Abstract
Study Objective:
To address the influence of gender and obstructive sleep apnea (OSA) on development of diabetes mellitus (DM) in a sleep clinic cohort.
Design:
A longitudinal observational study.
Participants:
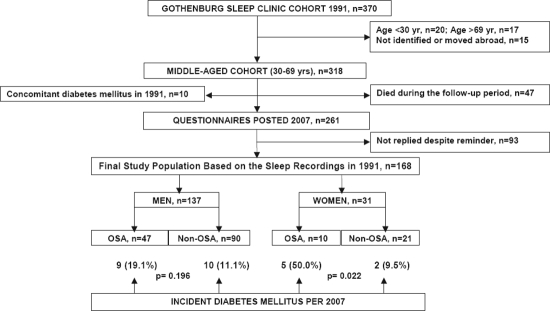
A consecutive middle-aged (30-69 years) sleep clinic cohort from 1991 (n = 318; 254 men, 64 women) with eligible baseline characteristics, clinical charts, and information from the Swedish Hospital Discharge Registry were identified. Ten individuals with DM at baseline and 47 patients who died during the follow-up period were excluded.
Measurements:
The remaining 261 subjects were asked to complete a postal questionnaire regarding concomitant diseases including DM, diagnosed by a physician.
Results:
In total, 168 patients (64.4%) replied. The incidence of DM was 24.9% in patients with OSA (overnight oxygen desaturations ≥ 30 in 1991) compared with 10.8% in subjects without OSA (p = 0.020). New-onset DM in men was 19.1% in OSA vs 11.1% in non-OSA (n.s.), while the corresponding values in women were 50.0% in OSA and 9.5% in non-OSA (p = 0.022). In a multivariate analysis, DM was predicted by OSA in women with an odds ratio (OR) of 11.8, but not by age, body mass index (BMI) at baseline, or weight change at follow-up. In men, only BMI (OR 1.16) predicted DM.
Conclusion:
The contribution of OSA to DM development seems to be gender-dependent and higher in women than in men.
Citation:
Celen YT; Hedner J; Carlson J; Peker Y. Impact of gender on incident diabetes mellitus in obstructive sleep apnea: a 16-year follow-up. J Clin Sleep Med 2010;6(3):244-250.
Keywords: Diabetes mellitus, gender, sleep apnea
Diabetes mellitus (DM) has been regarded as a major public health concern and one of the main causes of morbidity and mortality. The potential effect of gender on DM development has been extensively addressed in the literature. A clear male predominance of type 1 DM is seen across the age range. However, type 2 DM, which accounts for almost 90% of all DM cases, particularly in association with the increasing obesity rate, occurs more frequently in women.1 Moreover, many of the complications such as diabetic ketoacidosis, dyslipidemia, peripheral vascular disease, and lipodystropathy, as well as hypertension, coronary artery disease (CAD), and sudden death are more common in diabetic women compared to men with DM.1,2
BRIEF SUMMARY
Current Knowledge/Study Rationale: Cross-sectional studies suggest an independent association between obstructive sleep apnea and diabetes mellitus. Few data exist regarding the incident diabetes in sleep-clinic cohorts at long-term and impact of gender in this context.
Study Impact: This 16-year follow-up study demonstrates an increased incidence of physician diagnosed diabetes mellitus among patients with obstructive sleep apnea with significant oxygen desaturations. The contribution of intermittent hypoxemia to diabetes development seems to be gender dependent and higher in women than in men.
Obstructive sleep apnea (OSA) is characterized by repetitive collapse of the upper airway during sleep, which leads to progressive hypoxia and hypercapnia. The prevalence of OSA before the age of 60 is known to be two or three times as high in men as in women.3 Hormonal changes during menopause may be the cause of a change in this proportion at higher age. In sleep clinic cohorts, female OSA patients are usually older and more obese than male subjects with OSA at the time of the diagnosis.4
The risk factors age and central abdominal obesity are common both in OSA and DM. Several cross-sectional studies have identified a higher prevalence of OSA among adults with insulin resistance or DM.5,6 Likewise, there are data suggesting a higher prevalence of DM in subjects with OSA independent of age, gender, and obesity.7 In a recent report from Australia, moderate to severe OSA was suggested to be a significant risk factor for incident DM in a population-based cohort.8 Moreover, OSA and DM may constitute additional or synergistic risk factors for cardiovascular morbidity and mortality.9 Hence, the International Diabetes Federation Taskforce on Epidemiology and Prevention has recently recommended that health professionals working in both DM and OSA should ensure that a patient presenting with one condition is considered for the other.10
In general, several reports have displayed a link between sleep duration and impaired insulin metabolism suggesting that sleep loss could also contribute to development of DM.11 In one study, a relationship between short (≤ 6 h) or long (≥ 8 h) sleep duration and increased risk of DM was found in middle-aged women but not in men.12 Conversely, another study demonstrated a higher incidence of DM in men with short or long sleep duration.13 However, less is known regarding the impact of gender in the development of DM in OSA patients. Habitual snoring, a surrogate marker of OSA, was found to be a risk factor for the development of DM over 10 years, independent of confounding factors in a community-based male population.14 A more recent prospective study associated snoring and sleepiness with an elevated risk of DM in the female population,15 and this gender difference was also demonstrated for the relationship between DM and snoring/witnessed apnea.16 Thus, the existing evidence regarding the influence of gender on the development of DM in OSA patients is mostly based on the self-reported questionnaires rather than the objective measures of OSA.
In the current study, we addressed the impact of gender on development of DM in a consecutive sleep clinic cohort with OSA diagnosed by overnight polygraphy.
METHODS
Study Population
The study population has been described in detail elsewhere.17 In brief, 370 consecutive cases referred to the sleep laboratory with a history of snoring and/or witnessed apnea underwent an overnight polygraphic recording in the sleep laboratory. The patients were enrolled regardless of a history of excessive daytime sleepiness (EDS). By review of baseline data of this cohort, 20 young (age < 30 y) and 17 elderly (age > 69 y) subjects were excluded. Likewise, 15 individuals who had moved abroad or could not be identified and/or localized by the Population Register of the National Tax Board of Sweden were excluded (Figure 1). For the remaining 318 middle-aged (30-69 y) subjects, complementary information on health status was obtained from the Swedish Hospital Discharge Register (SHDR) via the Center for Epidemiology, National Board of Health and Welfare (see below) as well as from clinic charts from the event of the baseline recording. Ten patients who had DM at baseline were excluded. Each subject was followed up for 16 years from January 1, 1991, to December 31, 2007. Forty-seven patients died during the follow-up period. The remaining 261 subjects were asked to complete a questionnaire (see below). After one reminder by post, 168 (64.4%) replied by December 31, 2007, and were identified for the present study (Figure 1). The Ethics Committee of the Medical Faculty of the University of Gothenburg approved the study protocol.
Figure 1.
Patient log demonstrating the study cohort and the different subgroups
Baseline Investigations
All overnight sleep studies were assessed in the sleep laboratory. Investigations were initiated at approximately 23:00 and finalized at 06:00. Lights out and lights on were recorded along with the subjective sleep quality and subjective sleep duration. The mean estimated sleep time was empirically chosen to be 6 h. The investigation included a continuous recording of transcutaneous arterial oxygen saturation (SpO2) via a finger probe (BIOX 3700; Ohmeda, Louisville, CO), nasal and oral airflow recorded via a thermistor, and respiration and body movement monitored via a static charge sensitive bed (SCSB [Bio-matt; Biorec Inc., Raisio, Finland]). Signals were amplified and recorded on a filter pen recorder (Kipp & Zonen, Delft, Holland). An apnea was scored when SpO2 dropped by at least 4% from the immediately preceding baseline simultaneously with absence of nasal and oral airflow as well as presence of chest movements for > 10 s. Scoring was made manually from each recording strip by trained technicians unrelated to the study itself. The total number of significant oxygen desaturations (OD) as well as the minimal SpO2reached during the overnight recording (SpO2min) was determined. An overnight OD ≥ 30 was defined as OSA. This value was based on previously established diagnostic criteria18 of an apnea index ≥ 5 for the sleep apnea syndrome, which was accepted at the time of the baseline investigations. The oxygen desaturation index (ODI) was defined as the average number of OD per hour. Body weight and height was measured at the time of sleep investigation. Body mass index (BMI) was calculated according to the formula body weight divided by height squared.
Swedish Hospital Discharge Register
The Swedish Hospital Discharge Register (SHDR) covers all public inpatient care since 1987. In the SHDR, there are 4 different types of information: patient related data (personal identification number, sex, age, place of residence); hospital related data (county council, hospital, department); administration related data (date of admission, discharge, length of stay, acute or planned admission, admitted from, discharged to); and medical data (main diagnosis, secondary diagnoses, external cause of injury and poisoning, surgical procedures). The classification of diseases was implemented according to ICD-9 codes until 1997 and ICD-10 codes thereafter. Data from the SHDR was obtained for a 3-year extension period prior to as well as a 7-year period following the baseline investigation.
Questionnaires
The postal questionnaires, sent to 261 survivors in the beginning of 2007, included questions regarding current height and weight, history of smoking, concomitant diseases (including DM) diagnosed by a physician, as well as hospital admissions, ongoing medication, and treatment for snoring or OSA during the follow-up period. Information regarding self-reports of physician diagnosed DM was checked with the SHDR reports. Drugs that were registered include those listed within the anatomical therapeutic chemical classification system codes C01–C08.19 BMI gain was defined as a change in BMI values from 1991 to 2007.
Treatment of OSA
OSA treatment was initiated by different physicians according to the clinical routines depending on severity of the sleep related breathing disorder, the extent of EDS, and social aspects of loud snoring. Treatment with continuous positive airway pressure (CPAP), surgery (uvulopalatopharyngoplasty [UPPP]), oral device, or weight counseling was offered to the patients in accordance with the prevailing contemporary routines. All patients undergoing surgery were invited for postoperative sleep study to evaluate the effectiveness of treatment. In the event of therapeutic failure, patients were offered CPAP or oral device. Therapeutic CPAP titration was performed according to the prevailing manual standardized procedure, using CPAP nasal pressure monitoring in an overnight laboratory setting. The therapeutic effect of CPAP was routinely reinvestigated 3 and 12 months after the initiation of treatment. Objective evaluation of CPAP use was obtained from the device time-counter (hours divided by days since last recording). Incompletely treated cases were regarded as OSA patients without treatment or with remaining OSA in spite of treatment with UPPP, oral device, or daily CPAP run-time < 50% of estimated sleep time at the first year follow-up. OSA patients who had stopped using CPAP devices according to the 2007 follow-up questionnaire were also regarded as incompletely treated. Efficiently treated OSA patients were defined as patients with OD < 30 at the renewed sleep study following UPPP, on treatment with oral device, or on CPAP with an objective daily CPAP run-time ≥ 50% of estimated sleep hours. Subjects regarded as inefficiently treated at 1 year were included in the efficiently treated group at 16-year follow-up if they were on CPAP therapy according to the questionnaires.
Statistics
Statistical analysis was performed using the statistical analysis program SPSS 15.0 for Windows (SPSS, Chicago, IL). Continuous values are given as means ± SD. A p value (2-sided) ≤ 0.05 was regarded as statistically significant. The χ2 test and Fisher exact test were used for comparison between categorical variables; when the comparison involved continuous variables, the t test was used. The nonparametric test (Mann-Whitney) was used when the normal distribution criteria was not the case. The influence of multiple variables was analyzed using multiple logistic regression analysis. All significant variables that correlated with the outcome measures in the univariate analyses were subsequently included in a multiple logistic regression model, and adjusted odds ratios (OR) were calculated from the regression coefficients in men and women, respectively. All ORs are presented with their 95% confidence intervals (CI).
RESULTS
A total number of 168 participants without DM in 1991 (137 men, 31 women; mean age 48.2 ± 9.0 years, mean BMI 26.6 ± 3.8 kg/m2 at baseline) replied to the questionnaires in 2007 (Figure 1). The response rate did not differ significantly between men (64.3%) and women (64.5%). No significant difference was found between responders and nonresponders with regard to age (48.2 vs 46.8 y), BMI (26.6 vs 26.7 kg/m2), current smoking (38.1% vs 39.3%), OSA diagnosis (33.9% vs 29.9%), or severity of OSA in terms of ODI (6.3 vs 7.4 /h) and SpO2min (85.8% vs 86.4%) at baseline.
OSA was present at baseline in almost a third of the final male and female cohorts (Figure 1). Patients with OSA were older and had a higher BMI than the subjects without OSA at baseline, but the proportion of current smokers did not differ significantly between the groups (Table 1).
Table 1.
Demographic and clinical characteristics of the final study population in 1991 and 2007
| Variable | OSA (n = 57) | non-OSA (n = 111) | p value |
|---|---|---|---|
| Age at baseline, years | 50.2 ± 7.8 | 47.2 ± 9.46 | 0.030 |
| Male gender, n (%) | 47 (82.5) | 90 (81.1) | 0.828 |
| BMI in 1991, kg/m2 | 28.2 ± 3.9 | 25.8 ± 3.5 | < 0.001 |
| BMI in 2007, kg/m2 | 29.6 ± 4.9 | 27.5 ± 5.1 | 0.015 |
| Current smokers in 1991, n (%) | 19 (33.3) | 45 (40.5) | 0.362 |
| Current smokers in 2007, n (%) | 10 (17.5) | 22 (19.8) | 0.722 |
| ODI in 1991, n/h | 18.4 ± 16.7 | 1.7 ± 1.8 | < 0.001 |
| SpO2min in 1991, % | 80.3 ± 7.5 | 88.6 ± 4.3 | < 0.001 |
Continuous variables are expressed as mean ± SD, statistics by unpaired Student t test. Comparison of groups by χ2test.
OSA refers to obstructive sleep apnea; BMI, body mass index; ODI, oxygen desaturation index; SpO2min, minimum oxygen saturation
As shown in Table 2, both male and female OSA patients had a higher BMI at baseline than the respective non-OSA groups. OSA in women was also associated with a higher age. In subjects without OSA, there was no gender difference regardng age or BMI at baseline; whereas in the OSA group, women were significantly more obese and older than men (Table 2).
Table 2.
Demographic and clinical characteristics of males and females in 1991 and 2007 stratified by OSA
| Variable | OSA | non-OSA | p value |
|---|---|---|---|
| Males | |||
| N | 47 | 90 | |
| Age at baseline, years | 49.4 ± 7.8a | 47.0 ± 9.3 | 0.147 |
| BMI in 1991, kg/m2 | 27.6 ± 3.5b | 25.8 ± 3.5 | 0.002 |
| BMI in 2007, kg/m2 | 28.8 ± 4.3c | 27.4 ± 5.0 | 0.103 |
| Current smokers in 1991, n (%) | 15 (31.9) | 34 (37.8) | 0.497 |
| Current smokers in 2007, n (%) | 8 (17.0) | 15 (16.7) | 0.958 |
| ODI, n/h | 19.1 ± 17.2 | 1.8 ± 1.8 | < 0.001 |
| SpO2min, % | 81.0 ± 7.6 | 88.5 ± 3.8 | < 0.001 |
| Females | |||
| N | 10 | 21 | |
| Age at baseline, years | 54.3 ± 6.8a | 47.8 ± 10.3 | 0.047 |
| BMI in 1991, kg/m2 | 30.5 ± 5.0b | 26.0 ± 4.9 | 0.028 |
| BMI in 2007, kg/m2 | 33.1 ± 6.0c | 28.1 ± 5.9 | 0.044 |
| Current smokers in 1991, n (%) | 4 (40.0) | 11 (52.4) | 0.704 |
| Current smokers in 2007, n (%) | 2 (20.0) | 7 (33.3) | 0.677 |
| ODI, n/h | 15.3 ± 11.0 | 1.4 ± 2.0 | < 0.001 |
| SpO2min, % | 77.0 ± 6.4 | 88.6 ± 6.0 | < 0.001 |
Continuous variables are expressed as mean ± SD, statistics by unpaired Student's t test. Comparison of groups by χ2test or Fisher exact test (two-sided). OSA refers to obstructive sleep apnea; BMI, body mass index; ODI, oxygen desaturation index; SpO2min, minimum oxygen saturation;
p = 0.070;
p = 0.033;
p = 0.010.
After the baseline investigations, treatment of OSA was initiated with CPAP (n = 20) and/or UPPP (n = 26) and/or an oral device (n = 7), whereas no active treatment was considered in 14 (24.6%) patients with either mild OSA and/or absence of EDS. Among the CPAP-treated OSA patients, 6 (30.0%) cases returned the device or had low compliance with treatment at follow-up. Two of these received sucessful treatment with an oral device. Of the subjects undergoing UPPP, 15 (57.7%) still exhibited OSA at the follow-up recording. Three of these patients were given CPAP and 2 received oral devices. Among 7 subjects receiving an oral device, 4 were considered inefficiently treated, and 2 of these were allocated to CPAP therapy. Thus, in the whole cohort, 31 (54.4%) OSA patients were regarded as efficiently treated by CPAP and/or UPPP and/or oral device. No difference was observed with regard to gender in treatment effectiveness (55.3% in men vs50.0% in women).
Based on questionnaires, incident DM, diagnosed by a physician, was reported in 26 cases (15.5%). Of these, 14 had OSA (24.6%) and 12 did not (10.8%; p = 0.020). In the whole cohort, regardless of OSA diagnosis at baseline, 19 men (13.9%) and 7 women (22.6%; n.s) reported DM at follow-up. As illustrated in Figure 1, DM was reported by 9 OSA patients and by 10 non-OSA subjects in men (n.s) and by 5 OSA patients and 2 non-OSA subjects (p = 0.022) in women. When comparing the subgroups of OSA patients with incident DM with regard to gender, the women were older (mean age 56.0 ± 6.1 vs 48.0 ± 4.7 y; p = 0.018) and slightly more obese (mean BMI 30.1 ± 6.6 vs 29.0 ± 3.9 kg/m2; n.s), but had a markedly lower severity of OSA (mean ODI 8.4 ± 1.5 vs 30.8 ± 29.0/h; p = 0.050).
When analyzing the baseline non-OSA group, 9 men but none of the women reported new-onset OSA during the follow-up period, according to information obtained from questionnaires. Three of these 9 cases (33.3%) with new-onset OSA reported incident DM at the same time. Among the remaining 81 men without new-onset OSA, the incidence of DM was reported in 7 (8.6%; p = 0.059). Compared with the baseline values, there was a marked increase in BMI at follow-up (27.6 ± 4.9 vs 31.3 ± 10.8 kg/m2; p = 0.041) in the incident DM cases in the non-OSA group.
In a subanalysis, when the efficiently treated OSA patients (15 males and 4 females) were switched to the non-OSA group, there was a slight increase in incident DM among the OSA patients in both genders.
As shown in Table 3, males with incident DM had higher BMI than the subjects without DM and demonstrated higher ODI. In the female group, the incident DM patients were older than the non-DM cases. OSA was more prevalent and more severe in the incident DM cases compared to the subjects without DM (Table 3).
Table 3.
Demographic and clinical characteristics of males and females stratified by incident DM
| Variable | incident DM | no DM | p value |
|---|---|---|---|
| Males | |||
| N | 19 | 118 | |
| Age at baseline, years | 45.6 ± 6.2a | 48.2 ± 9.2 | 0.129 |
| BMI in 1991, kg/m2 | 28.1 ± 4.0 | 26.1 ± 3.2 | 0.016 |
| BMI in 2007, kg/m2 | 30.2 ± 2.2 | 27.5 ± 3.9b | 0.024 |
| Current smokers in 1991, n (%) | 5 (26.3) | 44 (37.3) | 0.445 |
| Current smokers in 2007, n (%) | 2 (10.5) | 21 (17.8) | 0.740 |
| OSA at baseline, n (%) | 9 (47.4) | 38 (32.2) | 0.196 |
| ODI, n/h | 15.8 ± 24.3 | 6.4 ± 10.1 | 0.048 |
| SpO2min, % | 83.6 ± 9.9c | 86.4 ± 5.7 | 0.296 |
| Females | |||
| N | 7 | 24 | |
| Age at baseline, years | 55.1 ± 6.6a | 48.4 ± 10.0 | 0.054 |
| BMI in 1991, kg/m2 | 28.4 ± 5.1 | 27.1 ± 5.4 | 0.583 |
| BMI in 2007, kg/m2 | 30.0 ± 5.5 | 29.7 ± 6.6b | 0.899 |
| Current smokers in 1991, n (%) | 3 (42.9) | 12 (50.4) | 1.000 |
| Current smokers in 2007, n (%) | 2 (28.6) | 7 (29.2) | 1.000 |
| OSA at baseline, n (%) | 5 (71.4) | 5 (20.8) | 0.022 |
| ODI, n/h | 7.0 ± 2.8 | 5.6 ± 10.3 | 0.027 |
| SpO2min, % | 76.6 ± 8.9c | 87.3 ± 6.3 | 0.006 |
Continuous variables are expressed as mean ± SD, statistics by unpaired Student's t test or Mann-Whitney test. Comparison of groups by χ2test or Fisher exact test (two-sided). OSA refers to obstructive sleep apnea; BMI, body mass index; ODI, oxygen desaturation index; SpO2min, minimum oxygen saturation;
p = 0.002;
p = 0.034;
p = 0.049.
When including a gender term and testing for interactions, there was a tendency for interaction between gender and OSA with regard to incident DM. Within the non-OSA group, difference between DM incidence among men and women was very small (11% vs 9%) while this difference was considerably greater within the OSA group (19% vs 50%). However, this tendency did not reach statistical significance when interaction term “gender x OSA” was tested in a multiple regression model (p = 0.172), probably because of the small number of women in the OSA group.
When addressing the significant predictors in men and women, respectively, incident DM was associated with BMI and ODI in men and with OSA and SpO2min in women in univariate analysis (Table 4). In a multivariate model, ODI was no longer significant after adjustment for age and BMI in men (Table 5). In women, both OSA and SpO2min remained as significant predictors. Including weight gain into the multivariate analysis did not change the outcome of the study. As the distribution of incident DM did not differ significantly between efficiently treated vs inefficiently treated OSA patients in men (5 vs 4) or women (2 vs 3), the treatment factor was not included in the multivariate analysis.
Table 4.
Variables associated with incident diabetes mellitus in a univariate logistic regression analysis
| Variable | Men |
Women |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p value | |
| Age, years | 0.97 | 0.91–1.02 | 0.244 | 1.09 | 0.98–1.22 | 0.116 |
| BMI in 1991, kg/m2 | 1.19 | 1.03–1.37 | 0.019 | 1.05 | 0.89–1.23 | 0.580 |
| BMI in 2007, kg/m2 | 1.10 | 1.00–1.21 | 0.044 | 1.01 | 0.88–1.16 | 0.894 |
| Weight gain | 1.06 | 0.93–1.21 | 0.399 | 0.91 | 0.69–1.20 | 0.513 |
| Current smoker in 1991 | 0.60 | 0.20–1.78 | 0.358 | 0.75 | 0.14–4.10 | 0.740 |
| Current smoker in 2007 | 0.54 | 0.12–2.53 | 0.437 | 0.97 | 0.15–6.25 | 0.976 |
| ODI, n/h | 1.04 | 1.01–1.07 | 0.014 | 1.02 | 0.93–1.11 | 0.708 |
| SpO2min, % | 0.95 | 0.89–1.01 | 0.098 | 0.84 | 0.73–0.96 | 0.010 |
| OSA | 1.89 | 0.71–5.05 | 0.201 | 9.50 | 1.40–64.4 | 0.021 |
OR refers to odds ratio; CI, confidence interval; BMI, body mass index; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; SpO2min, minimum oxygen saturation.
Table 5.
Baseline variables associated with incident diabetes mellitus in a multiple logistic regression analysis
| Variable | Men |
Women |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Model I | ||||||
| Age, years | 0.96 | 0.90–1.03 | 0.240 | 1.11 | 0.95–1.27 | 0.192 |
| BMI, kg/m2 | 1.16 | 1.00–1.35 | 0.050 | 0.89 | 0.69–1.14 | 0.347 |
| OSA | 1.58 | 0.55–4.58 | 0.395 | 11.78 | 1.14–121.7 | 0.038 |
| Model II | ||||||
| Age, years | 0.96 | 0.90–1.03 | 0.228 | 1.10 | 0.97–1.24 | 0.133 |
| BMI, kg/m2 | 1.13 | 0.97–1.32 | 0.110 | 0.98 | 0.80–1.20 | 0.835 |
| ODI, n/h | 1.03 | 1.00–1.06 | 0.057 | 1.01 | 0.92–1.12 | 0.834 |
| Model III | ||||||
| Age, years | 0.96 | 0.90–1.02 | 0.205 | 1.01 | 0.87–1.18 | 0.920 |
| BMI, kg/m2 | 1.15 | 0.99–1.33 | 0.062 | 0.80 | 0.57–1.11 | 0.184 |
| SpO2min, % | 0.96 | 0.89–1.03 | 0.207 | 0.77 | 0.60–0.98 | 0.033 |
OR refers to odds ratio; CI, confidence interval; BMI, body mass index; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; SpO2min, minimum oxygen saturation
DISCUSSION
This sleep clinic study has demonstrated a markedly increased incidence of DM in patients with OSA during a follow-up period of 16 years. Moreover, our results suggest that the contribution of OSA to DM development seems to be gender-dependent and higher in women than in men.
To our knowledge, this is the first long-term, observational study of incident DM with regard to gender difference in a sleep-clinic cohort. The strength of this study is the construction of an inception cohort with known OSA status, free of outcome measures at baseline as well as the use of SHDR from a well-organized public epidemiologic center providing reliable and complete data. As prospective cohort studies fulfill an important criterion for causality, our results provide an important contribution to the gender-related mechanisms involved in the development of DM in OSA patients.
The main weakness of this study is the lack of polysomnography for a fully accurate diagnosis of OSA and characterization of the links between the nocturnal changes in sleep apnea and glucose metabolism. However, an overnight OD of 30 or more was defined as OSA based on previously established diagnostic criteria,18 which were accepted at the time of the baseline investigations in 1991. Although the diagnosis was mainly based on the oximetry results, it was supported by data from oro-nasal thermistors as well as respiratory and body movements. The specificity and sensitivity of static charge-sensitive bed combined with pulse oximetry for an AI of more than 5 verified by polysomnograpy have previously been shown to vary between 67% and 100%.20 Although apnea events were identified, hypopneas could not be adequately detected at baseline in this study. This diagnostic procedure might explain the proportionally low prevalence of OSA (33.9%) in this sleep clinic cohort from 1991. Moreover, many of the subjects were referred from the Department of Otorhinolaryngology to the sleep laboratory for a polygraphic recording before surgical treatment of habitual snoring. This may also explain the relatively high proportion of the sleep apneics that underwent surgery. However, as we may have missed OSA subjects with mainly hypopneas and/or without desaturations, our results probably apply more robustly to a group of OSA patients characterised by a more pronounced hypoxic component.A second limitation is the self-reported data, which may include the risk of recall bias. Thus, our results refer only to the responders in this cohort. However, there was no significant difference in baseline characteristics of responders and non-responders. Another limitation refers to the objective evaluation of the reported new-onset OSA in the subjects regarded as non-OSA at baseline, and consequently, the lack of information regarding the incident DM in this subgroup. However, the information obtained from the questionnaires may reflect an additional support for the relationship between new-onset OSA and incident DM in this population.
Another limitation refers to the low number of female subjects with OSA in this sample. In this context, it might rather be expected that OSA would fail to predict incident DM. Interestingly, the results were significant even after entering age and BMI (though those were not predictive in univariate analysis) in the multivariate model. Unfortunately, because of the lack of information regarding waist circumference at the time of the baseline investigations in 1991, our results do not allow us to make further statistical adjustments for central obesity as a confounding factor.
It should also be kept in mind that, though the physician-diagnosed DM is not an uncommon method in the observational follow-up studies,7,8 our patients may have had more subtle abnormalities at baseline, including impaired glucose tolerance and elevated insulin levels. Consequently, it is likely that the DM process might have begun but not manifested yet as clinically diagnosable DM in some of the patients with OSA. Although those with apparent DM were eliminated from the baseline sample, it is likely that occult DM was present or underestimated as baseline. On the other hand, this possibility may apply to OSA as well as non-OSA subjects and both genders. Moreover, in our middle-aged cohort, DM diagnosis was present in ten subjects (3.2%) at baseline, similar to Swedish population surveys (20-79 years) with corresponding prevalence of 3.3% in men and 3.9% in women.21
In the current sleep cohort, there was a marked male predominance among the patients diagnosed with OSA. Moreover, the women were older and more obese than men within the OSA group, as indicated by previous clinical studies.4 This paradox was explained by the differences in fat distribution, upper airway length and collapsibility, neurochemical control mechanisms, level of chemoresponsiveness, arousal response, and an effect of sex hormones. Accordingly, men appear to be more vulnerable to the disease than women because of the increased deposition of fat around the airway at the same level of obesity with women.22 Some studies have suggested that vast majority of females with OSA are undiagnosed because they present with complaints such as fatigue or mood disturbance instead of symptoms commonly associated with OSA, such as witnessed apneas, heavy snoring, or daytime sleepiness.23 It has also been argued that partial upper airway obstruction with sleep fragmentation not manifested in the AHI may associate better with symptoms in women. The incidence and severity of OSA in women have therefore been reported to be underestimated when defined by AHI.24
Several studies have demonstrated a high prevalence of DM in patients with OSA.7 Moreover, Elmasry and coworkers suggested an increased incidence of DM in a prospective study of habitual snorers independent of age, weight, physical activity, smoking, and other confounders.14 A recent prospective study, addressing the gender difference in this context, demonstrated that the association between snoring with witnessed apnea and incident DM was stronger in women than in men.16 However, less is known regarding the development of DM in populations with objective sleep recordings at baseline. The 4-year follow-up data from the Wisconsin cohort suggested a causal relationship between OSA and physician-diagnosed incident DM, adjusted for age and gender, but this relationship lost statistical significance after adjustment for BMI.7 Although the current study included a lower number of subjects, our data support a significant association between OSA and physician-diagnosed DM in the multivariate analysis. The influence of BMI on the development of DM was demonstrated in our population as well, but interestingly restricted to men. Hence, the relative contribution of OSA to development of DM may be more pronounced in women than in men, supporting the results of Valham and coworkers mentioned above.16
There is also evidence in the literature for a dose-response relationship in the clinic-based cross-sectional studies, suggesting an association between the severity of OSA and the extent of impaired glucose metabolism as well as DM development in a manner independent of age, obesity, and hereditary predisposition.6,25,26 Although our study was not powered enough to address this topic, there was a significant relationship between the lowest oxygen saturation levels and incident DM in women This finding may suggest that the severity of intermittent hypoxemia rather than the frequency of apneas with desaturations may play an important role in the development of DM in women. There was also a tendency for a dose-response relationship between ODI and incident DM in men, but this was not significant after adjustment for BMI.
Mechanisms that potentially could contribute to development of insulin resistance in OSA have been discussed in detail elsewhere.10 In brief, sleep fragmentation and intermittent hypoxia appear to induce elevated sympathetic nervous system activity and altered hypothalamic pituitary adrenocortical axis function, as well as increased oxidative stress and activation of inflammatory pathways. Such mechanisms alone or in concert could clearly be implied in a reduced pancreatic β-cell function and development of insulin resistance.
Most of the previous studies addressing a gender influence on DM development have found a higher incidence of obesity (one of the most important risk factors for DM2) in women compared with men. It is possible that menopausal transition with excess weight gain, development of central obesity, changes in diet, and lack of exercise contribute to more rapid development of DM. Moreover, the benefits of estrogens on postprandial lipid metabolism are lost in postmenopausal women with DM.27 However, the persistence of significant associations after adjusting for BMI in our study suggests that mechanisms in addition to adiposity may be operational. Both endogenous hormones and obesity have been suggested to play an important role in the development of OSA in women, especially in postmenopausal period.28 For instance, testosterone, which is inversely associated with adiposity in men is positively related with adiposity in women.29 Sex hormone binding globulin, bioavailable testosterone, and estradiol are all shown to be associated with insulin resistance and glucose levels both in men and in women.29 Theorell-Haglow and coworkers recently demonstrated a stronger relationship between hypoxia and insulin sensitivity in older women compared with younger subjects, indicating that age may increase the sensitivity to hypoxia in women.30 As our female OSA patients to a large extent were peri- or postmenopausal at baseline, the additive impact of such mechanisms may have rendered them more susceptible to DM.
In conclusion, our observations from the current clinical cohort confirm previous findings of an independent association between OSA and DM. Though the small number of women who participated in the current study makes a proper gender analysis difficult, the significant impact of OSA on incident DM was independent of age and BMI in women, while this relationship was mainly dependent on BMI in men. Awaiting the longitudinal follow-up studies from larger populations, DM development and DM complications seem to depend on phenotypic and unknown genotypic factors, which should stimulate the future research in this area.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Yelda Turgut Celen is the recipient of a European Respiratory Society / European Lung Foundation Fellowship (Number 156). The study was supported by grants from the Swedish Heart and Lung Foundation and Research fund at Skaraborg Hospital. The authors thank statistician Salmir Nasic at the Research Center of the Skaraborg Hospital for help in analyzing the results, and gratefully acknowledge the research nurses, Jeanette Norum and Lena Engelmark for skillful assistance in data collection.
REFERENCES
- 1.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–8. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legato MJ, Gelzer A, Goland R, Ebner SA, Rajan S, Villagra V, Kosowski M Writing Group for The Partnership for Gender-Specific Medicine. Gender-specific care of the patient with diabetes: review and recommendations. Gend Med. 2006;3:131–58. doi: 10.1016/s1550-8579(06)80202-0. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Jordan AS, McEvoy RD. Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev. 2003;7:377–89. doi: 10.1053/smrv.2002.0260. [DOI] [PubMed] [Google Scholar]
- 5.Einhorn D, Stewart DA, Erman MK, Gordon N, Philis-Tsimikas A, Casal E. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr Pract. 2007;13:355–62. doi: 10.4158/EP.13.4.355. [DOI] [PubMed] [Google Scholar]
- 6.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 7.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall NS, Wong KK, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5:15–20. [PMC free article] [PubMed] [Google Scholar]
- 9.Lavie P, Herer P, Lavie L. Mortality risk factors in sleep apnea: a matched case-control study. J Sleep Res. 2007;16:128–34. doi: 10.1111/j.1365-2869.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 10.Shaw JE, Punjabi NM, Wilding JP, Alberti KG, Zimmet PZ. Sleep-disordered breathing and type 2 diabetes. A report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res Clin Pract. 2008;81:2–12. doi: 10.1016/j.diabres.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Meisinger C, Heier M, Loewel H. The MONICA/KORA Augsburg Cohort Study. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48:235–41. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- 12.Tuomilehto H, Peltonen M, Partinen M, et al. Sleep duration is associated with an increased risk for the prevalence of type 2 diabetes in middle aged women-the FIN-D2D survey. Sleep Med. 2008;9:221–7. doi: 10.1016/j.sleep.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–7. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 14.Elmasry A, Janson C, Lindberg E, et al. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Intern Med. 2000;248:13–20. doi: 10.1046/j.1365-2796.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 15.Lindberg E, Berne C, Franklin KA, Svensson M, Janson C. Snoring and daytime sleepiness as risk factors for hypertension and diabetes in women-A population based Study. Respir Med. 2007;101:1283–90. doi: 10.1016/j.rmed.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Valham F, Stegmayr B, Eriksso M, Hagg E, Lindberg E, Franklin KA. Snoring and witnessed sleep apnea is related to diabetes mellitus in women. Sleep Med. 2009;10:112–7. doi: 10.1016/j.sleep.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea. a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 18.Berry D, Webb W, Block A. Sleep apnea syndrome: a critical overview of the apnea index as a diagnostic criterion. Chest. 1984;86:529–31. doi: 10.1378/chest.86.4.529. [DOI] [PubMed] [Google Scholar]
- 19.Helsinki: Nordic Council on Medicines; 1985. Nordic statistics on medicines 1981–1983: guidelines for ATC classification. NCN Publication No. 16. [Google Scholar]
- 20.Svanborg E, Larsson H, Carlsson-Nordlander B, Pirskanen R. A limited diagnostic investigation for obstructive sleep apnea syndrome: oximetry and static charge sensitive bed. Chest. 1990;98:1341–5. doi: 10.1378/chest.98.6.1341. [DOI] [PubMed] [Google Scholar]
- 21.Estimated prevalence of diabetes and numbers of people with diabetes, 2003 and 2005, selected countries, the world. Brussels: International Diabetes Federation. 2003. [accessed 2006 Dec. 1]. (website of the British Heart Foundation). Available: www.heartstats.org/temp/TABsp12.8spwebo6.xls.
- 22.Buyse B, Markous NK, Cauberghs M, Van Klaveren R, Muls E, Demedts M. Effect of obesity and/or sleep apnea on chemosensitivty: differences between men and women. Respir Physiol Neurobiol. 2003;134:13–22. doi: 10.1016/s1569-9048(02)00202-1. [DOI] [PubMed] [Google Scholar]
- 23.Valipour A, Lothaller H, Rauscher H, Zwick H, Burghuber OC, Lavie P. Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: a clinical population study using the sleep disorders questionnaire. Sleep. 2007;30:312–9. doi: 10.1093/sleep/30.3.312. [DOI] [PubMed] [Google Scholar]
- 24.Anttalainen U, Saaresranta T, Kalleinen N, Toivonen J, Vahlberg T, Polo O. Gender differences in age and BMI distrubutions in partial upper airway obstruction during sleep. Respir Physiol Neurobiol. 2007;159:219–26. doi: 10.1016/j.resp.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Acartürk G, Unlü M, Yüksel S, Albayrak R, Köken T, Peker Y. Obstructive sleep apnea, glucose tolerance and liver steatosis in obese women. J Int Med Res. 2007;35:458–66. doi: 10.1177/147323000703500404. [DOI] [PubMed] [Google Scholar]
- 26.Tamura A, Kawano Y, Watanabe T, Kadota J. Relationship between the severity of obstructive sleep apnea and impaired glucose metabolism in patients with obstructive sleep apnea. Respir Med. 2008;102:1412–6. doi: 10.1016/j.rmed.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Masding MG, Stears AJ, Burdge GC, Wootton SA, Sandeman DD. The benefits of oestrogens on postprandial lipid metabolism are lost in post-menopausal women with Type 2 diabetes. Diabet Med. 2006;23:768–74. doi: 10.1111/j.1464-5491.2006.01867.x. [DOI] [PubMed] [Google Scholar]
- 28.Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 29.Netzer NC, Eliasson AH, Strohl KP. Women with sleep apnea have lower levels of sex hormones. Sleep Breath. 2003;7:25–9. doi: 10.1007/s11325-003-0025-8. [DOI] [PubMed] [Google Scholar]
- 30.Theorell-Haglöw J, Berne C, Janson C, Lindberg E. Obstructive sleep apnea is associated with decreased insulin sensitivity in women. Eur Respir J. 2008;31:1054–60. doi: 10.1183/09031936.00074907. [DOI] [PubMed] [Google Scholar]