency, including alpha, theta, or frequencies greater than 16 Hz (but not spindles) that lasted at least 3 seconds, with at least 10 seconds of stable sleep preceding the change.18 Other calculated variables included total sleep time, sleep efficiency (total sleep time divided by time in bed), arousal index, supine and nonsupine AHI, and the percentage of total sleep time with an arterial oxygen saturation (SaO2) of less than 90%. All of the polysomnogram studies were initially scored by a single senior technologist. The same author (SK) reviewed each study.
Statistical Analysis
Patient characteristics are presented as mean ± SD. All other data are represented as the median (interquartile range, range), unless otherwise specified. The design of the study was a 3-period cross-over. The study was an equivalence (noninferiority) trial, comparing effects on the AHI (events/h). The proportion of patients in each group experiencing an AHI of fewer than 5 events per hour of sleep was compared. The clinically meaningful difference to establish equivalency (noninferiority) was chosen to be 9%. The margin of 9% was based on the acceptance that, in the treatment of OSA, therapies that are shown to be more than 90% effective are considered to be clinically equivalent. The sample size of 38 was based on the between-treatment-group (baseline vs PD vs CPAP therapy) comparison of proportions at an α level of 0.05 (one-sided, 80% power). The proportions of patients in each treatment group having an AHI of fewer than 5 events per hour were analyzed using McNemars test for correlated proportions. If the difference between groups was within the 9% noninferiority margin, the PD was considered to be noninferior to CPAP therapy. A 2-way analysis of variance for repeated measures (treatment group and period) was used to compare various sleep measurements (including AHI) at baseline, with the PD and with CPAP therapy. Since all parameters failed the Wilk-Shapiro test for normality, the dependent variables were transformed into normalized ranks prior to analysis. Follow-up pairwise multiple comparisons were carried out between groups and included a Bonferroni correction. The oxygenation variables were described descriptively across treatment groups. All statistical analyses were carried out using SAS V9.1.3 software (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Two hundred and forty-five consecutive patients were screened to identify 40 patients with mild or moderate positional OSA on their baseline polysomnogram. One patient refused to participate in the study, and another dropped out after the CPAP night due to a death in the family. Therefore, 38 patients (25 men, aged 49 ± 12 years, body mass index 31 ± 5 kg/m2) agreed to participate and completed the study in a mean of 2.2 ± 1.3 months (Table 1). Twenty-nine were found to have mild OSA (10 ± 2 events/h) and 9 to have moderate OSA (21 ± 3 events/h), with an overall AHI of 13 ± 5 events per hour for the entire group. The supine AHI on the baseline study was 31 ± 19 events per hour, with a nonsupine AHI of 2 ± 1 events per hour. The mean time spent in the nonsupine position was 186 ± 80 minutes (56% ± 23% of total sleep time). The mean SaO2 during the night was 95% ± 2%, and the lowest SaO2 was 85% ± 4%. The percentage of total sleep time with an SaO2 less than 90% was 5% ± 10% (Table 1).
Table 1.
Characteristics of 38 patients
| Characteristic | Values |
|---|---|
| Age, y | 49 ± 12 |
| Men, no. | 25 |
| BMI, kg/m2 | 31 ± 5 |
| Baseline AHI, events/h | |
| Total | 13 ± 5 |
| Supine | 31 ± 19 |
| Nonsupine | 2 ± 1 |
| SaO2, % | |
| Mean | 95 ± 2 |
| Lowest | 85 ± 4 |
| TST with SaO2 < 90%, % | 5 ± 10 |
| TST, min | |
| Total | 336 ± 55 |
| Supine (% of TST) | 150 ± 83 (44 ± 23) |
| Nonsupine (% of TST) | 186 ± 80 (56 ± 23) |
| Sleep efficiency, % | 85 ± 12 |
| Spontaneous Arousal Index, arousals/h | 21 ± 12 |
| Sleep stage, % of TST | |
| 1 | 14 ± 11 |
| 2 | 56 ± 11 |
| 3 | 11 ± 8 |
| REM | 16 ± 8 |
Data are presented as mean ± SD or number of patients.
BMI refers to body mass index; AHI, apnea-hypopnea index; TST, total sleep time; REM, rapid eye movement.
Comparison of Positional Therapy with CPAP Therapy
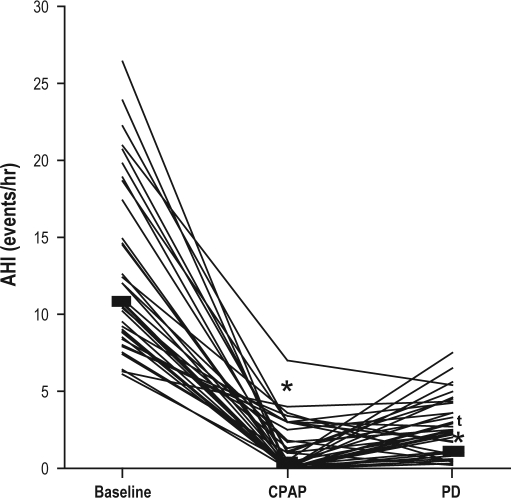
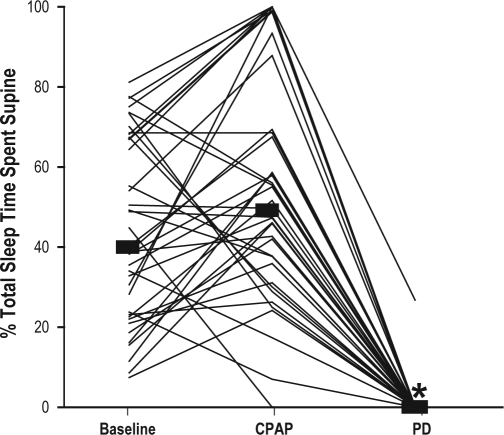
The proportion of patients that was able to normalize their AHI to fewer than 5 events per hour was equivalent with the PD (92%) and CPAP therapy (97%), (p = 0.16). When compared with baseline, both the PD and CPAP therapy (mean 10 ± 3 cm h2o) significantly decreased the AHI, from 11 (9-15, 6-26) events per hour to 2 (1-4, 0-8) and 0 (0-2, 0-7) events per hour with the PD and CPAP, respectively (p < 0.001), with a difference between the 2 treatments (p < 0.001) (Figure 2). The percentage of total sleep time spent in the supine position, when compared with baseline, was significantly decreased with the PD but unchanged with CPAP therapy, from 40% (23%-67%, 7%-81%) to 0% (0%-0%, 0%-27%) and 51% (36%-69%, 0%-100%), respectively (p < 0.001 comparing baseline with the PD) (Figure 3).
Figure 2.
Effects of the positional device (PD) and continuous positive airway pressure (CPAP) therapy on the apnea-hypopnea index (AHI)
When compared with baseline, both therapies significantly decreased the AHI (*p < 0.001), with a significant difference between the 2 treatments (tp < 0.001).
Figure 3.
When compared with baseline, the percentage of total sleep time spent in the supine position significantly decreased with the positional device (PD) (*p < 0.001) but was unchanged with continuous positive airway pressure (CPAP) therapy.
The mean SaO2 during the night was unchanged compared with baseline with the use of the PD but was increased with CPAP therapy from 95% (94%-96%, 92%-98%) to 95% (94%-96%, 91%-98%) and 96% (95%-97%, 93%-99%), respectively (p = 0.01). There was a increase in the lowest SaO2 during the night with both the PD and CPAP therapy, from 85% (83%-89%, 76%-93%) to 89% (86%-91%, 78%-95%) and 89% (87%-91%, 81%-95%), respectively (p = 0.02), with no difference between the 2 treatment modalities. The percentage of total sleep time with an SaO2 less than 90% was significantly decreased compared with baseline with the use of the PD and CPAP therapy, falling from 0.85% (0%-3.1%, 0%-51%) to 0.03% (0%-0.5%, 0%-37%) and 0% (0%-0.3%, 0%-12%), respectively (p < 0.001), with no significant difference between the 2 treatments.
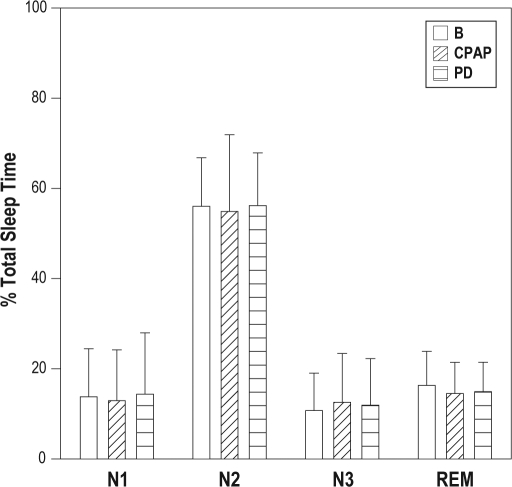
When compared with baseline, total sleep time did not change with the PD but decreased with CPAP therapy, falling from a baseline of 338 minutes (303-374, 159-449) to 334 minutes (287-336, 194-397) with the PD and 319 minutes (266-343, 170-386) with CPAP therapy (p = 0.02 compared with baseline). There was no change in sleep efficiency noted with either treatment, from a baseline of 89% (79%-93%, 38%-97%) to 88% (82%-94%, 48%-98%) and 85% (72%-92%, 46%-98%), with the PD and CPAP therapy, respectively (p = 0.17). Similarly, the spontaneous arousal index did not change from a baseline of 22 arousals per hour (11-28, 2-54) to 16 arousals per hour (10-24, 5-58) with the PD and 12 arousals per hour (9-19, 1-53) with CPAP (p = 0.06). The sleep architecture, expressed as a percentage of total sleep time, including stage 3 and rapid eye movement sleep, was not different, as compared with baseline, for either the PD or CPAP therapy (Figure 4).
Figure 4.
When compared with baseline (B), sleep architecture, expressed as a percentage of total sleep time, was unchanged with the positional device (PD) and continuous positive airway pressure (CPAP) therapy.
N1 refers to sleep stage 1; N2, sleep stage 2; N3, sleep stage 3; REM, rapid eye movement sleep.
Based on the questionnaire responses, 50% of patients preferred the PD, 34% preferred CPAP therapy, and 16% had no preference.
DISCUSSION
Despite the high prevalence of positional OSA, the effectiveness of positional therapy still needs to be defined. There were 4 major findings in this study: (1) in patients with positional OSA, positional therapy is equivalent to CPAP therapy at normalizing the AHI to fewer than 5 events per hour, in addition to decreasing the AHI by more than 50%; (2) positional therapy is similar to CPAP therapy in regard to effects on sleep quality and nocturnal oxygenation; (3) there is minimal night-to-night variability in the nonsupine AHI in patients with positional OSA; and (4) our PD is effective at maintaining patients in the nonsupine position throughout the night.
Positional OSA has been reported to be present in up to 50% to 60% of all patients with diagnosed OSA.9–13,19–21 However, most of the earlier studies, including those evaluating the effectiveness of positional therapy, simply defined positional OSA as a 50% reduction in the AHI when sleeping in the nonsupine position.9–13,19 Therefore, patients were often included who had an AHI that would still be considered mild to moderate OSA when the patients were not supine. More recently, Mador et al.14 examined the prevalence of positional OSA using a definition of normalizing the AHI to less than 5 when patients were in the nonsupine position. Using this narrow, possibly therapeutically more relevant definition, they also reported a high prevalence of positional OSA (27% overall), with 50% of patients with mild OSA and 19% of those with moderate OSA having positional OSA. Only 7% of patients with severe OSA met their criteria for positional OSA.14
A number of previous studies have examined the effects of positional therapy on sleep disordered breathing.9–13 Cartwright et al.9 examined the effects of sleep-position training using a position-based alarm in 10 patients with positional OSA. An initial decrease in the AHI with the alarm was noted to continue after 3 months of instruction to remain sleeping on their side at home (from 55 to 21 and 38 events/h, respectively). A follow-up study by the same investigators comparing 4 different methods of therapy demonstrated similar results in patients instructed to learn to sleep in the nonsupine position after 8 weeks.10 Similar findings were noted in a small observational study.12 Jokic et al.,11 in a randomized cross-over study of 13 patients, compared positional therapy using a backpack with a softball to CPAP therapy. After 2 weeks of treatment, both positional therapy and CPAP decreased the AHI (from 18 to 10 and 3 events/h, respectively), with the decrease with CPAP statistically more significant and associated with a normalization of the AHI (< 5 events/h). More recently, Skinner et al.,13 in a similar randomized, cross-over trial over 1 month of each treatment, compared a device designed to mimic the tennis-ball technique with CPAP therapy in 20 patients with positional OSA. As in the previous study, as compared with baseline, both the PD and CPAP decreased the AHI (from 23 to 12 and 5 events/h, respectively). A problem with all these prior studies relates to the liberal definition used to define positional OSA as a 50% reduction in the AHI while patients were in the nonsupine position rather than to normalize the AHI to fewer than 5 events per hour. As a result, although the results were statistically significant, many patients still demonstrated what would be considered mild to severe OSA with the use of positional therapy.9–13 In our study, we defined patients with positional OSA as having an AHI of fewer than 5 events per hour while in the nonsupine position as well as a decrease in the AHI by more than 50%. Positional therapy was equivalent to CPAP (92% vs 97%, respectively [p = 0.16]) at normalizing the AHI.
In the prior studies, in addition to including patients with an AHI of more than 5 events per hour in the nonsupine position based on the baseline study, difficulty normalizing the AHI in the lateral position was also related to the inability of the therapy to always eliminate supine sleep.9–13 In the study by Jokic et al.,11 3 of the 13 patients (23%) slept supine for at least 18 minutes. Cartwright et al.9 were more successful with the use of a positional alarm, with only 1 of the 10 patients sleeping supine for 18 minutes. However, at 3 months, 4 out of 10 patients slept supine for at least 32 minutes, demonstrating that the initial success was not sustainable. In their later study, Cartwright et al.10 also demonstrated that counseling on behavior modification and position modification was successful in eliminating supine sleep only 53% of the time. In the more recent study by Skinner et al.,13 patients spent 6% of their total sleep time in the supine position using their tennis-ball device. In the present study, our PD eliminated supine sleep in 37 of the 38 patients, with only a mean of 1 ± 4% of total sleep time spent supine. We believe that these results are related to the unique shape of the device and the hard foam material inside.
When evaluating the effects of positional therapy on sleep quality and nocturnal oxygenation, our findings are similar to those of prior studies. Jokic et al.11 demonstrated no difference in sleep quality between positional therapy and CPAP, as measured by total sleep time and sleep efficiency, with similar results noted by Skinner et al.13 No difference was noted in sleep architecture with either therapy in these studies,11,13 nor was a difference found in studies that have used a positional alarm.9 In regard to nocturnal oxygenation, Cartwright et al.9 showed a decrease in the number of desaturations and lowest SaO2during the night with use of a positional alarm. Jokic et al.11 noted no difference in mean SaO2, but the lowest SaO2was less with positional therapy, as compared with CPAP. Skinner et al.13 noted a clinically insignificant difference in mean SaO2 during the night between CPAP and positional therapy. We believe that one of the reasons we did not note a significant change in sleep quality with either CPAP or positional therapy is that we studied patients with only mild and moderate OSA (mean AHI = 13 ± 5 events/hr). Our results do support the finding that positional therapy can be used comfortably during the night without disrupting sleep quality.
There are a number of limitations with our study that need to be addressed. First, we only studied the acute 1-night effects of the PD, as compared with CPAP. Assessment of effectiveness would require the use of other outcome measures, such as daytime sleepiness, cognitive function, and quality of life, all of which would have to be evaluated in a randomized trial after more prolonged use and would be influenced by compliance. Compliance would be dependent on a number of factors, including comfort with the PD and perceived benefit. Whether prolonged use with this device will lead to sustained beneficial results will need to be determined. Second, we used both of the AASM recommended rules for scoring hypopneas, which may include events associated with arousals but without a decrease in SaO2.18 However, these are clinically accepted methods for scoring hypopneas, and we do not believe that this method inflated our AHI. In regard to possibly underscoring sleep disordered breathing events, it should be noted that we did not use esophageal catheters to rule out whether there was a conversion from apneas and hypopneas to flow-limited arousals when patients moved from the supine to lateral position. However, there was no flattening of the airflow signal leading to arousals, as measured with pressure transducers.22,23 In addition, the number of spontaneous arousals during the PD night was similar to that seen during both the baseline and CPAP therapy nights, at 16 (10-24, 5-58) arousals per hour, 22 (11-28, 2-54) and 12 (9-19, 1-53) arousals per hour, respectively (p = 0.06). Finally, we did not study patients with severe OSA. Results in this patient population will need to be verified. However, only 7% of patients with severe OSA have positional OSA, thus representing a small percentage overall of those with positional OSA.14
In conclusion, in patients with mild and moderate positional OSA, positional therapy is equivalent to CPAP therapy at normalizing the AHI. In addition, positional therapy is effective at maintaining sleep in the nonsupine position during the night and is similar to CPAP therapy in its effects on sleep quality and nocturnal oxygenation. Whether more prolonged use will maintain these effects and how positional therapy compares with CPAP in regard to cognitive function, compliance, and quality of life awaits further study.
DISCLOSURE STATEMENT
Financial support was provided by an Innovator Circle Grant from Abington Memorial Hospital, Abington, PA. Sleep Specialists, LLC, supplied the positional devices for the study and paid for a portion of the polysomnograms that were performed at Abington Memorial Hospital. Drs. Crocetti and Krachman have a financial interest in Sleep Specialists, LLC, makers of the Zzoma Positional Sleeper. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Work for this study was performed at Temple University Hospital Sleep Disorders Center—Temple University Health System, Philadelphia, PA; Jeanes Hospital Sleep Disorders Center—Temple University Health System, Philadelphia, PA; and Abington Memorial Hospital Sleep Disorders Center, Abington PA.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(1378):84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 6.Grunstein RR. Sleep-related breathing disorders: 5 nasal continuous positive airway pressure treatment for obstructive sleep apnoea. Thorax. 1995;50:1106–13. doi: 10.1136/thx.50.10.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMahon JP, Foresman BH, Chisholm RC. The influence of CPAP on neurobehavioral performance of patients with obstructive sleep apnea hypopnea syndrome. A systemic review. WMJ. 2003;102:36–43. [PubMed] [Google Scholar]
- 8.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 9.Cartwright RD, Lloyd S, Lilie J, Kravitz H. Sleep position training as treatment for sleep apnea syndrome: A preliminary study. Sleep. 1985;8:87–94. doi: 10.1093/sleep/8.2.87. [DOI] [PubMed] [Google Scholar]
- 10.Cartwright R, Ristanovic R, Diaz F, Caldarelli D, Alder G. A comparative study of treatments for positional sleep apnea. Sleep. 1991;14:546–52. doi: 10.1093/sleep/14.6.546. [DOI] [PubMed] [Google Scholar]
- 11.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea. Chest. 1999;115:771–81. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- 12.Kavey NB, Blitzer A, Gidro-Frank S, Korstanje K. Sleeping position and sleep apnea syndrome. Am J Otolaryngol. 1985;6:373–7. doi: 10.1016/s0196-0709(85)80015-6. [DOI] [PubMed] [Google Scholar]
- 13.Skinner MA, Kingshott RN, Filsell S, Taylor DR. Efficacy of the ‘tennis ball technique’ versus NCPAP in the management of position-dependent obstructive sleep apnoea syndrome. Respirology. 2008;13:708–15. doi: 10.1111/j.1440-1843.2008.01328.x. [DOI] [PubMed] [Google Scholar]
- 14.Mador MJ, Kufel TJ, Magalang UJ, et al. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128:2130–7. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 15.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research; the report of an American Academy of Sleep Medicine task force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 16.Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29:1031–5. [PubMed] [Google Scholar]
- 17.Epstein LJ, Kristo D, Strollo PJ, Friedman NF, Malhotra A, Patil AP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. Westchester, Ill: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 19.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients: Anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112:629–39. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 20.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7:110–114. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 21.Akita Y, Kawakatsu K, Hattori C, Hattori H, Suzuki K, Nishimura Posture of patients with sleep apnea during sleep. Acta Otolaryngol. 2003;550(Suppl):41–5. [PubMed] [Google Scholar]
- 22.Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer system. Am J Respir Crit Care Med. 1998;157:1461–67. doi: 10.1164/ajrccm.157.5.9708008. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PK, Edwards N, Gurgess KR, and Sullivan CE. Detection of increased upper airway resistance during overnight polysomnography. Sleep. 2005;28:85–90. doi: 10.1093/sleep/28.1.85. [DOI] [PubMed] [Google Scholar]