Abstract
Objective
We retrospectively evaluated the survival outcome of patients with brain metastasis from hepatocellular carcinoma (HCC).
Methods
Between 1991 and 2007, a total of 20 patients were diagnosed as having brain metastasis from HCC. The mean age of the patients was 55 ± 13 years, and 17 (85.0%) were men. Seventeen (85.0%) patients had already extracranial metastases. The median time from diagnosis of HCC to brain metastasis was 18.5 months. Fourteen (70.0%) patients had stroke-like presentation due to intracerebral hemorrhage (ICH). Ten (50.0%) patients had single or solitary brain metastasis. Among a total of 34 brain lesions, 31 (91.2%) lesions had the hemorrhagic components.
Results
The median survival time was 8 weeks (95% CI, 5.08-10.92), and the actuarial survival rates were 85.0%, 45.0%, 22.5%, and 8.4% at 4, 12, 24, and 54 weeks. Age < 60 years, treatment of the primary and/or extracranial lesions, and recurrent ICH were the possible prognostic factors (p = 0.044, p < 0.001, and p = 0.111, respectively). The median progression-free survival (PFS) time was 3 months (95% CI, 0.95-5.05).
Conclusion
The overall survival of the patients with brain metastasis from HCC was very poor with median survival time being only 8 weeks. However, the younger patients less than 60 years and/or no extracranial metastases seem to be a positive prognostic factor.
Keywords: Brain metastasis, Hepatocellular carcinoma, Survival outcome, Prognostic factor
INTRODUCTION
Hepatocellular carcinoma (HCC) is a notorious cancer due to its aggressive clinical course and short survival time in Asian populations, and over the past two decades it has been a growing public health problem worldwide because its incidence has substantially increased5,7,21,22). Therefore, HCC currently ranks as the fifth most common type of cancer in men worldwide, though it was formerly considered to be a rare disease in Western and European countries6,7,22). In addition, recent therapeutic advances in surgical technique, transarterial chemoembolization (TACE), and various chemotherapeutic agents have contributed to the improved survival rate in these patients, which has increased the incidence of extrahepatic metastases1,10).
However, even in the endemic areas, brain metastasis from HCC is so rare that the incidence was reported to be only about 0.2%10). Only few exclusive studies reported that the prognosis is so poor when HCC spreads into the intracranial structures, including the skull and/or the brain, that the median survival time was just 1-2 months1,3). Thus, the authors performed this retrospective study in an effort to identify both the overall survival time and the prognostic factors for patients with brain metastasis from HCC.
MATERIALS AND METHODS
Patients and inclusion criteria
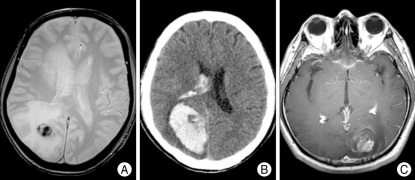
Between 1991 and 2007, a total of 20 patients were diagnosed as having brain metastasis from HCC. In this study, we included only the patients who had one or more brain parenchymal lesions with rim enhancement, homogeneous enhancement, or lobar hemorrhage suggesting neoplastic hemorrhage with enhancing solid portion (Fig. 1C) rather than hypertensive hemorrhage on brain computed tomography (CT) scan or magnetic resonance (MR) scan in the setting of histologically-confirmed HCC.
Fig. 1.
A : Gradient echo magnetic resonance (MR) image showing a hemorrhagic component in the tumor as lesions with low signal intensities. B : A large intracerebral hemorrhage (ICH) is identified on computed tomography scan of a patient with sudden headache and visual disturbance. The area near the midline in the hematoma with lower density than hematoma is brain metastasis from hepatocellular carcinoma. C : An ICH and edema around the heterogeneously enhancing mass on T1 gadolinium-enhance MR image.
All patients underwent surgical resection or biopsy for the liver mass, and the histological confirmation of the HCC diagnosis was made in every case. All patients had had a history of liver cirrhosis after hepatitis B virus (HBV) infection in 17 (85.0%) patients, after HCV infection in 2 (10.0%), and after Clonorchis sinensis infection in one (5.0%). Brain lesion was histologically diagnosed as a metastasis from HCC in 7 (35.0%) patients, and the remaining 13 (65%) patients whose diagnosis of brain lesion was made only radiologically had the evidence of neoplastic hemorrhage with enhancing solid portion on MR images, suggesting a metastasis from HCC, and/or had multiple extracranial metastases in the lung, bone, and/or lymph nodes.
During the aforementioned period, a total of 41,400 patients were diagnosed as having HCC in our institute. Thus, the incidence of brain metastasis from HCC was 0.05%.
Data collection and treatments for brain metastasis
All patient data were based on information contained in hospital charts and radiological studies and were collected in accordance with the case record form approved by the institutional review board. Clinical data, including information on age, Child-Pugh classification12,19), the level of alpha-fetoprotein (AFP), the Eastern Cooperative Oncology Group (ECOG) performance status15), the RTOG recursive partitioning analysis (RPA) classification8), radiological characteristics, treatment modality, and survival were collected. Any data that were missing from the medical records due to follow-up loss were obtained through a telephone interview with the patient or with his or her relatives, if deceased, after obtaining their permission.
The initial treatment modalily and/or the adjuvant treatment for brain lesions was selected after our considering the patients' will and/or patients' status, such as presence of intracerebral hemorrhage (ICH) and its mass effect, possibility of surgical resection, and medical and functional condition of the patients. Conventional radiotherapy had been mainly selected as an initial or adjuvant treatment according to the physician's decision before 1998, when a Gamma Knife unit was installed in our institute.
Whole brain radiotherapy and/or Gamma Knife radiosurgery
Whole brain radiotherapy (WBRT) was initiated 1-2 weeks after surgery or diagnosis of brain metastasis with a total dose of 30-45 Gy and 3.0 Gy per fraction with five fractions per week. WBRT or Gamma Knife radiosurgery (GKS) was used only as an adjuvant treatment after failure of each modality.
GKS was performed using the Leksell Gamma Knife (Elekta Instrument AB, Stockholm, Sweden) model B or C. Treatment planning was performed using thin-sliced MR images. The radiosurgery isodose, maximum dose, and marginal dose were decided based on contrast-enhanced lesion volume, calculated during dose planning with the best-fit isodose method. Therefore, we prescribed about 20 Gy for small lesions less than 6 cm3 in volume, and reduced the dose gradually to 10 Gy according to increase of the lesion volume. The treatments were designed to deliver 45-70% of the maximum dose at the margins of the lesion.
Statistical analysis
The overall survival and prognostic factors were analyzed. Overall survival was defined as the interval between the date of diagnosis of brain metastasis and the data of death. The Kaplan-Meier method was used to estimate the overall survival distributions. A log-rank test was used to test for differences in overall survival between the groups according to a given covariate. All covariates were analyzed in the form of a categorical variable, such as age > 60 years, AFP > 400 ng/mL, ECOG performance status 3/4 or not, and RPA class 1/2 or not. The patients were also stratified into two groups according to whether or not surgical resection for brain metastasis was performed, and were stratified according to whether or not treatment for the primary and/or extracranial lesions was performed. To compare the distribution of the binomial variables, the Mann-Whitney U test were performed. All statistical analyses were performed at an α level of 0.05, using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical and radiological features
The mean age of the patients was 55 ± 13 years (range, 22-76), and 17 (85.0%) were men. In 14 (70.0%) patients, brain metastasis was identified by work-up for neurological symptoms during follow-up of HCC. Metastasis to the lung and/or spine or para-aortic lymph nodes preceded brain metastasis in 10 patients, and metastasis only to the paraaortic lymph node and spine was identified before brain metastasis in each one of two patients. In the remaining two patients, there was no evidence of extracranial metastasis at the time of brain metastasis. In six (30.0%) patients, neurological symptoms were the first manifestation of HCC. The median time from diagnosis of HCC to brain metastasis was 18.5 months.
Five patients already had extracranial metastases discovered concurrently with development of neurological symptoms; four patients with lung and/or bone metastases and one with only bone metastasis. The rest one patient had no extracranial metastasis. Thus, overall 17 (85.0%) patients had already extracranial metastases. Only 3 (15.0%) had no evidence of extracranial metastasis after a thorough systemic work-up, and interestingly one female patient of them did not have any evidence of a viable tumor even in the liver and survived 165 weeks, the longest survival time of this series.
Presenting symptoms were hemiparesis and/or headache in 9 (45.0%) patients, visual field cut and headache in 5 (25.0%) patients, only headache in 3 (15.0%), and loss of consciousness in 3 (15.0%). Fourteen (70.0%) patients had stroke-like presentation due to ICH, and among them 12 patients were those whose brain metastases were identified during follow-up of HCC (p = 0.022).
At the time of brain metastasis, the severity of liver cirrhosis was classified as Child-Pugh grade A12,19) in 16 (80.0%) patients and B in the remaining 4 (20.0%) patients. None of the patients were classified as grade C, and no patient had a delayed prothrombin time. Among 18 patients whose serum AFP level was evaluated at the time of diagnosis of brain metastasis from HCC, the serum AFP level was within normal rage (0-20 ng/mL) in 6 (33.3%) patients.
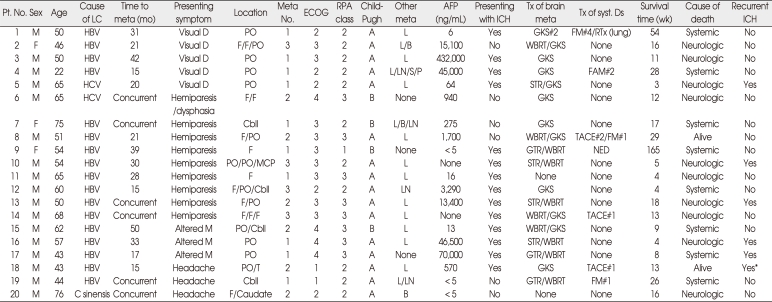
Ten (50.0%) patients had single or solitary brain metastasis and the remaining half of the patients had multiple lesions up to three. Among a total of 34 brain lesions, 15 (44.1%) were located in the parieto-occipital area, 12 (35.3%) in the frontal lobe, 4 (11.8%) in the cerebellar hemisphere, and each one (2.9%) of the remaining three lesions in the temporal lobe, middle cerebellar peduncle, and the head of the caudate nucleus. The mean longest diameter of brain lesions defined as contrast-enhancing portion on MR images was 21 ± 13 mm (range, 4-50). The hemorrhagic components in or near the mass were radiologically identified in 31 (91.2%) lesions on the gradient echo MR images and/or CT scan (Fig. 1). The characteristics of the patients and the details of their management are summarized in Table 1.
Table 1.
Characteristics of the patients and details of their management
*Recurrent ICH developed in the contralateral parietal lobe in 10 days. AFP : alpha-fetoprotein, C sinensis : Clonorchis sinensis, caudate : the head of the caudate nucleus, cbll : the cerebellum, Child-Pugh : Child-Pugh classification12,19), ECOG : Eastern Cooperative Oncology Group performance status15), F : the frontal lobe, FAM : 5-fluorouracil, adriamycin, mitomycin, GKS : gamma knife radiosurgery, GTR : complete resection, HBV : hepatitis B virus infection, ICH : intracerebral hemorrhage, L/B/LN/S/P : lung/bone/regional lymph node/stomach/pancreas, LC : liver cirrhosis, meta : metastasis, MCP : the middle cerebellar peduncle, meta No : number of metastatic lesions, NED : no evidence of systemic disease, other meta : extracranial metastasis except the brain metastasis, PO : the parieto-occipital area, Pt. No. : patient number, RPA class : the RTOG recursive partitioning analysis classification8), STR : incomplete resection, syst. Ds : systemic disease including the primary and extracranial lesions, T : the temporal lobe, TACE : transarterial chemoembolization, Tx : treatment, visual D : visual disturbance including field-cut, WBRT : whole brain radiotherapy, #number : number of cycles or treatment
Treatments
Seven (35.0%) patients underwent surgical resection of brain metastasis and/or ICH; three patients had complete resection and four patients had incomplete resection based on radiological findings after surgery; CT scan in three patients and MR imaging in four. The cause of incomplete resection in four patients was a profound bleeding from the metastatic lesions during surgery. Then, they were treated with WBRT in 6 patients and GKS in one patient. Among them, one patient underwent one cycle of chemotherapy using 5-fluorouracil and mitomycin (FM#1) for treatment of primary and/or extracranial lesions, and one had no evidence of disease after thorough systemic work-up at the time of brain metastasis.
Eleven (55.0%) patients were initially treated with radiation, such as GKS in 7 patients and WBRT in 4 patients. Among them, five patients underwent treatments for their primary and/or extracranial lesions; two patients were treated with one session of TACE, one with two sessions of TACE and FM#1, one with two cycles of chemotherapy using 5-fluorouracil, adriamycin, and mitomycin, one with FM#4 and radiotherapy to the lung metastases (3,750 cGy in 15 fractions). The remaining two (10.0%) patients (patient No. 11 and 20) refused any treatments for their primary lesion and brain metastases.
Overall survival and progression free survival
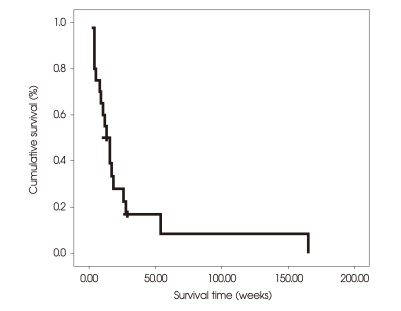
As of January 2, 2008, a total of 18 (90.0%) patients died, and two (10.0%) were still alive with progressive disease in the brain parenchyma. In terms of the cause of death as determined using the protocol described by Patchell et al.17), there were 10 (50.0%) neurologic deaths and 8 (40.0%) systemic deaths. The median survival time was 8 weeks (95% CI, 5.08-10.92), and the actuarial survival rates were 85.0%, 45.0%, 22.5%, and 8.4% at 4, 12, 24, and 54 weeks (Fig. 2).
Fig. 2.
Kaplan-Meier estimates of overall survival in the whole series (n = 20). The median survival time is 8 weeks (95% CI, 5.08-10.92), and the actuarial survival rates are 85.0%, 45.0%, 22.5%, and 8.4% at 4, 12, 24, and 54 weeks.
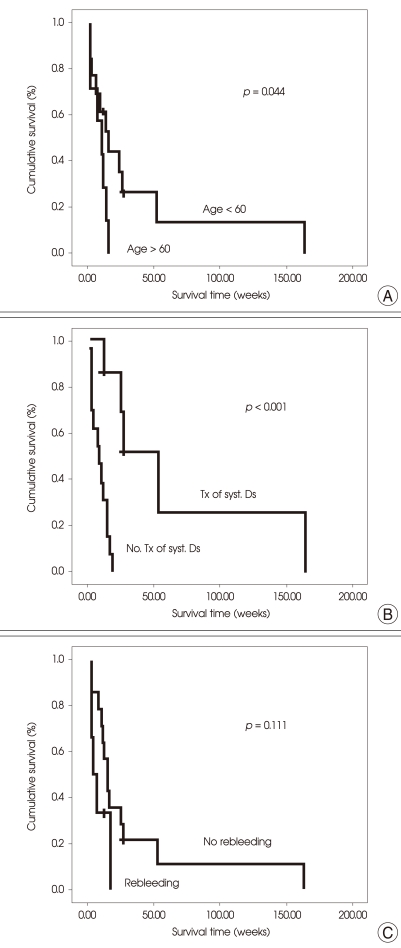
The patients younger than 60 years had a longer median survival time than the others [18 weeks (95% CI, 6.87-29.13) vs. 12 weeks (95% CI, 4.30-19.70); p = 0.044] (Fig. 3A). The patients whose primary and/or extracranial lesions were treated had a significantly longer median survival time that those without treatment [54 weeks (95% CI, 27.31-80.69) vs. 9 weeks (95% CI, 1.96-16.05); p < 0.001] (Fig. 3B). Patients with recurrent ICH after treatment had a poorer median survival time than that of those without it [5 weeks (95% CI, 0.20-9.80) vs. 16 weeks (95% CI, 10.569-21.44); p = 0.111] (Fig. 3C), however, the difference in survival time did not reach the statistical significance. The patients treated with radiation only using WBRT and/or GKS had a longer median survival time (16 weeks; 95% CI, 9.93-22.07) than that of those who underwent surgical resection of brain metastases followed by adjuvant therapy (8 weeks; 95% CI, 0.30-15.70), however this difference in survival time also did not reach the statistical significance (p = 0.667). Manifestation with ICH, RPA class 1/2 or not, liver function define by Child-Pugh classification, AFP level (> 400 ng/mL), and multiplicity of the lesions also did not have a significant effect on survival.
Fig. 3.
Kaplan-Meier estimates to test for differences in overall survival between groups according to a given covariate using a log-rank test. A : The patients younger than 60 years show a longer median survival time than those older. [18 weeks (95% CI, 6.87-29.13) vs. 12 weeks (95% CI, 4.30-19.70); p = 0.044]. B : The patients whose primary and/or extracranial lesions were treated show a significantly longer median survival time that those without treatment for the primary and/or extracranial lesions [54 weeks (95% CI, 27.31-80.69) vs. 9 weeks (95% CI, 1.96-16.05); p < 0.001]. 'syst. Ds' in the figure means the primary and/or extracranial lesions. C : Patients with recurrent ICH after treatment show a poorer median survival time than that of those without it [5 weeks (95% CI, 0.20-9.80) vs. 16 weeks (95% CI, 10.569-21.44); p = 0.111].
Seven (35.0%) patients died without any follow-up imaging studies; six patients died of very rapid progression of the neurological status, and the remaining one patient died of upper gastro-intestinal (GI) bleeding. In three (23.1%) of 13 patients with one or more follow-up imaging studies, new lesions appeared outside the treated areas, and 5 (38.5%) patients had regrowth of the treated lesions. The median progression-free survival (PFS) time was 3 months (95% CI, 0.95-5.05). The actuarial PFS rates were 69.2%, 49.5%, and 16.5% at 1, 3, and 5 months, respectively. The presence of extracranial metastases, AFP > 400 ng/mL, and the presence of multiple brain lesions showed the negative association with PFS (p = 0.089, p = 0.187, and p = 0.062, respectively), however, they all did not reach the statistical significance.
Additional treatments and complications
Six (30.0%) patients had additional treatment for brain metastases; one patient underwent repeated radiosurgery for new lesions outside the initially treated lesion, four patients were treated with radiosurgery for progression of the brain metastasis or the new lesions outside the first brain lesion after WBRT, and the last one patient experienced surgery due to the recurrent ICH during WBRT after incomplete surgical resection.
No patients among 4 patients who underwent chemotherapy experienced hematologic toxicities assessed using World Health Organization common toxicity criteria version 2.0.
Six (30.0%) patients experienced recurrent ICH during follow-up period; four patients among them died or underwent repeat surgery due to recurrent ICH, and the remaining two patients were treated conservatively. The majority of recurrent ICHs developed within 2 weeks (4 days-8 weeks) after diagnosis of brain metastasis from HCC. Three (15.0%) patients experienced aggravation of edema around the brain lesion resulted in aggravation or development of hemiparesis after WBRT and/or GKS. Three (15.0%) patients experienced GI bleeding during the follow-up period and were treated with endoscopic esophageal varix ligation. And, each one of other three patients was treated due to hepatitis after herb medication, hepatic encephalopathy, and pneumonia, which were not directly related with treatments.
DISCUSSION
Though brain metastasis from HCC is a rare occurrence even in the endemic areas, it may become a major health problem in the near future due to the increasing incidence of HCC, especially in the western and European countries, and the prolonged survival due to the recent development of advanced therapeutic techniques5,7,10,11,20-22). And, the incidence of brain metastases from HCC seems to be more higher than the previous estimations of 0.26% of the study of Chang et al.1) considering the report of 2.2% in Japanese autopsy series14).
Clinical and radiological features
In the present study, overall 17 (85.0%) patients already had extracranial metastases to the lung, bone, and/or regional lymph nodes at the time of diagnosis of brain metastasis from HCC. Thus, brain metastasis from HCC seems to develop at the end stage of disease progression of HCC with dismal 5-year survival rate of less than 5 percent7). Accordingly, the overall survival was very poor that the median survival time was only 8 weeks, and the actuarial survival rate was approximately 8% at one year. These findings may suggest that a more effective therapy for systemic HCC is required before any progress will be made with brain metastases.
Such a poor survival outcome may also result from some characteristic features of HCC brain metastasis. One of the noticeable features is that brain metastasis from HCC had a tendency of bleeding as in the previous reports1,2,4,9,10,13,14,16,18,21), though no patients were classified as Child-Pugh grade C, and had a delayed prothrombin time in this study. Thus, 70.0% of the patients had stroke-like presentation due to tumor bleeding, and over 90% of the lesions had the hemorrhagic components in or near the mass on MR images and/or CT scan. In addition, six (42.9%) of 14 patients who had presented with overt ICH experienced recurrent bleeding from the tumors after treatments, which resulted in fatal outcomes in those patients. This high rate of ICH after treatments seemed to mainly result from the incomplete resection of the tumor in the patients who experienced surgical resection and also was partly caused by the insufficient liver function that alters the hemostasis. Furthermore, because the majority of the lesions were located near the sensory-motor cortex, complete surgical resection of the lesions were difficult and bleeding from the tumor had usually resulted in neurological deficits and functional decline of the patients, which made those patients not being eligible for further adjuvant treatments.
Prognostic factors for overall survival
Age less than 60 years and treatments for the primary and systemic extracranial metastases seems to be positively associated with overall survival of the patients with brain metastasis from HCC. This result may be in accordance with those of the studies dealing with patients with brain metastasis from other primary sites, such as lung, breast, kidney, colon, and so on; however, there may be some possible confounding variables in this result, such as the functional status of the patients and the state of the systemic disease, including the state of intrahepatic primary tumor and extracranial metastasis. Namely, treatments for the primary and systemic extracranial metastases might be possible only for the patients with relatively limited extent of disease and favorable general condition. Nevertheless, we did not perform the multivariate analysis to correct the possible confounding variables because its statistical power was expected to be low due to the small sample size.
One of the noticeable findings about brain metastasis from HCC is that it has a tendency of bleeding, and it can rebleed after treatment. In fact, recurrent bleeding from tumor itself is one of the possible prognostic factors found in this study, though it did not reach the statistical significance. However, it was most problematic event during management of patients with brain metastasis from HCC. In addition, the cause of 4 deaths among 10 neurological deaths was recurrent tumor bleeding (Table 1). Thus, recurrent tumor bleeding seems to be the only evident cause of neurologic death after treatment of brain metastasis from HCC.
Such a tendency of bleeding may be due to the pathological feature of brain metastasis of HCC of a trabecular or sinusoidal pattern and interspersed vascular channels21). Considering that all four patients with incomplete surgical resection experienced recurrent ICH during or immediately after adjuvant WBRT, complete surgical resection should be obtained. If there are remnant tumors or unresectable lesions, GKS seems to be more useful than WBRT in terms of the time necessary for completion of each modality considering rapid progression of the neurological status and relatively early rebleeding from brain metastasis though it is obscure for GKS to prevent the recurrent bleeding and tumor progression rather than other modalities. However, one of the most important findings of this study is that decision of any treatments including surgical resection, WBRT, GKS, and chemotherapy should be cautiously made in selected patients, especially without evidence of extracranial metastases and/or any viable tumor in the liver, considering the poor clinical course and short-survival time in patients with brain metastases from HCC, suggesting no response to any treatments used in this study.
CONCLUSION
The overall survival of the patients with brain metastasis from HCC was very poor with median survival time being only 8 weeks, and the actuarial survival rate was approximately 8% at one year. Recurrent bleeding from tumor is one of the most problematic events in management of patients with brain metastasis from HCC. However, the younger patients less than 60 years and/or no extracranial metastases seem to be a positive prognostic factor.
Acknowledgements
This study was supported by Nuclear Research and Development Program of the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean Government (MEST) (Grant Code; M2010-0017604).
References
- 1.Chang L, Chen YL, Kao MC. Intracranial metastasis of hepatocellular carcinoma : review of 45 cases. Surg Neurol. 2004;62:172–177. doi: 10.1016/j.surneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Cho DC, Yi HJ, Ko Y, Oh SJ, Lee SR, Paik SS. Recurrent metastatic hepatocellular carcinoma presenting as consecutive "mirror image" intracerebral haematomas. J Clin Neurosci. 2005;12:699–702. doi: 10.1016/j.jocn.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Choi HJ, Cho BC, Sohn JH, Shin SJ, Kim SH, Kim JH, et al. Brain metastases from hepatocellular carcinoma : prognostic factors and outcome : brain metastasis from HCC. J Neurooncol. 2009;91:307–313. doi: 10.1007/s11060-008-9713-3. [DOI] [PubMed] [Google Scholar]
- 4.Del Ben M, Caporale A, Feole K, Alessandri C, Angelico F. Intracranial hemorrage due to brain metastases in an Italian HCV patient with hepatocellular carcinoma. J Exp Clin Cancer Res. 2003;22:641–644. [PubMed] [Google Scholar]
- 5.Deuffic S, Poynard T, Buffat L, Valleron AJ. Trends in primary liver cancer. Lancet. 1998;351:214–215. doi: 10.1016/S0140-6736(05)78179-4. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Hepatocellular carcinoma : an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 8.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi K, Matsuo T, Kurihara M, Daikoku M, Kitange G, Shibata S. Skull metastasis of hepatocellular carcinoma associated with acute epidural hematoma : a case report. Surg Neurol. 2000;53:379–382. doi: 10.1016/s0090-3019(00)00208-1. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Na DL, Park SH, Jeon BS, Roh JK. Nervous system involvement by metastatic hepatocellular carcinoma. J Neurooncol. 1998;36:85–90. doi: 10.1023/a:1005716408970. [DOI] [PubMed] [Google Scholar]
- 11.Kim SR, Kanda F, Kobessho H, Sugimoto K, Matsuoka T, Kudo M, et al. Hepatocellular carcinoma metastasizing to the skull base involving multiple cranial nerves. World J Gastroenterol. 2006;12:6727–6729. doi: 10.3748/wjg.v12.i41.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucey MR, Brown KA, Everson GT, Fung JJ, Gish R, Keeffe EB, et al. Minimal criteria for placement of adults on the liver transplant waiting list : a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transpl Surg. 1997;3:628–637. doi: 10.1002/lt.500030613. [DOI] [PubMed] [Google Scholar]
- 13.McIver JI, Scheithauer BW, Rydberg CH, Atkinson JL. Metastatic hepatocellular carcinoma presenting as epidural hematoma : case report. Neurosurgery. 2001;49:447–449. doi: 10.1097/00006123-200108000-00034. [DOI] [PubMed] [Google Scholar]
- 14.Murakami K, Nawano S, Moriyama N, Sekiguchi R, Satake M, Fujimoto H, et al. Intracranial metastases of hepatocellular carcinoma : CT and MRI. Neuroradiology. 1996;38:S31–S35. doi: 10.1007/BF02278115. [DOI] [PubMed] [Google Scholar]
- 15.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 16.Otsuka S, Fukumitsu T, Yamamoto T, Komori H, Shirane H. Brain metastasis of hepatocellular carcinoma presenting with hemorrhage--case report. Neurol Med Chir (Tokyo) 1987;27:654–657. doi: 10.2176/nmc.27.654. [DOI] [PubMed] [Google Scholar]
- 17.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 18.Peres MF, Forones NM, Malheiros SM, Ferraz HB, Stávale JN, Gabbai AA. Hemorrhagic cerebral metastasis as a first manifestation of a hepatocellular carcinoma. Case report. Arq Neuropsiquiatr. 1998;56:658–660. doi: 10.1590/s0004-282x1998000400023. [DOI] [PubMed] [Google Scholar]
- 19.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 20.Salvati M, Cimatti M, Frati A, Santoro A, Gagliardi FM. Brain metastases from hepatocellular carcinoma. A case report. J Neurosurg Sci. 2002;46:77–80. discussion 80. [PubMed] [Google Scholar]
- 21.Seinfeld J, Wagner AS, Kleinschmidt-DeMasters BK. Brain metastases from hepatocellular carcinoma in US patients. J Neurooncol. 2006;76:93–98. doi: 10.1007/s11060-005-4175-3. [DOI] [PubMed] [Google Scholar]
- 22.Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-94. Lancet. 1997;350:1142–1143. doi: 10.1016/S0140-6736(05)63789-0. [DOI] [PubMed] [Google Scholar]