Abstract
The rule of diagnostic parsimony—otherwise known as “Ockham's Razor”—teaches students of medicine to find a single unifying diagnosis to explain a given patient's symptoms. While this approach has merits in some settings, a more comprehensive approach is often needed for patients with chronic, nonspecific presentations for which there is a broad differential diagnosis. The cardinal manifestations of sleep disorders—daytime neurocognitive impairment and subjective sleep disturbances—are examples of such presentations. Successful sleep medicine clinicians therefore approach every patient with the knowledge that multiple diagnoses—rather than simply one—are likely to be found. Teaching an integrated and comprehensive approach to other clinicians in an organized and reproducible fashion is challenging, and the evaluation of effectiveness of such teaching is even more so. As a practical aid for teaching the approach to—and evaluation of—a comprehensive sleep medicine encounter, five functional domains of sleep medicine clinical problem-solving are presented as potential sources for sleep/wake disruption: (1) circadian misalignment, (2) pharmacologic factors, (3) medical factors, (4) psychiatric/psychosocial factors, and (5) primary sleep medicine diagnoses. These domains are presented and explained in an easy-to-remember “five finger” format. The five finger format can be used in real time to evaluate the completeness of a clinical encounter, or can be used in the design of standardized patients to identify areas of strength and potential weakness. A score sheet based upon this approach is offered as an alternative to commonly used Likert scales as a potentially more objective and practical measure of clinical problem-solving competence, making it useful for training programs striving to achieve or maintain fellowship accreditation.
Citation:
McCarty DE. Beyond Ockham's Razor: redefining problem-solving in clinical sleep medicine using a “five-finger” approach. J Clin Sleep Med 2010;6(3):292-269.
Keywords: Sleep disorders, medical education, clinical competence, problem solving, educational measurement
OCKHAM'S RAZOR, SEARCH SATISFICING ERRORS, AND THE MULTIDIMENSIONAL NATURE OF SLEEP MEDICINE
Pluralitas non est ponenda sine necessitate.
(Plurality should not be assumed unnecessarily)
—William of Ockham
Quodlibeta (c1324) No. 5, Question 1, Art. 21
Most of us were taught as medical students to seek a single diagnosis to explain a patient's collection of symptoms, the lesson being that a simple diagnosis is more likely than a complex collection of unrelated diseases, even if that simple diagnosis is relatively rare. William of Ockham, a 14th century logistician and Franciscan friar, likely did not invent the law of parsimony, but the idea of the simplest solution being correct is often attributed to him, with the term Ockham's Razor referring to this strategy's ability to “cut away” extraneous information.
In our current environment of chronic disease management, polypharmacy, and multiple medical problems, however, clinging to the belief that a single unifying diagnosis will tie together all a patient's complaints often leads to a decision-making stumble termed the “search satisficing” error.2 “Satisficing” is a portmanteau (or “blended word”), combining the terms satisfy and suffice, and is used in decision-making logic to imply that the decision-making process stops when an adequate (though possibly suboptimal) solution is identified.3 In other words, once a plausible diagnosis is found, the clinician ceases all efforts to identify other contributors to the problem. In such a case, the “simple” explanation may fall short of a comprehensive assessment, potentially leading to inappropriate or ineffective treatment strategies.
From a clinical problem-solving perspective, one of the more important developments in the field of sleep medicine is the concept that disturbances of the states experienced as “sleep” and “wake” not only have a differential diagnosis, but that commonly more than one diagnosis is likely to contribute to the patient's complaints.4 For example, a patient with obstructive sleep apnea may present with daytime sleepiness and disturbed nocturnal sleep. After taking a careful history, the astute clinician may discover circadian misalignment; contributions to sleep onset insomnia from alerting medications; contributions to daytime sleepiness from sedating medications; or sleep disturbances due to medical problems, such as reflux, cough, or pain. Sleepiness out of proportion to polysomnographic evidence of sleep disruption may be a useful clue to consider an underlying primary hypersomnia. In cases like these, it would not be realistic to expect a complete resolution of the patient's complaints by addressing the sleep disordered breathing alone. Moreover, compliance and tolerability of an intervention such as positive airway pressure will prove problematic if other sleep-disrupting forces exist and are not identified and addressed. However, unless a comprehensive approach is taken for every patient—particularly those with apparently clear-cut diagnoses—the likelihood of the “search satisficing error” will be high, with missed opportunities for treatment.
THE FIVE FINGER APPROACH
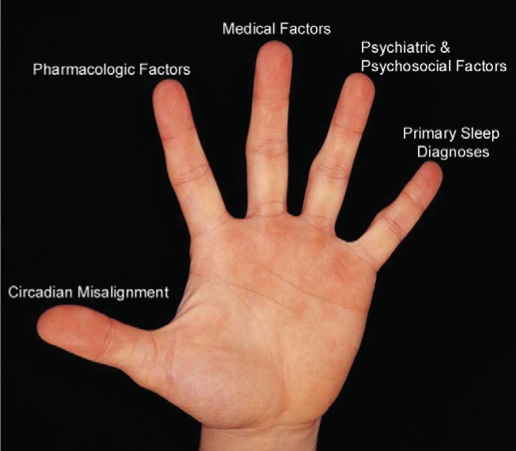
In the training of our sleep medicine fellows, we recognized the importance of teaching a methodical and comprehensive approach to all patients, particularly for those with “obvious” diagnoses. To do this, we developed a Five-Finger construct (see Figure 1), a mnemonic similar to the Five-Finger approach devised by renowned teacher and clinician W. Proctor Harvey for the clinical evaluation of a cardiology patient.5 Dr. Harvey's approach stratified the importance of five basic elements in cardiology clinical reasoning—history, physical exam, ECG, radiographic studies, and lab tests—providing a model that would become the standard for generations of clinicians.6 In the sleep medicine Five-Finger approach, each digit of the hand represents a potential source of disturbance to the quality of a patient's sleeping or waking experience. The task of the clinician, therefore, is to thoroughly evaluate the patient to determine the potential factors within each domain that may adversely affect sleep or wake.
Figure 1.
The five domains of clinical sleep medicine (see text)
The Thumb: Circadian Misalignment
The thumb represents the domain of circadian misalignment, stressing the cornerstone-like importance of obtaining a careful history of sleep/wake scheduling, including weekend schedules and shift work. Patients who report irresistible urges to sleep during the day and who have difficulty falling asleep at night may have an underlying circadian phase delay. Those who routinely doze prior to their usual bedtime and who report early terminal awakening may have an advanced sleep phase. Parsing out circadian misalignment and adequately addressing it is a fundamental step to helping patients achieve normalcy with their sleep/wake habits.
The Index Finger: Pharmacologic Factors
Polypharmacy is strikingly common, particularly in older patients.7 Furthermore, many common medications have disruptive effects on sleep, wake, or both. In addition to alerting and sedating effects, medications may induce pain syndromes (e.g., statins8,9), cough (e.g., ACE Inhibitors10), restless legs symptoms (e.g., antidepressants, neuroleptics, and antihistamines11), or REM sleep behavior disorder (e.g., antidepressants, MAO-B inhibitors12), all of which have potential to dramatically disrupt sleep. The contribution of over-the-counter and social pharmacologic agents (such as caffeine, alcohol, and tobacco) must always be considered as well, both in terms of their acute pharmacologic effects as well as symptoms of between-dose withdrawal. It is in emphasis of both the importance of this domain, and in recognition of the fact that it is often overlooked, that pharmacologic factors is listed second, the index finger within our Five-Finger rubric. An annotated selection of common medications that adversely affect sleep and wake, along with associated clinical syndromes and suggested practical solutions are presented in supplementary Tables S1 and S2 (the supplementary tables are available online only at : www.aasmnet.org/jcsm).
The Middle Finger: Medical Factors
The middle finger represents medical factors and is the third domain in our Five-Finger” construct. Medical disorders often impact the quality of sleep, and can also lead to numerous daytime impairment symptoms, as well. Common examples include such familiar entities as gastroesophageal reflux, musculoskeletal pain syndromes (e.g., chronic arthritis, fibromyalgia), allergic rhinitis, benign prostatic hypertrophy (leading to frequent nocturia), and heart failure. In pediatric patients, atopic dermatitis is a common problem, and is frequently associated with sleep disturbances due to sleep-related itching.13,14
Medical disorders can impact the perceived quality of wakefulness as well. Chronic pain can lead to reports of fatigue and depression,15 possibly affecting the physician's perception of daytime neurocognitive impairment. Parkinson's disease may cause daytime sleepiness and nocturnal sleep disruption as a result of the neurodegenerative process itself.16 Chronic cardiopulmonary disease is often perceived by patients as a pervasive sense of fatigue.17 Hypovitaminosis D is associated with chronic pain syndromes,18–21 muscle weakness,22 and daytime neurocognitive impairment.23–25 The list could go on and on.
Teasing out the medical issues which contribute to sleep-wake complaints has practical implications. For instance, for a patient with obstructive sleep apnea, the mandate for definitive treatment of the sleep disordered breathing is informed in large part by the degree to which the problem represents a cardiovascular risk, and by the severity of the subjective disturbances of sleep and wake. A typical example helps to illustrate this point:
A 73-year-old man is referred for polysomnography by his primary care physician, after reporting symptoms of poor quality sleep, snoring, and daytime sleepiness. His Epworth Sleepiness Scale score is 18/24, indicating a pathologic degree of daytime sleepiness. An overnight polysomnogram reveals heavy sleep discontinuity, poor sleep efficiency, and occasional obstructive apneas and hypopneas, primarily in REM sleep, with a total sleep time apnea hypopnea index (AHI) of 8 per hour of sleep with a minimum oxygen saturation of 89%. By definition, this patient meets diagnostic criteria for obstructive sleep apnea syndrome.4 The patient undergoes a CPAP titration study but does not tolerate the intervention, stating that CPAP only makes the quality of his sleep worse. Despite numerous attempts with the device, using different interfaces and pressures, the patient eventually declines treatment with CPAP altogether.
Given the magnitude of this patient's daytime impairment symptoms, it would seem at first that more aggressive measures to overcome the sleep disordered breathing would be appropriate, even if they carried some risk to the patient. Indeed, it is not uncommon for sleep medicine physicians to be placed in a position of recommending second-tier treatments for sleep apnea, such as surgery (which carries the physical risk of pain and complications) or oral appliances (which can represent a financial hardship to patients who may need to purchase the device out-of-pocket due to insurance limitations). Logic would argue that the greater the functional impairment from sleep apnea, the more aggressively a definitive solution should be sought. In this example, however, further questioning reveals that this patient suffers from severe back pain and gastroesophageal reflux symptoms, both of which greatly contribute to his nocturnal sleep disruption and subsequent daytime impairment. In such a setting, the comprehensive approach would favor optimizing treatment for these medical disorders prior to more aggressive management strategies for the sleep disordered breathing, particularly since the disease-specific mortality risk for this degree of sleep apnea in this age group does not constitute a strong rationale for definitive treatment.26
The strategy for addressing factors within the medical domain is two-fold. First, the sleep medicine physician must partner with other participating physicians to ensure that the patient's medical problems are optimally managed. Next, in the event that—despite optimization of therapy—medical factors continue to disturb the patient's sleeping or waking experience, the sleep medicine physician must then consider the possibility of symptom-specific interventions. For example, the prescribing of a hypnotic agent may be a reasonable option for the patient with chronic insomnia due to arthritis pain. So too, the judicious use of an alerting medication may improve the quality of the waking day for the patient with hypersomnia due to Parkinson's disease. In each case, the physician is obligated to assist the patient with assessing the risks of therapy and providing guidance as to whether such risks are justified, based upon the severity and functional impact of the symptom reported. For example, severe insomnia due to arthritis pain may be treatable with prescription hypnotics coupled with narcotic pain relievers, but the potential for induction of sleep disordered breathing, as well as increased likelihood of accidents and injury must be factored into the decision-making calculus and follow-up plan.
The Ring Finger: Psychiatric and Psychosocial Factors
The ring finger represents psychiatricand psychosocial factors which impact the quality of sleep or wake. The scope of this domain is vast, and includes primary psychiatric diagnoses (such as depression, anxiety, bipolar disease, or attention deficit disorder), social and behavior patterns with regard to sleep, as well as patient expectations, concerns, and beliefs about their sleeping habits. The unifying factor among these elements is that this domain reminds the clinician to seek answers to the following question: “Does this patient have mental health issues, psychological processes, or social problems that contribute to sleep disturbances or daytime impairment?”
For example, the patient with poorly controlled generalized anxiety disorder may complain of insomnia, as may the patient who has the unreal expectation of a 14-hour sleeping period, though clearly the treatment recommendations for these two patients would be vastly different. A patient with a noisy or (worse) abusive bedpartner may complain of insomnia or simply suffer from nonrestorative sleep and complain of daytime impairment. Another patient struggling to make ends meet with two jobs and chronic behavioral sleep restriction may complain of excessive daytime sleepiness. As stated in the section above, the mandate for definitive treatment of a disorder like sleep apnea is informed in part by the degree of impairment of sleep and wake. The careful clinician must therefore be conscientiously circumspect about where to assign “blame” for these symptoms. By methodically considering the possibility of contributing factors within this domain, the clinician is less likely to overlook opportunities for effective interventions.
The Pinky: Primary Sleep Diagnoses
The fifth finger represents traditionally-described primary sleep diagnoses. This is intentionally listed last, not to suggest that these diagnoses are unimportant, but to emphasize that, common as they may be in a busy sleep medicine practice, they are not all-important. Included within this domain are “bread and butter” diagnoses familiar to all sleep medicine physicians such as obstructive sleep apnea, narcolepsy, restless legs syndrome, and others (Table 1). By considering these last, one is less likely to overlook other diagnoses contributing to the patient's symptoms.
Table 1.
Primary sleep diagnoses
| Category | Selected Diagnoses |
|---|---|
| Sleep related breathing disorders | Obstructive sleep apnea |
| Central sleep apnea | |
| Sleep related hypoventilation | |
| Cheyne-Stokes respirations | |
| Sleep related movement disorders | Restless legs syndrome |
| Periodic limb movement disorder | |
| Parasomnias | REM behavior disorder |
| Sleepwalking | |
| Confusional arousals | |
| Primary hypersomnias | Narcolepsy with cataplexy |
| Narcolepsy without cataplexy | |
| Idiopathic CNS hypersomnia with long sleep time | |
| Idiopathic CNS hypersomnia without long sleep time | |
| Recurrent hypersomnia | |
| Insomnia | Idiopathic insomnia |
| Paradoxical insomnia |
A recent patient in our clinic nicely illustrates the importance of careful consideration of this domain with all patients, even for those in whom the diagnosis appears “certain”:
A 51-year-old woman presented to an academic sleep disorders center to establish care for a diagnosis of sleep apnea. She had been given the diagnosis of OSA several years prior at an outside facility, and had been using nocturnal CPAP ever since. At her initial appointment, the patient had few complaints, and stated that the device was reasonably comfortable. However, as part of her intake questionnaire, she completed an Epworth Sleepiness Scale, and her score of 22/24 indicated a continuing degree of pathologic daytime sleepiness. On further questioning, the patient did admit to episodes of cataplexy, sleep paralysis, and sleep related hallucinations. A polysomnogram without CPAP revealed a few scattered respiratory effort related arousals, but few actual apneas or hypopneas. The MSLT revealed a mean sleep latency of less than four minutes, with two sleep-onset REM periods. The patient's daytime sleepiness symptoms were attributed to the diagnosis of narcolepsy, with upper airway resistance features possibly contributing to a minor degree, if at all. She was subsequently started on standard treatments for narcolepsy, and her clinical status improved.
USE OF THE FIVE FINGER APPROACH IN AN ACADEMIC SLEEP MEDICINE FELLOWSHIP PROGRAM
One of the challenges faced by the program directors of all academic Sleep Medicine fellowship training programs is to develop a curriculum that successfully teaches young clinicians to approach patients comprehensively. In practice, this teaching is usually undertaken in the clinic under the supervision of experienced physicians, with a multidimensional approach modeled for trainees in a way that, ultimately, (it is hoped) will result in trainees developing these habits for themselves. We have found that the Five-Finger approach provides an easily recalled structure for reviewing all aspects of a clinical presentation, and assists the staff in knowing where further practice is needed. For example, a sleep medicine fellow who completed a psychiatric residency may have ample familiarity with elements within the psychiatric and psychosocial domain, but may be less comfortable with the notion of considering chronic medical problems within the decision-making process. Using the Five-Finger construct allows teaching faculty to more readily identify areas for improvement, and better customize the educational experience for each fellow.
Indeed, this approach lends itself to teaching and learning in other environments as well. Seminal work by Barrows and Tamblyn reintroduced the medical decision-making process as a phenomenon they termed clinical reasoning, emphasizing the importance of parallel processing of information, and, most importantly, identifying simulated patient encounters as a method of imparting not only factual knowledge about disease, but also about the process of medical problem solving and self-directed learning.27 As an exercise to augment clinical experience, we have developed a standardized patient script which includes factors from within each of the five domains as part of the presentation. Trainees interview the “patient,” who answers questions in a standardized fashion according to a written script. Following the interview, fellows are asked to provide counseling to the patient commensurate with their findings, and then to formally document their history, diagnoses, and plans. At the completion of the exercise, fellows are provided a copy of the standardized patient script, as well as written feedback regarding missed opportunities for intervention. In our short experience, a single practice standardized patient exercise using the Five Finger approach during the first quarter of the program resulted in a noticeable improvement in fellows' skills in the clinic, with a broader scope of problem-solving more closely mirroring that of more experienced clinicians. The question of whether this short experience would be borne out by more rigorous testing would be an interesting subject for further research.
Lastly, and importantly, the Five-Finger approach affords an easy structure for documenting competence within an increasingly complex, multidisciplinary field. Indeed, “clinical competence” is a nebulous term, the documentation of which is typically populated by equally nebulous subjective Likert rating scales. The Five-Finger method allows for rapid documentation of more objective measures of cross-dimensional competency, a feature which assists in programs aiming for American Council of Graduate Medical Education (ACGME) accreditation of their teaching programs. For example, following a case presentation, the attending faculty asks the fellow to not only enumerate the sleep and wake-related complaints, but also to list the factors within each domain that potentially contribute to them. This list is then compared to the gold standard produced by more experienced physicians (or, in the case of a standardized patient, with a standard score sheet), with strengths and areas for improvement clearly documented. An example of a documentation form which could be used in this regard is provided in the addendum to this paper (the addendum is available online only at www.aasmnet.org/jcsm).
CONCLUSION
Most graduate medical education programs are grappling with the development of a standardized curriculum incorporating elements of the six ACGME competencies.28–30 For a specialty spanning multiple disciplines like sleep medicine, it is critical to emphasize the importance of developing a process of methodical decision-making that naturally leads to a comprehensive assessment, lest the proverbial forest be lost for the trees. Indeed, the identity of sleep medicine as a standalone specialty is one of a discipline which recognizes the multidimensional nature of every patient,31,32 calling attention to the notion that diagnostic multiplicity is not an anomaly, but an expectation. Within such a construct, the age-old axiom of Ockham's Razor may increase the possibility of error via “search satisficing.” However, replacing such an indelible fundamental paradigm of problem-solving is not easily done.
The Five Finger approach described here is a simple mnemonic which helps to capture the complexity of an ideal comprehensive sleep medicine encounter, providing a methodical structure for the reflective questioning familiar to every wise clinician: “What else could this be?”33 In an academic training environment, it also provides a structure for formal feedback, a tool for documentation of clinical competency, and a rubric for development of simulation-based training tools. Outcomes-based research could show whether this construct truly improves a clinician's ability to identify multiple dimensions of care for a given sleep medicine patient, thereby increasing overall clinical effectiveness by fostering comprehensive consultations with fewer missed opportunities for intervention.
DISCLOSURE STATEMENT
This was not an industry-supported study. The author has indicated no financial conflict of interest.
ACKNOWLEDGMENT
The author wishes to thank Dr. Andrew L. Chesson Jr. for guidance, mentorship, and editing assistance with this manuscript.
Table S1.
Selected common medications with potential to disrupt sleep
| Drug Class | Examples | Typical Indications | Patient Complaints | Mechanism | Potential Solution |
|---|---|---|---|---|---|
| Selective serotonin reuptake inhibitors | sertraline, fluoxetine, citalopram | Major depression, anxiety disorders, postmenopausal hot flashes | Restless legs symptoms Sleep onset insomnia, Nonrestorative sleep, Dream enactment behavior or “sleepwalking” | Drugs may have alerting side effects.1 Increases restless legs symptoms and periodic limb movements of sleep,2, 3 increases likelihood of REM without atonia (may lead to clinical REM behavior disorder)4 | Reassess original indication, consider alternate agent (bupropion not associated with increased RLS symptoms) |
| Tricyclic antidepressants | amitriptyline, imipramine, protriptyline | Major depression, anxiety, insomnia, neuropathic pain | Restless legs symptoms, sleep onset insomnia, nonrestorative sleep | Increases restless legs symptoms and periodic limb movements of sleep,2 protriptyline is strongly adrenergic and has an alerting side effect profile5,6 | Reassess original indication, consider alternate agent |
| ACE Inhibitors | lisinopril, enalapril | Hypertension, diabetic proteinuria, congestive heart failure | Nocturnal cough Worsening sleep apnea | Increased bradykinin production leads to airway irritation, possible increased airway edema7 | Consider substitution with angiotensin receptor blocker or other drug class |
| Norepinephrine and dopamine reuptake inhibitors | venlafaxine, desvenlafaxine, bupropion | Major depression, anxiety, postmenopausal hot flashes | Sleep onset insomnia | Increased activity of alerting neurotransmitters8 | Reassess original indication, consider alternate agent |
| Beta-2 agonists | albuterol, salmeterol | Asthma, COPD | Sleep onset insomnia Sleep maintenance insomnia | Alerting side effects of adrenergic medications9 | Reassess original indication, educate patient regarding nocturnal use of medication, consider use of hypnotic if nocturnal usage is unavoidable |
| Beta blockers | Metoprolol, propranolol | Hypertension, tachyarrhythmias, migraine prophylaxis | Nightmares | Not known, likely related to central β-adrenergic blockade, lipophilic β-blockers may be more problematic10,12 | Consider switch to less lipophilic agent (e.g., atenolol), or alternate drug class |
| Corticosteroids | prednisone, methylprednisolone | Rheumatologic disorders, COPD | Sleep onset insomnia Sleep maintenance insomnia13Abnormal dreams14 | Unknown | Consider non-steroid alternatives if medically reasonable, consider low dose hypnotic if corticosteroid medication is medically mandatory |
| Non-nucleoside reverse transcriptase inhibitors (anti-retroviral) | efavirenz | HIV disease | Abnormal dreams15 | Unknown | Consider alternate agent if severely troubling, consider low-dose hypnotic with low potential for drug-drug side effects (e.g., doxepin)15 |
| Statins | atorvastatin, simvastatin, pravastatin, rosuvastatin | Hyperlipidemia | Sleep onset insomnia or frequent awakenings due to muscle pain | Statin induced myopathy, possible statin induced arthralgia16,17 | Reassess original indication, reassess treatment goals, consider decreased dose, consider alternate agent |
| Opiates | methadone, oxycodone, morphine | Chronic pain, restless legs syndrome | Frequent awakenings, Nocturnal breathlessness, Nonrestorative sleep | Increased risk of obstructive and central sleep apnea18 | Consider non-opiate alternatives, consider polysomnography with positive airway pressure if sleep disordered breathing is severe |
| CNS stimulants | methylphenidate, dextro-amphetamine | Narcolepsy, Idiopathic CNS hypersomnia, Attention deficit hyperactivity disorder | Sleep onset insomnia, frequent awakenings | Central stimulation of dopaminergic alerting system | Consider earlier dosing, use of immediate release formulations for later day dosing, replacement with modafinil |
| Social drugs | Caffeine tobacco/ nicotine | n/a | Sleep onset insomnia Sleep maintenance insomnia Snoring | Caffeine may have hold-over stimulatory effects lasting into the nocturnal timeframe; nicotine used at night can produce CNS stimulation; heavy smokers during daytime hours can experience nocturnal withdrawal during sleep-induced abstinence, leading to physical discomfort. Smoking increases upper airway inflammation, which can worsen sleep disordered breathing | Decrease or discontinue use |
| Social drugs | Alcohol | n/a | Sleep maintenance insomnia Snoring Worsening sleep apnea | Alcohol tends to worsen propensity for sleep disordered breathing, possibly by altering upper airway tone and by increasing arousal threshold; though it shortens sleep latency, pre-bedtime use often results in insomnia in the second half of the night. | Decrease or discontinue use |
Table S2.
Selected common medications with potential to disrupt wake
| Drug Class | Examples | Typical Indications | Patient Complaints | Mechanism | Potential Solutions | |
|---|---|---|---|---|---|---|
| Selective serotonin reuptake inhibitors | sertraline, paroxetine | Major depression, anxiety, postmenopausal hot flashes | Daytime sleepiness | Sedating effects of medication19 | Reassess original indication, consider taper or substitution of an agent with a more alerting side effect profile (e.g., venlafaxine or bupropion) | |
| Tricyclic antidepressants | amitriptyline | Insomnia, major depression, anxiety, chronic neuropathic pain | morning grogginess nonrestorative sleep daytime sleepiness | Sedating effects of medication Prolonged half-life for some medications leads to next-day “hangover” | Reassess original indication, consider dose decrease, consider nocturnal dosing schedule, consider alternate agent | |
| Benzo-diazepines | diazepam, clonazepam, flurazepam | Insomnia, anxiety, muscle spasms, REM behavior disorder | morning grogginess, nonrestorative sleep, daytime sleepiness | Sedating effects of medication; prolonged half-life for some medications leads to next-day “hangover” | Reassess original diagnosis, consider alternate agent, consider addition of daytime alerting agent if agent is considered medically necessary | |
| Anticonvulsants | gabapentin, phenytoin, levetiracetam | Seizure disorder, neuropathic pain, migraine prophylaxis | Daytime sleepiness | Sedating effects of medication | Consider dose decrease, consider alternate agent, consider addition of daytime alerting agent if agent is considered medically necessary | |
| Neuroleptics | quetiapine, risperidone, haloperidol | Psychotic disorders, major depression, attention deficit disorder | Daytime sleepiness | Sedating effects of medication | Reassess original diagnosis, consider alternate agent, consider addition of daytime alerting agent if agent is considered medically necessary | |
| Beta blockers | metoprolol, propranolol, bisoprolol | Hypertension, tachyarrhythmias migraine prophylaxis | Daytime fatigue | Central adrenergic blockade→fatigue or drowsiness20,21 | Consider less lipophilic agent (e.g., atenolol), consider alternate drug class | |
| Statins | atorvastatin, simvastatin, pravastatin, rosuvastatin | Hyperlipidemia | Daytime fatigue, poor exercise tolerance due to muscle pain | Statin induced myopathy, possible statin induced arthralgia16,17,20 | Reassess original diagnosis, reassess treatment goals, consider lower dose, consider alternate agent | |
| Antihistamines | Diphenhydramine, hydroxyzine | Allergic reactions, anxiety, pruritic conditions, insomnia | Daytime fatigue Daytime sleepiness Poor attention and concentration22 | Blockade of central histaminergic receptors→ drowsiness, anticholinergic effects on basal forebrain decreases concentration and information processing ability | Consider lower dose, consider nonsedating alternatives, consider addition of daytime alerting agent if agent is considered medically necessary | |
| Social drugs | Alcohol | n/a | Daytime fatigue Headaches Depression Anxiety | Alcohol can lead to daytime impairment symptoms by virtue of its effects on sleep (see Table S1), by direct CNS sedative effects, toxicity (“hangover”) effects, or due to withdrawal symptoms. | Taper and discontinue use |
Addendum. Sleep medicine as a multidimensional encounter - observation form
References
- 1.Brambilla P, Cipriani A, Hotopf M, Barbui C. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38:69–77. doi: 10.1055/s-2005-837806. [DOI] [PubMed] [Google Scholar]
- 2.Yang C, Winkelman JW. Iatrogenic Restless Legs Syndrome. In: Ondo WG, editor. Restless Legs Syndrome: Diagnosis and Treatment. New York: Informa Healthcare; 2007. pp. pp. 255–67. [Google Scholar]
- 3.Hargrave R, Beckley DJ. Restless leg syndrome exacerbated by sertraline. Psychosomatics. 1998;39:177–8. doi: 10.1016/S0033-3182(98)71370-2. [DOI] [PubMed] [Google Scholar]
- 4.Parish JM, Parish JM. Violent dreaming and antidepressant drugs: or how paroxetine made me dream that I was fighting Saddam Hussein. Journal of Clinical Sleep Medicine. 2007;3:529–31. [PMC free article] [PubMed] [Google Scholar]
- 5.Guilleminault C, Fromherz S. Narcolepsy: Diagnosis and Management. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2005. pp. 780–90. [Google Scholar]
- 6.Malow B. Approach to the Patient with Disordered Sleep. In: Kryger MH, Thomas R, Dement William C, editors. Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier; 2005. pp. 589–93. [Google Scholar]
- 7.Cicolin A, Mangiardi L, Mutani R, Bucca C. Angiotensin-converting enzyme inhibitors and obstructive sleep apnea. Mayo Clin Proc. 2006;81:53–5. doi: 10.4065/81.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Hewett K, Chrzanowski W, Schmitz M, et al. Eight-week, placebo-controlled, double-blind comparison of the antidepressant efficacy and tolerability of bupropion XR and venlafaxine XR. Journal of Psychopharmacology. 2009;23:531–8. doi: 10.1177/0269881108089602. [DOI] [PubMed] [Google Scholar]
- 9.Pierson WE, Shapiro GG, Furukawa CT, Bierman CW. Albuterol syrup in the treatment of asthma. Journal of Allergy & Clinical Immunology. 1985;76:228–33. doi: 10.1016/0091-6749(85)90707-9. [DOI] [PubMed] [Google Scholar]
- 10.Thompson DF, Pierce DR. Drug-induced nightmares. Annals of Pharmacotherapy. 1999;33:93–8. doi: 10.1345/aph.18150. [DOI] [PubMed] [Google Scholar]
- 11.Maebara C, Ohtani H, Sugahara H, et al. Nightmares and panic disorder associated with carvedilol overdose. Annals of Pharmacotherapy. 2002;36:1736–40. doi: 10.1345/aph.1A476. [DOI] [PubMed] [Google Scholar]
- 12.Reeves RR, Liberto V, Reeves RR, Liberto V. Precipitation of PTSD with metoprolol for hypertension. Psychosomatics. 2003;44:440–2. doi: 10.1176/appi.psy.44.5.440. [DOI] [PubMed] [Google Scholar]
- 13.Lozada F, Silverman S, Jr., Migliorati C. Adverse side effects associated with prednisone in the treatment of patients with oral inflammatory ulcerative diseases. J Am Dent Assoc. 1984;109:269–70. doi: 10.14219/jada.archive.1984.0349. [DOI] [PubMed] [Google Scholar]
- 14.Adams H. Awareness, dreaming or steroid-induced psychosis. Anaesthesia. 2007;62:198. doi: 10.1111/j.1365-2044.2007.04965.x. [DOI] [PubMed] [Google Scholar]
- 15.Omonuwa TS, Goforth HW, Preud'homme X, Krystal AD. The Pharmacologic Management of Insomnia in Patients with HIV. Journal of Clinical Sleep Medicine. 2009;5:251–62. [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar AJ, Wong SK, Andrew G, Kumar AJS, Wong SK, Andrew G. Statin-induced muscular symptoms: a report of 3 cases. Acta Orthopaedica Belgica. 2008;74:569–72. [PubMed] [Google Scholar]
- 17.Campion J, Western A, Campion J, Western A. Statins and joint pain. British Journal of Clinical Pharmacology. 2008;66:570–1. doi: 10.1111/j.1365-2125.2008.03218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alattar MA, Scharf SM. Opioid-associated central sleep apnea: a case series. Sleep & Breathing. 2009;13:201–6. doi: 10.1007/s11325-008-0221-7. [DOI] [PubMed] [Google Scholar]
- 19.Oberndorfer S, Saletu-Zyhlarz G, Saletu B. Effects of selective serotonin reuptake inhibitors on objective and subjective sleep quality. Neuropsychobiology. 2000;42:69–81. doi: 10.1159/000026676. [DOI] [PubMed] [Google Scholar]
- 20.Eagles CJ, Kendall MJ. The effects of combined treatment with beta 1-selective receptor antagonists and lipid-lowering drugs on fat metabolism and measures of fatigue during moderate intensity exercise: a placebo-controlled study in healthy subjects. British Journal of Clinical Pharmacology. 1997;43:291–300. doi: 10.1111/j.1365-2125.1997.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristal-Boneh E, Melamed S, Bernheim J, Peled I, Green MS. Reduced ambulatory heart rate response to physical work and complaints of fatigue among hypertensive males treated with beta-blockers. Journal of Behavioral Medicine. 1995;18:113–26. doi: 10.1007/BF01857864. [DOI] [PubMed] [Google Scholar]
- 22.Izumi N, Mizuguchi H, Umehara H, et al. Evaluation of efficacy and sedative profiles of H(1) antihistamines by large-scale surveillance using the visual analogue scale (VAS) Allergology International. 2008;57:257–63. doi: 10.2332/allergolint.O-07-525. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Oxford University Press . Oxford English dictionary. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- 2.Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9:1184–204. doi: 10.1111/j.1553-2712.2002.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 3.Garst J, Kerr NL, Harris SE, Sheppard LA. Satisficing in hypothesis generation. Am J Psychol. 2002;115:475–500. [PubMed] [Google Scholar]
- 4.American Academy of Sleep Medicine . The international classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 5.Chizner MA, editor. Classical teachings in clinical cardiology. Cedar Grove, NJ: Laennec Publishing; 1996. [Google Scholar]
- 6.March SK. W. Proctor Harvey: a master clinician-teacher's influence on the history of cardiovascular medicine. Tex Heart Inst J. 2002;29:182–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Fialova D, Topinkova E, Gambassi G, et al. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA. 2005;293:1348–58. doi: 10.1001/jama.293.11.1348. [DOI] [PubMed] [Google Scholar]
- 8.Campion J, Western A, Campion J, Western A. Statins and joint pain. Br J Clin Pharmacol. 2008;66:570–1. doi: 10.1111/j.1365-2125.2008.03218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar AJ, Wong SK, Andrew G, Kumar AJS, Wong SK, Andrew G. Statin-induced muscular symptoms: a report of 3 cases. Acta Orthop Belg. 2008;74:569–72. [PubMed] [Google Scholar]
- 10.Cicolin A, Mangiardi L, Mutani R, Bucca C. Angiotensin-converting enzyme inhibitors and obstructive sleep apnea. Mayo Clin Proc. 2006;81:53–5. doi: 10.4065/81.1.53. [DOI] [PubMed] [Google Scholar]
- 11.Yang C, Winkelman JW. Iatrogenic restless legs syndrome. In: Ondo WG, editor. Restless legs syndrome: diagnosis and treatment. New York: Informa Healthcare; 2007. pp. pp. 255–67. [Google Scholar]
- 12.Hoque R, Chesson AL., Jr Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and rapid eye movement behavior disorder/REM sleep without atonia: a literature review, qualitative scoring and comparative analysis. J Clin Sleep Med. 2010 [PMC free article] [PubMed] [Google Scholar]
- 13.Bender BG, Ballard R, Canono B, et al. Disease severity, scratching, and sleep quality in patients with atopic dermatitis. J Am Acad Dermatol. 2008;58:415–20. doi: 10.1016/j.jaad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Bender BG, Leung SB, Leung DY, Bender BG, Leung SB, Leung DYM. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: an objective life quality measure. J Allergy Clin Immunol. 2003;111:598–602. doi: 10.1067/mai.2003.174. [DOI] [PubMed] [Google Scholar]
- 15.Iliffe S, Kharicha K, Carmaciu C, et al. The relationship between pain intensity and severity and depression in older people: exploratory study. BMC Fam Pract. 2009;10:54. doi: 10.1186/1471-2296-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comella CL. Sleep disturbances and excessive daytime sleepiness in Parkinson disease: an overview. J Neural Transm. 2006;(Supplementum):349–55. doi: 10.1007/978-3-211-45295-0_53. [DOI] [PubMed] [Google Scholar]
- 17.Baghai-Ravary R, Quint JK, Goldring JJ, et al. Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respir Med. 2009;103:216–23. doi: 10.1016/j.rmed.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Russell JA. Osteomalacic myopathy. Muscle Nerve. 1994;17:578–80. doi: 10.1002/mus.880170603. [DOI] [PubMed] [Google Scholar]
- 19.Lotfi A, Abdel-Nasser AM, Hamdy A, Omran AA, El-Rehany MA. Hypovitaminosis D in female patients with chronic low back pain. Clin Rheumatol. 2007;26:1895–901. doi: 10.1007/s10067-007-0603-4. [DOI] [PubMed] [Google Scholar]
- 20.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78:1463–70. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 21.Glerup H, Mikkelsen K, Poulsen L, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66:419–24. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 22.Ziambaras K, Dagogo-Jack S. Reversible muscle weakness in patients with vitamin D deficiency. West J Med. 1997;167:435–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–65. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 24.Berk M, Sanders KM, Pasco JA, et al. Vitamin D deficiency may play a role in depression. Med Hypotheses. 2007;69:1316–9. doi: 10.1016/j.mehy.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Bracha HS, Ralston TC, Matsukawa JM, Williams AE, Bernstein DM. Diminished stress resilience in institutionalized elderly patients: is hypovitaminosis D a factor? Am J Geriatr Psychiatry. 2004;12:544–5. doi: 10.1176/appi.ajgp.12.5.544. [DOI] [PubMed] [Google Scholar]
- 26.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25:514–20. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 27.Barrows HS, Tamblyn RM. Problem-based learning: an approach to medical education. New York: Springer; 1980. [Google Scholar]
- 28.Swing SR, Swing SR. Assessing the ACGME general competencies: general considerations and assessment methods. Acad Emerg Med. 2002;9:1278–88. doi: 10.1111/j.1553-2712.2002.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 29.Lynch DC, Swing SR, Horowitz SD, et al. Assessing practice-based learning and improvement. Teach Learn Med. 2004;16:85–92. doi: 10.1207/s15328015tlm1601_17. [DOI] [PubMed] [Google Scholar]
- 30.Swing SR, Swing SR. The ACGME outcome project: retrospective and prospective. Med Teach. 2007;29:648–54. doi: 10.1080/01421590701392903. [DOI] [PubMed] [Google Scholar]
- 31.Malow B. Approach to the patient with disordered sleep. In: Kryger MH, Thomas R, Dement William C, editors. Principles and practice of sleep medicine. Philadelphia, PA: Elsevier; 2005. pp. 589–93. [Google Scholar]
- 32.Pevernagie D, Stanley N, Berg S, et al. European guidelines for the certification of professionals in sleep medicine: report of the task force of the European Sleep Research Society. J Sleep Res. 2009;18:136–41. doi: 10.1111/j.1365-2869.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- 33.Groopman JE. How doctors think. Boston: Houghton Mifflin; 2007. [Google Scholar]