Abstract
Anti-neutrophil cytoplasmic antibodies against proteinase 3 (PR3-ANCA) are used as diagnostic tools for patients with small vessel vasculitis (AASV). We have produced chimeric mouse/human PR3 molecules and investigate changes in reactivity over time and the possible relationship between epitope specificity and clinical course. Thirty-eight PR3-ANCA-positive patients diagnosed between 1990 and 2003 were followed until December 2005. Plasma was collected at each out-patient visit and older samples were retrieved retrospectively. Patients reacted with multiple epitopes at the time of diagnosis. At subsequent relapses 12 patients shifted reactivity, in 11 cases from epitopes located in the C-terminal towards epitopes in the N-terminal. Patients with reactivity against N-terminal parts of PR3 at diagnosis had a significantly lower relapse rate, 30% compared to 78% in the group with predominantly C-terminal reactivity (P = 0·04). The reactivity pattern did not correlate to outcome measured as death, end-stage renal disease or vasculitis activity index score (VDI) at 5 years. Further research is necessary to conclude if this is a general phenomenon.
Keywords: ANCA, epitope, proteinase 3, vasculitis, Wegener's granulomatosis
Introduction
The detection of anti-neutrophil cytoplasmic antibodies (ANCA) is an established tool for diagnosing patients with small vessel vasculitis such as Wegener's granulomatosis (WG), microscopic polyangiitis (MPA) and Churg–Strauss syndrome (CSS); these are now often referred to as ANCA-associated small vessel vasculitis (AASV). In vasculitis ANCA are directed against proteinase 3 (PR3) or myeloperoxidase (MPO), enzymes harboured in the azurophilic granules of neutrophils and in monocytes. In the first report linking ANCA to WG it was already stated that ANCA levels are related to disease activity [1]. Since then, many studies have been performed to examine the relationship between ANCA levels and disease activity, some of them finding ANCA levels valuable in the follow-up of patients with AASV [2–6], while others state that ANCA levels cannot be used to guide immunosuppressive therapy [7].
Various methods are used for PR3-ANCA detection, most frequently indirect immunoflurescence (IIF) and various enzyme-linked immunosorbent assays (ELISA), and the results of the different assays do not always match. The reason for the variable diagnostic yields with different assays is not known, but a plausible explanation is a differential exposure of epitopes on the PR3 antigen, and that antibodies directed at certain epitopes have a higher diagnostic potential. Several studies have been carried out to map the epitopes recognized by the PR3-ANCA. It has been shown that ANCA recognize conformational epitopes on PR3 [8], and subsequently studies using overlapping synthetic peptides, in order to find linear epitopes have been inconclusive [9,10]. An alternative approach is to express different parts of the antigenic molecule in a homologous but non-antigenic framework. This approach was used successfully in Goodpasture's disease to locate epitopes on the NC1 domain of the α3 chain and parts of the non-antigenic α1 chain in our laboratory [11]. We have also shown that chimeric molecules of human PR3 (hPR3) and murine PR3 (mPR3) can be expressed as recombinant proteins, recognized both by WG patient PR3-ANCA and mouse anti-human-PR3 monoclonal antibodies [12].
The aim of this study was to investigate epitope specificity in patients with PR3-ANCA-positive vasculitis at the time of diagnosis and to explore if epitope shifts occurred over time. We also wanted to explore the clinical utility of mouse/human chimeric proteins, when used as antigens in ELISA, for the diagnosis and follow-up of patients.
Methods
Patients and plasma
A cohort was generated in January 2003 consisting of patients with PR3-ANCA-positive AASV, diagnosed between 1990 and 2003, and followed at the departments of Nephrology in Lund and Malmö. The cohort was followed prospectively until December 2005, and a plasma sample was drawn at each out-patient visit. Samples were stored at −20°C at the kidney research laboratory in Lund until analysed. In addition, older samples drawn for other research studies or for clinical purposes were retrieved from our research laboratory as well as from Wieslab AB, Lund. All patients fulfilled the classification criteria for systemic vasculitis as indicated in the European Medicines Agency (EMEA) classification proposal and were classified according to an algorithm in the same report [13]. Of 44 identified patients, five were excluded because of missing serum samples at the time of diagnosis, and one patient was lost to follow-up. Plasma from 24 healthy blood donors, stored at −20°C until analysed, were used as negative controls.
Clinical data from patient charts were collected both prospectively and retrospectively. From the time of diagnosis the following data were retrieved: age, gender, clinical signs, creatinine and disease activity as assessed by the Birmingham Vasculitis Activity Score (BVAS). BVAS was created by a group in Birmingham, UK in 1994 [14] to measure disease activity in patients with a variety of systemic vasculitides. It scores abnormality ascribable to the presence of active vasculitis; the maximum possible score is 63. Only recent activity that has occurred afresh or deteriorated within the previous months, and is concluded as caused by active vasculitis, is measured.
The patients were followed until December 2005. During follow-up the following data were recorded: time of follow-up, time and cause of death, time of end-stage renal disease (ESRD), time of and symptoms at relapse and Vasculitis Damage Index (VDI) at 1 and 5 years. The VDI was created in 1997 [15] to aid in the separation of damage from disease activity in systemic vasculitis. The VDI scores damage due to non-healing scars from any cause that has occurred since the onset of vasculitis. It is a cumulative assessment of organ dysfunction, damage or scarring and either remains stable or increases with time.
Clinical remission was defined as absence of vasculitic activity as indicated as a BVAS of 0. Clinical relapse was defined as return of vasculitic activity to an extent yielding a BVAS of 4 or more. ANCA levels were not considered when assessing disease activity.
A sample from the time of diagnosis was selected from each patient and, when available, once every 6 months in remission, one at relapse and one 3 months before relapse. In patients without any relapses the numbers of selected samples were limited to four during the remission period. However, the most recent available sample was always chosen for analysis. A total of 342 samples were analysed using purified PR3 from human neutrophils (nHPR3) and recombinant proteins.
Ethics
The study was approved by the Lund University Ethics committee and informed consent was obtained from all the subjects enrolled.
Recombinant proteins
Recombinant PR3 were produced based on different combinations of the PR3 gene sequence from man and mouse. Eight constructs have been described previously [12]. Two of them, designated rmPR3 and rHPR3, are based entirely upon the mouse and human sequences, respectively. The remaining six, designated mHH, mHm, mmH, Hmm, HmH and HHm, are chimeric constructs, with one-third of the sequence from one species and two-thirds from the other species. Two new chimeric proteins, designated mH and Hm, were constructed to consist of half the human and half the mouse PR3 sequences. The following primers were used when producing the new constructs: GCT AAA GCT TGT GGG CGG GCA CGA GGC G; GCT AGC GGC CGC TCA GGG GCG GCC CTT GGC; ACA CAA AGC TTG TAG GTG GGC ACG; GTG TGT GCG GCC GCG CCC CAG CTT TAG GCT GC; GTC CAG CTG CCA CAG CAG GAC CAG ACT CTG TCC CAG; CTG GGA CAG AGT CTG GTC CTG CTG TGG CAG CTG GAC; GCT TCT CTG CCC CAG GAC CAG CCA GTG CCC CAC; and GTG GGG CAC TGG CTG GTC CTG CTG GGG CAG AGA AGC, using double overhang splicing polymerase chain reaction (PCR) as described previously [12].
All 10 recombinant proteins were produced in HEK-293 cells and purified using 6xHisTag, as described previously. The amino acid sequences for human PR3 (hPR3), murine PR3 (mPR3) and the eight different chimeric PR3 used in this study are shown in Fig. 1.
Fig. 1.
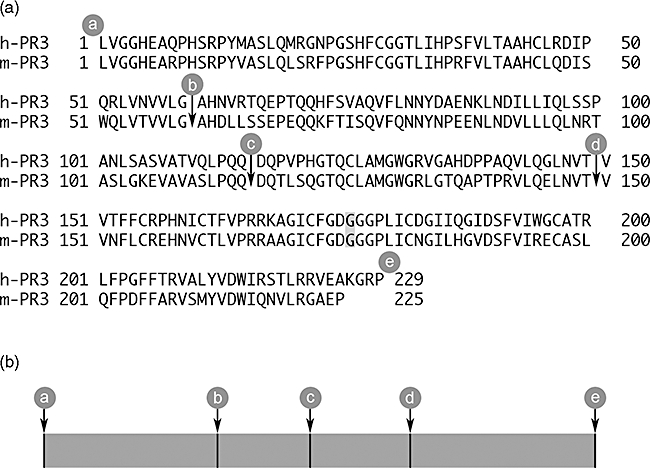
(a) The amino acid sequences for human proteinase 3 (h-PR3) and murine PR3 (m-PR3) used as templates for the recombinant proteins. The signal peptides and propeptides are removed and a HindIII site is introduced at the 5′ end and a NotI site is introduced at the 3′ end. All the recombinant molecules were mutated at the active site by changing Ser to Gly (shaded) to create enzymatically inactive mutants. The cleavage sites for the different constructs are marked a, b, c, d, and e. (b) The chimeric constructs were named according to the origin of the respective proportions of the molecule, were H stands for hPR3 and m for mPR3; for example, mmH is comprised of murine sequence from a to d followed by human sequence from d to e.
ELISA
Polystyrene microtitre plates (Nunc Immunoplate, Roskilde, Denmark) were coated overnight at room temperature (RT) with 0·05 µg/well of purified recombinant protein or native human neutrophil PR3 (nHPR3) (Wieslab, Lund, Sweden) in coating buffer (50 mM Na2CO3, 0·02% NaN3, pH 9·6). The plates were washed three times with washing buffer [0·15 mM NaCl, 0·05% (v/v) Tween 20] and then incubated for 1 h at RT with 100 µl/well of human plasma diluted 1/100 in PBS–bovine serum albumin (BSA) [1·5 mM KH2PO4, 8 mM Na2HPO4, 0·14 M NaCl, 2·7 mM KCl, 0·02% (w/v) NaN3, 0·05% (v/v) Tween 20, containing 0·2% (w/v) BSA, pH 7·3]. After three new washes the plates were incubated for 1 h with 100 µl/well of alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG) diluted 1/10 000 in PBS–BSA. The amount of bound antibodies was detected by the use of P-nitrophenyl phosphate (Sigma Chemical Company, St Louis, MO, USA) (1 mg/ml) in substrate buffer (1 M diethanolamine, 0·5 mM MgCl2, pH 9·8). Colour development was measured spectrophotometrically at 405 nm after 1 h. All assays were performed in duplicate, and when a standard error larger than 10% was found the sample was reanalysed. Results were expressed as optical density (OD) values after the subtraction of the mean value of control samples ± two standard deviations (s.d.). The s.d. (0·05) was calculated using the arithmetic mean of the s.d. from 10 runs with one positive control plasma sample and 24 runs with plasma samples from negative controls, with all assays using recombinant proteins as well as purified neutrophil PR3 in direct ELISA.
Patterns of reactivity
The OD values from the different assays were put into the following formula: (Hm + HHm + Hmm) – (mH + mHH + mmH). A cut-off level of 0·11 was chosen based on two times the average s.d. obtained with control plasma. A sample was considered to have a predominant N-terminal reactivity (N-pattern) if the formula yielded a positive result above 0·11, and a predominant C-terminal reactivity (C-pattern) if the result was below –0·11. Sample results whose values were between >−0·11 and <+0·11 were sorted into an intermediate pattern category (I-pattern).
Statistics
Comparisons between the reactivity patterns at diagnosis and relapse rate were carried out with χ2 test for independence, using GraphPad InStat version 3·0. The interquartile ranges were calculated using Prism 5·0 GraphPad software.
Results
Patient characteristics
Thirty-eight patients with PR3-ANCA-positive AASV were included in this study; 34 were classified as WG and four as MPA. Demographic and clinical data at the time of diagnosis are shown in Table 1 and organ involvement is shown in Fig. 2. Kidneys were the organ systems involved most frequently, followed by the upper respiratory tract. All patients in this study achieved initial disease control with standard treatment. Follow-up data are also shown in Table 1. The median follow-up time was 8·5 years (range 2–15·6 years). Twenty-two patients experienced one or more relapses and the time from diagnosis to the first relapse was a median 2·5 years (range 0·3–9·0 years). From diagnosis to the end of the study the total number of relapses was 36; nine of these relapses occurred during the prospective phase of the study (2003–05). The most frequent organ involvement at first relapse was again renal and upper respiratory tract involvement. The median BVAS at first relapse was 12 (range 4–22). Three patients died during the prospective period, at 4·5, 8·5 and 10·2 years after diagnosis. Two patients were on renal replacement therapy at the start of the prospective period. One of them started treatment with haemodialysis 6·2 years after diagnosis and the other patient had already started haemodialysis at the time of diagnosis and received a renal transplant 2 years later.
Table 1.
Clinical data at time of diagnosis and numbers of subsequent relapses for the 38 patients with proteinase 3-anti-neutrophil cytoplasmic antibodies (PR3-ANCA)-positive small vessel vasculitis, divided according to reactivity pattern at diagnosis
| Reactivity pattern at diagnosis |
||||
|---|---|---|---|---|
| All | C-pattern | N-pattern | I-pattern | |
| Number of patients | 38 | 18 | 10 | 10 |
| Male/female | 22/16 | 12/6 | 3/7 | 7/3 |
| Age, years† | 58 (10–78) | 58 (10–78) | 52 (23–75) | 59 (26–78) |
| WG/MPA | 34/4 | 17/1 | 9/1 | 8/2 |
| Creatinine, µmol/l† | 89 (47–777) | 86 (47–777) | 114 (55–547) | 170 (47–684) |
| BVAS† | 19 (5–32) | 21 (6–32) | 17 (6–25) | 17 (5–29) |
| Patients with relapse n (%) | 22 (58) | 14 (78)* | 3 (30)* | 5 (50) |
P = 0·04.
Median (range). BVAS: Birmingham vasculitis activity score; WG/MPA: Wegener's granulomatosis/microscopic polyangiitis.
Fig. 2.
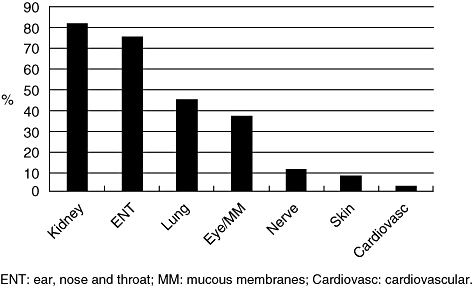
Organ involvement at diagnosis for the 38 patients with proteinase 3-anti-neutrophil cytoplasmic antibodies (PR3-ANCA)-positive small vessel vasculitis.
Patterns of epitope reactivity
Individual samples exhibited a wide variety of reactivity against the different chimeric constructs and none of the ELISAs with recombinant PR3 performed better than ELISA with native human PR3. Patient plasma reacted with all chimeric constructs, but the reactivity for some constructs was generally low, i.e. for mHm and HmH. However, during an exacerbation episode no patient switched epitope recognition pattern. Figure 3a shows reactivity of plasma from one patient, with three exacerbations with six different chimeric constructs. In this example there is reactivity mainly to the N-terminal parts of PR3 during the entire time-period. In order to describe and compare the reactivity patterns, we combined the results from the chimeric assays and divided the reactivities into groups based upon whether reactivity was directed mainly against the N-terminal or C-terminal parts of the PR3 molecule, according to the formula in the Methods section. At the time of diagnosis, 18 patients exhibited the C-pattern, 10 patients the N-pattern and 10 patients the I-pattern (Fig. 4a).
Fig. 3.
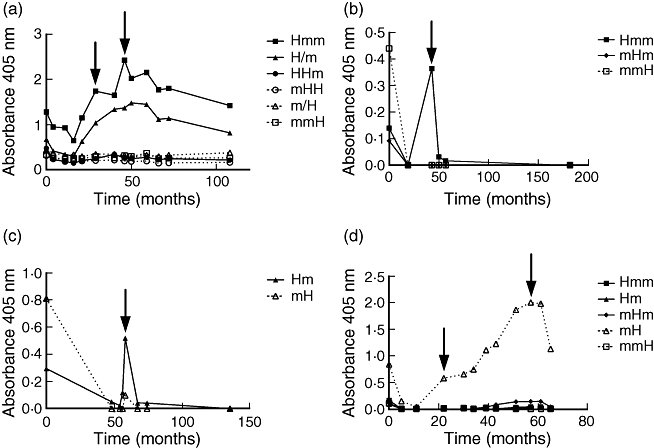
Reactivity of plasma from four patients with different chimeric proteinase 3 (PR3) in enzyme-linked immunosorbent assay. Time 0 is the time of diagnosis. Arrows indicate relapses. Note that values are not proportionate between diagrams.
Fig. 4.
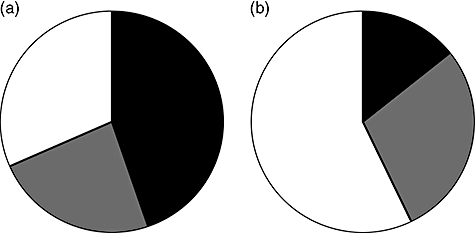
Reactivity patterns at (a) diagnosis (38 patients) and (b) at first relapse (21 patients). Black: C-terminal reactivity; grey: N-terminal reactivity; white: intermediate reactivity.
Epitope shifts
When patients went into remission, reactivity against the constructs generally diminished. At relapse the reactivity increased again and the reactivity pattern changed considerably; that is, from N-terminal pattern to C-terminal or vice versa, in 12 patients. One patient changed his reactivity pattern three times. The other 11 patients all exhibited only one shift – interestingly, in all 11 patients this shift was from the C-terminal pattern to the N-terminal pattern. Figure 3b,c shows examples of patients whose reactivity changed from predominantly C-terminal reactivity towards mainly N-terminal reactivity at relapse. Figure 3d shows a patient with antibodies towards only the C-terminal part of PR3 during the entire course of disease, including two relapses (at 22 months and 57 months). Figure 4b shows the distribution of reactivity patterns at first relapse for 21 patients, indicating a major shift in reactivity compared to onset (Fig. 4a).
Sensitivity to detect and predict relapses
Serum or plasma samples were available from 29 of the 36 instances of relapses occurring during the entire study period (1990–2005). As mentioned above, the recombinant PR3 performed worse than ELISA using PR3 purified from human neutrophils. Reactivity above the cut-off level with recombinant human PR3 was found for only 50% of the sera, and 8% exhibited positive results for recombinant mouse PR3 at time of relapse. Results using chimeric constructs differed between 16% and 47%, with a construct having the human sequence in the C-terminal end (mHH), showing the highest rate of positive results. We concluded that no single chimeric constructs offered any improvement when trying to diagnose relapse by serological assays.
Prognostic information
At diagnosis 18 patients exhibited the predominant C-terminal pattern of reactivity, 10 patients exhibited the predominant N-terminal pattern, while 10 patients were considered to have an intermediate pattern (Table 1). We found no differences in basal clinical characteristics from the time of diagnosis when comparing patients with different patterns of reactivity. However, there was a higher tendency for relapse among patients with the C-terminal pattern. During a mean follow-up time of 8·6 years, 14 (78%) of the patients with the C-terminal pattern experienced at least one relapse, while the corresponding figure was three (30%) during a mean follow-up of 8·8 years among patients with the N-terminal pattern (P = 0·04). Five (50%) patients with the intermediate pattern experienced relapses. Despite the different relapse tendencies we did not record any significant difference in VDI at 5 years, ESRD or patient death during follow-up (data not shown).
Discussion
We produced chimeric human/mouse PR3 molecules to reveal epitope specificity of PR3-ANCA in order to increase our understanding about the immunization process and with the hope of constructing more effective diagnostic assays. Because PR3-ANCA recognize conformational epitopes [8] it is very important that the three-dimensional structure is correct, and studies with linear peptides have not been successful in finding the epitopes for PR3-ANCA [9,10,16,17]. Recombinant PR3 have been expressed in different eukaryotic cell systems. PR3-ANCA from patients with WG react with recombinant PR3 expressed in the rat basophilic mast cell line RBl-1 and the murine myeloblast-like cell line 32D [18], or expressed in the human mast cell line HMC-1 [19] and the human epithelial cell line 293 [20]. However, none of the recombinant proteins investigated in our study offered any improvement in diagnostic yield compared to standard methods for ANCA detection. Recombinant human PR3 was recognized by serum from only 50% of the patients with systemic vasculitis at the time of diagnosis, while 87% were positive using an assay with native human PR3 in direct ELISA.
The expression of recombinant PR3 in HEK 293 cells has been studied in detail [20]; it was found that the N-terminal activation dipeptide of PR3 is not cleaved off in this cell type, and some PR3-ANCA react with PR3 only after cleavage of this propeptide. This was circumvented in our study by constructing cDNA without the propeptide. Another discovery in that study was that the recombinant PR3 secreted into the culture media had an approximate molecular mass of 32–38 kDa; after removal of the aspargine-linked sugar moieties the molecular mass was reduced to about 29 kDa, which indicates that the recombinant PR3 is glycosylated. The same group has shown recently that glycosylation occurs at both aspargine-linked glycosylation sites (Asn-102 and Asn-147) in human neutrophil PR3, and when expressing recombinant PR3 in HMC-1 cells unglycosylated PR3 becomes secreted preferentially into media supernatants [21]. This unglycosylated variant was not recognized by all PR3-ANCA-positive sera in capture ELISA but, conversely, a subset of ANCA displayed increased binding to recombinant PR3 without a glycan at Asn-147. This shows that there is heterogeneity in the ANCA response and that ANCA are sensitive to minimal changes in the conformation of PR3. We have not analysed to what extent our recombinant proteins are glycosylated and part of the differences between our recombinant human PR3 and native human PR3 might be due to disparate glycosylation.
Even though the chimeric constructs had a low sensitivity to detect active disease, disqualifying them as potential antigens for ELISAs in clinical practice, other relevant findings can be determined from the present study. When analysing the results of individual assays we saw that reactivity shifted during the course of disease. When analysing samples drawn from one exacerbation episode the autoantibodies seemed directed to the same target but when patients relapsed, sometimes many years later, often a different set of epitopes seemed to be targeted. Epitope shifts in WG have been reported in a small study [22] where it was shown that patients could inhibit their own PR3-ANCA. In this study, samples from the time of diagnosis were made to compete with samples from relapse in the same patient; with this method epitope spreading was observed in two patients, while in two others epitope narrowing was seen. If PR3-ANCA is the consequence of molecular mimicry based on shared epitopes with a certain as yet unidentified microorganism, and if relapse is a consequence of re-exposure to this organism, it is strange that patients react with different epitopes at relapses. When we created a formula to analyse the epitope reactivity pattern we found that there was a clear trend showing that most epitope shifts occurred from a C-terminal towards a more N-terminal reactivity pattern. We have no explanation for this finding.
Another interesting piece of information relating to epitope reactivity was that the reactivity pattern at the time of diagnosis seemed to reveal prognostic information. It must be stressed, however, that this was a serendipitous finding. The statistical significance can be questioned as the study was small, and no such hypothesis was formulated in advance. Patients who exhibited the C-terminal pattern at the time of diagnosis experienced more relapses. If patients at lower risk of relapse could be selected for milder treatment, side effects could be diminished. Patients at higher risk of relapse could be observed more attentively to detect early signs of disease activity, before permanent organ damage has occurred. To confirm these speculations, larger studies are required with better chimeric PR3.
In conclusion, our studies show that different patients recognize different epitopes at time of diagnosis. Twelve of 19 patients shifted epitope reactivity at subsequent relapses, 11 from C-terminal to N-terminal reactivity. Patients with an N-terminal pattern at diagnosis seem to have a lower risk of relapse. The frequent occurrence of epitope shifts seen in this patient group indicates an activation of new B cell clones during the course of disease. This may be of importance when designing new therapy regimens.
Acknowledgments
This study was supported by grants from the Swedish Research Council (#15152), The Crafoord Foundation and Lund University Hospital funds.
Disclosure
None.
References
- 1.van der Woude FJ. Anticytoplasmic antibodies in Wegener's granulomatosis. Lancet. 1985;2:48. doi: 10.1016/s0140-6736(85)90105-9. [DOI] [PubMed] [Google Scholar]
- 2.Tervaert JW, van der Woude FJ, Fauci AS, et al. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149:2461–5. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 3.Jayne DR, Gaskin G, Pusey CD, Lockwood CM. ANCA and predicting relapse in systemic vasculitis. Q J Med. 1995;88:127–33. [PubMed] [Google Scholar]
- 4.Boomsma MM, Stegeman CA, van der Leij MJ, et al. Prediction of relapses in Wegener's granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum. 2000;43:2025–33. doi: 10.1002/1529-0131(200009)43:9<2025::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Segelmark M, Phillips BD, Hogan SL, Falk RJ, Jennette JC. Monitoring proteinase 3 antineutrophil cytoplasmic antibodies for detection of relapses in small vessel vasculitis. Clin Diagn Lab Immunol. 2003;10:769–74. doi: 10.1128/CDLI.10.5.769-774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders JS, Huitma MG, Kallenberg CG, Stegeman CA. Prediction of relapses in PR3-ANCA-associated vasculitis by assessing responses of ANCA titres to treatment. Rheumatology (Oxf) 2006;45:724–9. doi: 10.1093/rheumatology/kei272. [DOI] [PubMed] [Google Scholar]
- 7.Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med. 2007;147:611–9. doi: 10.7326/0003-4819-147-9-200711060-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bini P, Gabay JE, Teitel A, Melchior M, Zhou JL, Elkon KB. Antineutrophil cytoplasmic autoantibodies in Wegener's granulomatosis recognize conformational epitope(s) on proteinase 3. J Immunol. 1992;149:1409–15. [PubMed] [Google Scholar]
- 9.Griffith ME, Coulthart A, Pemberton S, George AJ, Pusey CD. Anti-neutrophil cytoplasmic antibodies (ANCA) from patients with systemic vasculitis recognize restricted epitopes of proteinase 3 involving the catalytic site. Clin Exp Immunol. 2001;123:170–7. doi: 10.1046/j.1365-2249.2001.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Der Geld YM, Simpelaar A, Van Der Zee R, et al. Antineutrophil cytoplasmic antibodies to proteinase 3 in Wegener's granulomatosis: epitope analysis using synthetic peptides. Kidney Int. 2001;59:147–59. doi: 10.1046/j.1523-1755.2001.00475.x. [DOI] [PubMed] [Google Scholar]
- 11.Hellmark T, Segelmark M, Unger C, Burkhardt H, Saus J, Wieslander J. Identification of a clinically relevant immunodominant region of collagen IV in Goodpasture disease. Kidney Int. 1999;55:936–44. doi: 10.1046/j.1523-1755.1999.055003936.x. [DOI] [PubMed] [Google Scholar]
- 12.Selga D, Segelmark M, Wieslander J, Gunnarsson L, Hellmark T. Epitope mapping of anti-PR3 antibodies using chimeric human/mouse PR3 recombinant proteins. Clin Exp Immunol. 2004;135:164–72. doi: 10.1111/j.1365-2249.2004.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watts R, Lane S, Hanslik T, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66:222–7. doi: 10.1136/ard.2006.054593. R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Q J Med. 1994;87:671–8. [PubMed] [Google Scholar]
- 15.Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–80. doi: 10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- 16.Williams RC, Jr, Staud R, Malone CC, Payabyab J, Byres L, Underwood D. Epitopes on proteinase-3 recognized by antibodies from patients with Wegener's granulomatosis. J Immunol. 1994;152:4722–37. [PubMed] [Google Scholar]
- 17.Chang L, Binos S, Savige J. Epitope mapping of anti-proteinase 3 and anti-myeloperoxidase antibodies. Clin Exp Immunol. 1995;102:112–9. doi: 10.1111/j.1365-2249.1995.tb06644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garwicz D, Lindmark A, Hellmark T, Gladh M, Jogi J, Gullberg U. Characterization of the processing and granular targeting of human proteinase 3 after transfection to the rat RBL or the murine 32D leukemic cell lines. J Leukoc Biol. 1997;61:113–23. doi: 10.1002/jlb.61.1.113. [DOI] [PubMed] [Google Scholar]
- 19.Specks U, Fass DN, Fautsch MP, Hummel AM, Viss MA. Recombinant human proteinase 3, the Wegener's autoantigen, expressed in HMC-1 cells is enzymatically active and recognized by c-ANCA. FEBS Lett. 1996;390:265–70. doi: 10.1016/0014-5793(96)00669-2. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, Fass DN, Viss MA, et al. A proportion of proteinase 3 (PR3)-specific anti-neutrophil cytoplasmic antibodies (ANCA) only react with PR3 after cleavage of its N-terminal activation dipeptide. Clin Exp Immunol. 1998;114:320–6. doi: 10.1046/j.1365-2249.1998.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Specks U, Fass DN, Finkielman JD, et al. Functional significance of Asn-linked glycosylation of proteinase 3 for enzymatic activity, processing, targeting, and recognition by anti-neutrophil cytoplasmic antibodies. J Biochem (Tokyo) 2007;141:101–12. doi: 10.1093/jb/mvm008. [DOI] [PubMed] [Google Scholar]
- 22.Rarok AA, van der Geld YM, Stegeman CA, Limburg PC, Kallenberg CG. Diversity of PR3-ANCA epitope specificity in Wegener's granulomatosis. Analysis using the biosensor technology. J Clin Immunol. 2003;23:460–8. doi: 10.1023/b:joci.0000010422.73892.b5. [DOI] [PubMed] [Google Scholar]


