Abstract
Specific immunotherapy (SIT) is a well-established and clinically effective treatment for allergic diseases. A pollen allergoid formulated with the T helper type 1 (Th1)-inducing adjuvant monophosphoryl lipid A (MPL) facilitates short-term SIT. Little is known about mechanisms of tolerance induction in this setting. In a prospective study, 34 patients allergic to grass pollen (25 male, nine female, median age 10·2 years) received a total of 44 SIT courses (20 in the first, 24 in the second) with MPL-adjuvanted pollen allergoids. Immunogenicity was measured by levels of specific immunoglobulin G (IgGgrass) and IgG4grass by antibody blocking properties on basophil activation, and by induction of CD4+, CD25+ and forkhead box P3 (FoxP3+) regulatory T cells (Treg). Specific IgG and IgG4 levels increased only slightly in the first year of SIT. In the second year these changes reached significance (P < 0·0001). In keeping with these findings, we were able to show an increase of Treg cells and a decreased release of leukotrienes after the second year of treatment. In the first year of treatment we found little evidence for immunological changes. A significant antibody induction was seen only after the second course of SIT. Short-course immunotherapy with pollen allergoids formulated with the Th1-inducing adjuvant MPL needs at least two courses to establish tolerance.
Keywords: allergoids, blocking antibodies, monophosphoryl lipid A (MPL), regulatory T -cells, ultra-short-course specific immunotherapy
Introduction
Immunoglobulin (Ig)E-mediated allergic disorders such as asthma and allergic rhinitis have become a growing epidemic in industrialized countries, affecting 20–30% of the population [1]. Atopic disease nowadays is a major burden to public health, demanding an ongoing research to prevent and treat allergic conditions. Modern pharmacotherapy can only mitigate the symptoms of allergic diseases; to date, specific immunotherapy (SIT) is the only safe and efficacious treatment with the potential to similarly ameliorate symptoms and alter the natural course of the disease. Gradually increasing quantities of specific allergen are given to induce immunotolerance and reduce symptoms to that allergen upon subsequent exposure [2–4]. Tolerance to allergens can be defined as persistence of efficacy after discontinuation of treatment, implying an altered allergen-specific memory T and B cell response [5,6]. Ever since the introduction of SIT in 1911, continuing development has improved safety and efficacy. The vaccines are now better standardized and characterized, according to new regulatory requirements [7]. This goal was achieved by addressing manufacturing quality (better characterized and standardized allergen extracts), optimal dosing, allergen modification (to reduce allergenicity while maintaining immunogenicity), adjuvant adsorption (to control release) and adjuvant activity (to assist immunomodulatory action).
It was shown that l-tyrosine is a safe adjuvant for human use [8] and has both an adsorptive role (as a short-term depot) and a favourable immunological effect [9,10]. Monophosphoryl lipid A (MPL®) is an adjuvant derived from the lipopolysaccharide of Salmonella minnesota R 595. The lipid A portion of the endotoxin has long been known to be a potent adjuvant, but unacceptable toxicity has limited its clinical use. The removal of a phosphate and fatty acid group from lipid A produced a molecule, MPL®, that retained the adjuvant properties of lipid A but reduced its toxicity significantly [11,12]. The adjuvant activity of MPL® promotes primarily a T helper type 1 (Th1) response [13–15]. MPL® has been shown to be well tolerated and to enhance both humoral and cellular immune responses.
One formulation (Pollinex® Quattro) is employing glutaraldehyde modified pollen (allergoids) adsorbed onto l-tyrosine and MPL®. This provides a vaccine that is efficacious with only four doses, in contrast to longer administration schedules in use for other allergy vaccines [16]. Short-term SIT offers a convenient option and supports compliance in children and adolescents.
So far there is no consensus regarding the mechanism of successful SIT, but it is thought to involve a redressed Th1/Th2 cell balance [17] by depressing the Th2 cellular response [interleukin (IL)-4, IL-5 and IL-13] in favour of a more Th1-like response [interferon (IFN)-γ, IL-2 and tumour necrosis factor (TNF)-α], the induction of regulatory T cells [18] and formation of specific IgG, especially IgG4 antibodies [19–22].
IgG antibodies induced by means of immunotherapy block allergen-induced IgE-dependent histamine release by basophils [23] and suppress allergen-specific T cell responses in vitro by inhibiting binding of IgE allergen complexes to antigen-presenting cells [24,25]. It has been shown that blocking IgG antibodies, which have been induced by allergen-specific immunotherapy, inhibit IgE-facilitated allergen presentation and can result in decreased T cell proliferation and reduced production of IL-4 and IL-5 [26]. Furthermore, IL-10 and transforming growth factor (TGF)-β cooperate in the regulatory T cell response to mucosal allergens in short-term immunotherapy (SCIT) via an antigen-specific suppressive activity in CD4+CD25+ T cells of allergic individuals. Regulatory T cells (Tregs) are able to inhibit the development of allergic Th2 responses and their up-regulation plays a major role in allergen-specific immunotherapy (SIT) [27,28]. This association was found because forkhead box P3 (FoxP3)-deficient subjects suffer among other findings from atopic disease [29]. Subsets of Treg cells are the thymus-selected CD4+CD25+FoxP3+ T cells [30,31]. FoxP3 is a member of the forkhead/winged-helix family of transcription factors and acts as the master regulator for the development and function of Tregs[32]. Tregs selectively expressing FoxP3 [33] show suppressive properties on effector T cells [34,35]. This suppressive capacity of Tregs is impaired in atopic patients, especially during the pollen season [36,37]. The demonstration of local FoxP3+CD25+ T cells in the nasal mucosa and their increased numbers after immunotherapy supported the role of Treg cells in the induction of allergen-specific tolerance in human subjects [38]. Their increased numbers correlate with clinical efficacy and suppression of seasonal allergic inflammation. Therefore, they have become a prime target for strategies aimed at inducing tolerance. Children who outgrew cow's milk allergy had increased numbers of circulating Tregs compared with children with persistent active allergic disease [39]. Consequently, the formation of specific IgG antibodies and the induction of regulatory T cells can be used as surrogate markers for successful SIT. In our study we investigated the time–course of specific IgG and IgG4 antibodies, their property to act as blocking antibodies and the induction of CD4+, CD25+ and FoxP3+ T cells (Treg) in the first and second years of a specific immunotherapy.
Methods
Participants
Thirty-four patients [25 male, nine female, 6–17 years old, mean age 10·2 years, standard deviation (s.d.) 3·4] were enrolled into the study (Table 1). Criteria for inclusion were as follows: signed informed consent, participants ≥ 6 and < 18 years of age who gave a clinical history of troublesome symptoms of seasonal allergic rhinoconjunctivitis (SAR) and/or allergic asthma grades I–II according to the Global Initiative for Asthma (GINA) guidelines [40] during the grass pollen season, raised serum allergen-specific IgE [radioallergosorbent test (RAST) ≥ II, ≥ 0·7 kU/ml] to grass pollen and no significantly abnormal findings on physical examination.
Table 1.
Patient characteristics.
| Gender (male : female) | 25 : 9 |
| Age (years) | 10·2(5–17) |
| Lung function | |
| Vital capacity | 95·4(70–147) |
| Forced expiratory volume(mean %) | 102(79–158) |
| Exhaled nitric oxide(ppB) | 24.1(4–125) |
| Total immunoglobulin E(kU/l) | 460(49–2000) |
| Immunoglobulin E specific to grass(kU/l) | 105(1–525) |
Reasons for exclusion included the following: forced expiratory volume in 1 s (FEV1) less than 70% of the predicted value prior to enrollment, concomitant therapy with inhaled corticosteroids (ICS) of > 500 µg/day of budesonide or beclomethasone or 250 µg/day of fluticasone for at least 3 months, and the use of systemic steroids. Further patients with serious underlying conditions were excluded from the study.
Human guidelines of good clinical practice, the German Drug Act and the declarations of Helsinki (1964) and Edinburgh (2000) were followed in the conduct of the trial. The study was approved by the local ethics committee. All parents and all patients older than 14 years of age provided written informed consent.
Study design
All patients received a standardized allergy vaccine containing l-tyrosine and the adjuvant monophosphoryl Lipid A (MPL®), to which a mixture of pollen allergoids from grasses were adsorbed (Pollinex® Quattro; Bencard Allergie, Munich, Germany). The treatment cycles were conducted off-season between September and March. Included patients received four injections subcutaneously prior to the pollen season comprising a total of 5100 standardized grass pollen units [visit 1: 300 standardized units (SU); visit 2: 800 SU; visit 3: 2000 SU; visit 4: 2000 SU] per treatment cycle. The injections were administered on a weekly basis.
Prior to the first injection (week 1), directly after the fourth injection (week 4) and – in a subset of patients – four weeks later (week 8), serum levels of specific IgE to grasses (IgEgrass), specific IgG to grasses (IgGgrass) and specific IgG4 to grasses (IgG4grass) were determined as described below. Tregs were analysed by flow cytometry. In a subset of patients we analysed further the induction of blocking antibodies.
This open trial encompassed 34 patients receiving a total of 44 treatment cycles (consisting of four injections each) over a time-period of 2 years. Ten patients were followed over two treatment cycles (first and second years of treatment), 10 over the first year of treatment only, and 14 over the second year of treatment only (the first treatment cycle was given before the patients were enrolled into this study). In total we were able to analyse 20 treatment cycles of the first year and 24 of the second year of treatment (Fig. 1.)
Fig. 1.
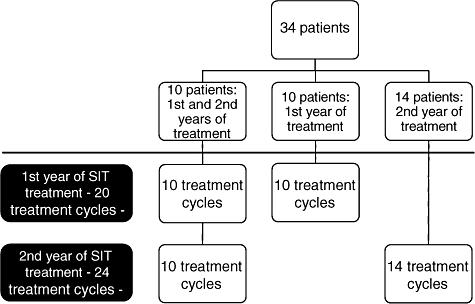
Flowchart of the patients in the conduct of this study.
Clinical procedures
In order to characterize our subjects, at visit 1 a skin prick test was performed using biological standardized allergens including Dermatophagoides pteronyssinus and farinae, cat, dog, mould mix, grass- and tree-pollen-mix (Allergopharma, Reinbek, Germany). Pulmonary function tests were performed with the MasterScreen® bodyplethysmograph by VIASYS Healthcare GmbH (Höchberg, Germany).
During the 4 weeks of therapy patients underwent weekly visits for updosing and maintenance therapy. All injections were administered in the upper arm, and patients were observed for half an hour after each injection.
Specific antibodies
Serum was collected at weeks 1, 4 and 8 and stored at −80°C until testing. Samples were analysed for specific antibodies against Phleum pratense L. (IgEgrass, IgGgrass) using a two-sided chemiluminescent assay (Immulite DPC, Bad Nauheim, Germany). Specific IgG4grass was detected by enzyme-linked immunosorbent assay (ELISA) technique (DST, Schwerin, Germany). Analysis was performed according to the manufacturer's instructions.
Blocking antibodies
Blocking antibodies were analysed in a subset of nine patients. Blood samples were taken before SIT and after the fourth injection in the first year and before SIT, after the fourth injection, and 4 weeks after the fourth injection in the second year of treatment. Cellular antigen stimulation test (CAST; Buehlmann Laboratories, Allschwill, Switzerland) was used to analyse inhibitory properties of serum antibodies. Briefly, leucocytes were isolated by dextrane sedimentation from ethylenediamine tetraacetic acid (EDTA) blood. Simultaneously, patient's serum was incubated with grass extract (2 ng/ml) for 1 h at room temperature. This mixture of patient's serum and grass extract was then used for cellular stimulation for 40 min. Supernatants were decanted and stored at −80°C. Ionomycin and anti-IgE-receptor antibody solutions were used as positive controls and patient's serum without grass extract was used as negative control. Cysteinyl leucotrienes (cysLT) were determined by ELISA technique according to the manufacturer's instructions.
Detection of Tregs
Peripheral blood mononuclear cell (PBMCs) lymphocytes from patients were obtained by centrifugation on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). Isolated PBMCs were surface-stained with a cocktail of anti-CD4-fluorescein isothiocyanate (FITC) (OKT-4) and anti-CD25-phycoerythrin (PE) (BC96) monoclonal antibodies. After fixation and permeabilization, the cells were blocked with 2% normal rat serum and intracellular staining was performed using anti-human FoxP3 allophycocyanin (APC) (PCH101) antibody (Natutec, Frankfurt am Main, Germany). On each sample, 10 000 events were analysed by a flow cytometer (Becton Dickinson, San Jose, CA, USA). Data analysis was performed with CellQuest software (Becton Dickinson, San Jose, CA, USA).
Recording of side effects
Participants were given diary cards and were asked to record side effects directly after administration and each of the following 4 days after administration of specific immunotherapy. We asked specifically for local swelling and pain, asthma symptoms, urticaria and other signs of anaphylaxis. Of the 176 injections, the diary data of 167 participants are available. The participants recorded their local and systemic side effects as being present (1) or not (2). The symptoms rated were local redness, diameter of swelling, local pain, urticaria, asthma, tissue irritation and symptoms of anaphylaxis. The results of each visit (visit 1 equals first injection) are shown in Table 2. In case of allergic symptoms, subjects had free access to relief medication (dimetindenmaleate locally, oral cetirizine, inhaled formoterol, oral prednisone and subcutaneous adrenaline) in a stepwise fashion, depending on the persistence and severity of the symptoms.
Table 2.
Symptom scores following subcutaneous administration of specific immunotherapy.
| Diameter of swelling |
||||
|---|---|---|---|---|
| Redness | < 5 cm | > 5 cm | Local pain | |
| Visit 1: Injections with symptoms | 15 | 17 | 8 | 11 |
| % positive | 35 | 40 | 19 | 26 |
| Visit 2: Injections with symptoms | 13 | 15 | 10 | 18 |
| % positive | 31 | 36 | 24 | 43 |
| Visit 3: Injections with symptoms | 11 | 14 | 9 | 11 |
| % positive | 27 | 34 | 22 | 27 |
| Visit 4: Injections with symptoms | 5 | 10 | 4 | 10 |
| % positive | 12 | 24 | 10 | 24 |
| % positive (all injections) | 26·3 | 33·5 | 18·5 | 30·0 |
Statistical analysis
Data were analysed using the statistical software SPSS version 11·0 (SPSS Inc., Chicago, IL, USA). Within-group comparison was performed using a two-sided Wilcoxon matched-pairs test, and differences between baseline and other time-points were assessed by the Friedmann non-parametric repeated-measures test. Comparisons between groups were performed using a Mann–Whitney U-test. Induction of blocking antibodies was calculated by unpaired Student's t-test. Values of P < 0·05 were considered statistically significant.
Results
Specific IgE
In total, 44 therapy courses with patients allergic to grass pollen were monitored. The median IgEgrass was 33·4 (range 1–310, n = 20) before the start of the therapy; immediately after the SCIT (after the fourth injection) the median was 48·3 IU/ml (range 1–493, n = 20; P < 0·0001). Four weeks later the median was 14·8 (range 1–96·7, n = 7). This change was not statistically significant (Fig. 2).
Fig. 2.
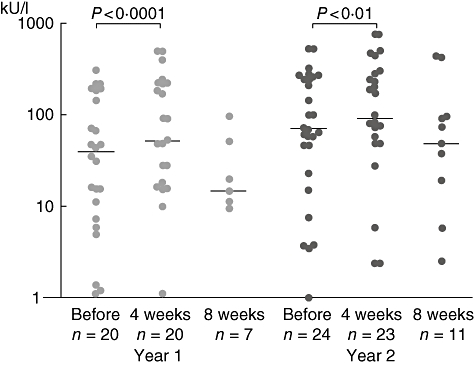
Levels of specific immunoglobulin (Ig)E to grass pollen before and after specific immunotherapy.
Before the second year of treatment the median of specific IgEgrass was 85·05 kU/l (1–525, n = 20) and 100 (0·9–493; n = 20, P < 0·001) after the fourth injection. Four weeks later the median was 48·4 (n = 7; 1–436). This again did not reach statistical significance.
Specific IgG
The median of specific IgGgrass was 9 KU/l (2·9–31, n = 20) before SIT and 11 (n = 19; 2·8–47; P < 0·05) after the fourth injection. Four weeks later the median was 9·7 [n = 7; 2·8–23·8; not significant (n.s.)]. Before the second year of treatment the median of specific IgGgrass was 13 KU/l (n = 24; 2·8–23) and 33·5 (n = 24; 3·8–101; P < 0·001) immediately after the fourth injection. Four weeks later the median was 39·5 (n = 11; 6–86·7; P < 0·05).
The medians 4 weeks after immunotherapy between years 1 and 2 differed significantly (cycles 1 and 2: P < 0·001; Fig. 3).
Fig. 3.
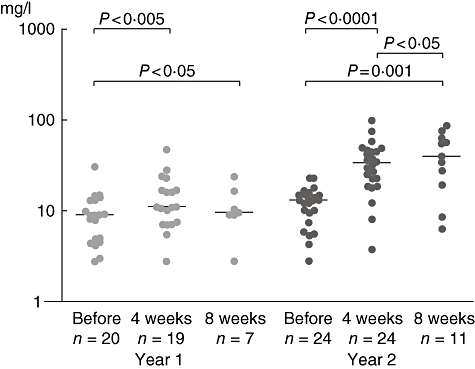
Levels of specific immunoglobulin (Ig)G to grass pollen before and after specific immunotherapy.
Specific IgG4
The median of specific IgG4grass was 0·4 U/ml (n = 20; 0·4–0·4) before SIT and 0·4 (n = 20; 0·4–93·4; n.s.) after the fourth injection. Four weeks later the median was 0·4 (n = 7, 0·4–197; n.s.).
Before the second year of treatment the median of specific IgG4grass was 2·1 U/ml (n = 24, 0·4–59·7) before the SIT and 48·8 (n = 24, 0·4–1362; P < 0·001) immediately after the fourth injection. Four weeks later the median was 23·8 (n = 11, 0·4–705; P < 0·05). These changes reached statistical significance (P < 0·001, Fig. 4).
Fig. 4.
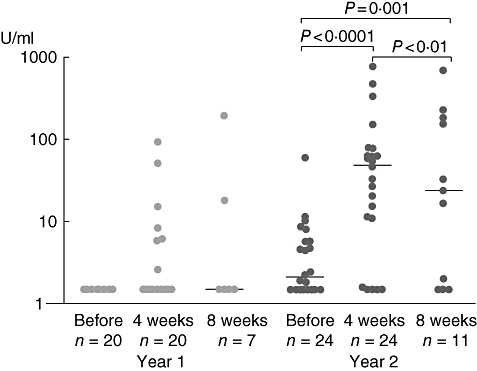
Levels of specific immunoglobulin (Ig)G4 to grass pollen before and after specific immunotherapy.
The antibody levels (IgEgrass, IgGgrass, and IgG4grass) of those patients who had been observed for 2 years demonstrated a similar course to the overall values.
Tregs
Tregs were detected by the simultaneous expression of CD4, CD25 and FoxP3, comparing the percentage of cells before and 24 h after the fourth injection in the first and second years (Fig. 5). The percentage of Tregs raised significantly after the fourth injection in the second year (before: 2·1%, 1·27–4; 24 h after: 3·6%, 2·0–4·5; P < 0·005), but not in the first year of treatment (before: 2·1%, 0·5–3·0; 24 h after: 2·7%, 0·3–4·63).
Fig. 5.
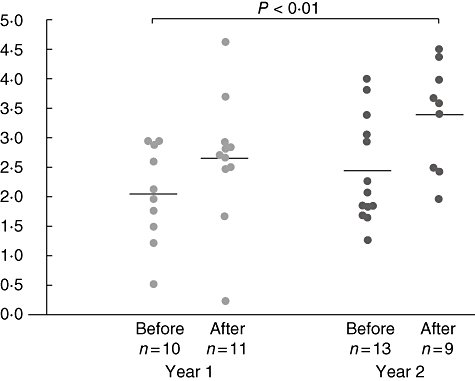
Percentage of CD4+CD25+forkhead box P3+ regulatory T cells of all T cells before and after specific immunotherapy.
Induction of blocking antibodies
In a subset of nine patients, we looked further for blocking activity of the specific antibodies by analysing the levels of cysLT before and 4 weeks after treatment in the first and second years (Fig. 6). We found a continuous decrease in cysLT release during the whole treatment period, which became significant after 4 weeks after the last injection in the second year compared to the time before SIT (before: 1383 pg/ml, ± 121; last injection: 1198 pg/ml, ± 133; P < 0·05).
Fig. 6.
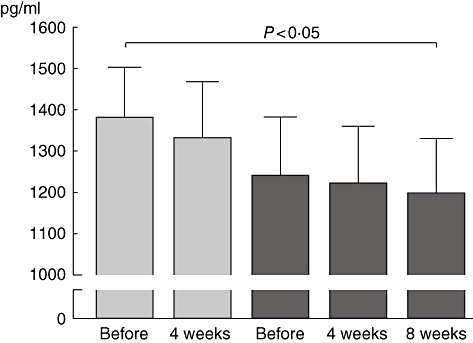
Levels of leukotrienes in supernatant as a surrogate marker for the induction of blocking antibodies.
Local and systemic side effects following subcutaneous specific immunotherapy
Participants showed local redness, swelling and pain in approximately 30% of all injections. These side effects were transient and did not require any systemic treatment. However, two patients experienced a local urticarial rash. One patient suffered from a mild form of anaphylaxis after the first injection with mild symptoms of asthma and urticaria. After administration of prednisone orally the symptoms resolved within 30 min. Further injections showed only local side effects.
Overall, we were not able to show any correlation between antibody induction and local or systemic side effects following injection (data not shown).
Discussion
Allergen-specific immunotherapy (SIT) leads to significant clinical improvement of allergic rhinitis, conjunctivitis and asthma in affected patients [41]. These effects last for at least 10 years after discontinuation of therapy in conventional SIT [42]. The immunological mechanisms underlying these effects are being clarified continuously. A rise in specific IgG and IgG4 antibodies, a reduction in tissue numbers and/or decrease in mediator release of eosinophils, basophils and mast cells are involved similarly in successful SIT as a switch from a Th2 to a more Th1 allergen-specific immune response with the induction of Tregs[5].
SIT has the disadvantage that its efficacy only builds up with time. In the data published so far there is controversy about when and to what extent the clinical effects can be expected [43–46]. Especially in the first year of treatment, the clinical effects are limited. In a study by Rolinck-Werninghaus et al. [47], treatment with SIT alone before the first grass pollen season was unable to reduce either symptom score or the use of rescue medication significantly in children when compared with the reference group of SIT with an irrelevant allergen.
Consistent with the weak clinical effects after the first treatment cycle, we only found a modest increase of specific IgG and IgG4 antibodies in this study.
However, we could demonstrate a pronounced increase in specific IgG and IgG4 antibodies after the second course of SIT without a persistent rise of total IgE levels, as reported in successful conventional SIT [48]. Interestingly, the antibody levels rose quickly and were already detectable after 3 weeks of therapy. The fast and profound rise in the second and third years of treatment suggests immunological priming. These IgG antibodies were described first as blocking antibodies in 1935 [49]. In particular, IgG4 induced by means of immunotherapy blocks allergen-induced IgE-dependent histamine release by basophils and suppresses allergen-specific T cell responses in vitro by inhibiting binding of IgE–allergen complexes to antigen-presenting cells [24].
There are contradictory reports on the role of IgG on the efficacy of the immunotherapy [50,51]. The issue of whether there is a cause-and-effect relationship between the increase in IgG levels and the symptomatic improvement conferred by immunotherapy, or if this is just a surrogate marker of exposure to the SIT, remains unresolved. However, higher IgG concentrations are associated independently with symptomatic improvement in many studies, implying a linkage between immunological and clinical changes [48,52].
As there is a lack of correlation between IgG4 antibody levels and the degree of clinical improvement it is suggested rather to measure the blocking activity of allergen-specific IgG than the crude levels in sera [53]. In our work we were able to show a significant increase of blocking activity in the serum measured by reduced leukotriene release in treated patients.
Another attractive hypothesis for the immunological basis of specific immunotherapy is its effect on Tregs[54]. Tregs are able to inhibit the development of allergic Th2 responses and play a major role in allergen SIT [55].
IL-10 derived from Tregs generates tolerance in T cells, regulates specific isotype formation and skews the specific response from an IgE- to an IgG4-dominated phenotype. Description of Treg goes back to Groux et al. in 1997 [56]. They are critical players in peripheral immune tolerance [57]. Further, they have the ability to suppress allergen-specific T cell responses and to regulate allergen-specific IgG4 and IgE synthesis [27,58]. Normally, 5–10% of peripheral T lymphocytes are Tregs[59]; our study revealed a diminished basal frequency of Tregs of 2–3·6%. Previous studies have shown that the capacity of CD4+, CD25+ and FoxP3+ T cells from allergic individuals are reduced or even absent [37,60]. Hartl et al. found a significant increase in CD25– high CD4+ T cells in peripheral blood and in bronchoalveolar lavage fluids after corticoid therapy [61]. In our work a significant increase in the proportion of CD4+, CD25+ and FoxP3+ cells was seen after the second treatment cycle compared to baseline. This finding is in keeping with a study by Pereira-Santos et al., who showed a significant increase in FoxP3+ T cells 6 and 12 months after venom immunotherapy in adolescents and adults (mean age 47, range 16–65) [62].
Due to the relevant allergen challenge in SIT, IgE levels rise frequently at the beginning. This effect is transient, and is followed by a gradual decrease in the course of the therapy [63]. These changes are also found in our study with short-term immunotherapy. The IgE levels increase slightly during the therapy and decrease 4 weeks after discontinuation in the first and second years of treatment.
The success of SIT led to extensive research to improve efficacy and convenience for the patients. Ultra short-term immunotherapy (uSCIT) demonstrates some shortcomings in convenience, as one-third of our patients suffered from local side effects. In comparison to other types of SIT, this increase of side effects could be caused by MPL®. Other immunomodulators, such as cytosine-guanine dinucleotide (CpG) motifs, are in development, while new targets, such as the Notch protein/receptor interaction, may eventually give further improvement [64]. The excellent safety profile of the sublingual route of administration of allergy vaccines could lead to the wider use of SIT, and locally active immunomodulators could make SIT a therapy of choice for many more patients than at present. Besides clinical outcomes, i.e. bronchial hyperreactivity, or symptom scores, IgG- and IgG4 antibodies could similarly be helpful tools to evaluate efficacy of SIT. The same applies to the amount of Tregs and the induction of blocking antibodies. The long-term efficacy of new therapy regimens has to be elucidated in further studies.
Conclusion
Our study demonstrates immunological changes after short-term immunotherapy in childhood and adolescence. In the first year of treatment we found only moderate evidence for immunological tolerance. A significant induction of blocking antibodies was seen only after the second course of SIT. Short-course immunotherapy with pollen allergoids formulated with the Th1-inducing adjuvant MPL needs at least two courses to establish tolerance in allergic patients.
Disclosure
Stefan Zielen has disclosed receiving a research grant from Allergy Therapeutics and MSD; he also received fees for speaking and consulting for Novartis, Allergy Therapeutics, Symbio Vaccines, HAL, MSD, GSK and Wyeth. All the other authors have disclosed that they have no relevant financial interests.
References
- 1.Pearce N, Aït-Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2007;62:758–66. doi: 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finegold I. Allergen immunotherapy: present and future. Allergy Asthma Proc. 28:44–9. doi: 10.2500/aap.2007.28.2971. [DOI] [PubMed] [Google Scholar]
- 3.Passalacqua G, Durham SR. Allergic rhinitis and its impact on asthma update: allergen immunotherapy. J Allergy Clin Immunol. 2007;119:881–91. doi: 10.1016/j.jaci.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet J, Lockey R, Malling HJ, et al. Allergen immunotherapy: therapeutic vaccines for allergic diseases. World Health Organization, American Academy of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1998;81:401–5. doi: 10.1016/s1081-1206(10)63136-5. [DOI] [PubMed] [Google Scholar]
- 5.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119:780–91. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 7.van Ree R, Chapman MD, Ferreira F, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008;63:310–26. doi: 10.1111/j.1398-9995.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- 8.Baldrick P, Richardson D, Woroniecki SR, et al. Pollinex Quattro ragweed: safety evaluation of a new allergy vaccine adjuvanted with monophosphoryl lipid A (MPL) for the treatment of ragweed pollen allergy. J Appl Toxicol. 27:399–409. doi: 10.1002/jat.1223. [DOI] [PubMed] [Google Scholar]
- 9.Baldrick P, Richardson D, Elliott G, et al. Safety evaluation of monophosphoryl lipid A (MPL): an immunostimulatory adjuvant. Regul Toxicol Pharmacol. 2002;35:398–413. doi: 10.1006/rtph.2002.1541. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler AW, Woroniecki SR. Modern approaches to therapeutic vaccination as treatment for type 1 respiratory hypersensitivity (allergy) treatment. Exp Rev Vaccines. 2006;5:27–31. doi: 10.1586/14760584.5.1.27. [DOI] [PubMed] [Google Scholar]
- 11.Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103–7. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- 12.Alving CR. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology. 1993;187:430–46. doi: 10.1016/S0171-2985(11)80355-4. [DOI] [PubMed] [Google Scholar]
- 13.Thompson HS, Davies ML, Watts MJ, et al. Enhanced immunogenicity of a recombinant genital warts vaccine adjuvanted with monophosphoryl lipid A. Vaccine. 1998;16:1993–9. doi: 10.1016/s0264-410x(98)00088-7. [DOI] [PubMed] [Google Scholar]
- 14.Moore A, McCarthy L, Mills KH. The adjuvant combination monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1. Vaccine. 1999;17:2517–27. doi: 10.1016/s0264-410x(99)00062-6. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki S, Tsuji T, Hamajima K, et al. Monophosphoryl lipid A enhances both humoral and cell-mediated immune responses to DNA vaccination against human immunodeficiency virus type 1. Infect Immun. 1997;65:3520–8. doi: 10.1128/iai.65.9.3520-3528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drachenberg KJ, Wheeler AW, Stuebner P, et al. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498–505. doi: 10.1034/j.1398-9995.2001.056006498.x. [DOI] [PubMed] [Google Scholar]
- 17.Durham SR, Till SJ. Immunologic changes associated with allergen immunotherapy. J Allergy Clin Immunol. 1998;102:157–64. doi: 10.1016/s0091-6749(98)70079-x. [DOI] [PubMed] [Google Scholar]
- 18.Akdis CA, Blaser K. Mechanisms of allergen-specific immunotherapy. Allergy. 2000;55:522–30. doi: 10.1034/j.1398-9995.2000.00120.x. [DOI] [PubMed] [Google Scholar]
- 19.Aalberse RC, van der Gaag R, van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–6. [PubMed] [Google Scholar]
- 20.Lambin P, Bouzoumou A, Murrieta M, et al. Purification of human IgG4 subclass with allergen-specific blocking activity. J Immunol Methods. 1993;165:99–111. doi: 10.1016/0022-1759(93)90111-j. [DOI] [PubMed] [Google Scholar]
- 21.Moingeon P, Batard T, Fadel R, et al. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006;61:151–65. doi: 10.1111/j.1398-9995.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 22.Rossi RE, Monasterolo G, Coco G, et al. Evaluation of serum IgG4 antibodies specific to grass pollen allergen components in the follow up of allergic patients undergoing subcutaneous and sublingual immunotherapy. Vaccine. 2007;25:957–64. doi: 10.1016/j.vaccine.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 23.García BE, Sanz ML, Gato JJ, et al. IgG4 blocking effect on the release of antigen-specific histamine. J Invest Allergol Clin Immunol. 3:26–33. [PubMed] [Google Scholar]
- 24.van Neerven RJ, Wikborg T, Lund G, et al. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–52. [PubMed] [Google Scholar]
- 25.Till SJ, Francis JN, Nouri-Aria K, et al. Mechanisms of immunotherapy. J Allergy Clin Immunol. 2004;113:1025–34. doi: 10.1016/j.jaci.2004.03.024. quiz 1035. [DOI] [PubMed] [Google Scholar]
- 26.Visco V, Dolecek C, Denépoux S, et al. Human IgG monoclonal antibodies that modulate the binding of specific IgE to birch pollen Bet v 1. J Immunol. 1996;157:956–62. [PubMed] [Google Scholar]
- 27.Akdis CA, Blesken T, Akdis M, et al. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jutel M, Akdis M, Budak F, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–14. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 29.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–5. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Robinson DS, Larché M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004;114:1389–97. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akdis CA, Blaser K, Akdis M. Genes of tolerance. Allergy. 2004;59:897–913. doi: 10.1111/j.1398-9995.2004.00587.x. [DOI] [PubMed] [Google Scholar]
- 32.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 33.Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 34.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–5. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 35.Read S, Powrie F. CD4(+) regulatory T cells. Curr Opin Immunol. 2001;13:644–9. doi: 10.1016/s0952-7915(01)00273-4. [DOI] [PubMed] [Google Scholar]
- 36.Grindebacke H, Wing K, Andersson A, et al. Defective suppression of Th2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season. Clin Exp Allergy. 2004;34:1364–72. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 37.Ling EM, Smith T, Nguyen XD, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–15. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 38.Radulovic S, Jacobson MR, Durham SR, et al. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121:1467–72. doi: 10.1016/j.jaci.2008.03.013. 1472.e1. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. J Exp Med. 2004;199:1679–88. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–78. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 41.Möller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–6. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 42.Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 43.Varney VA, Gaga M, Frew AJ, et al. Usefulness of immunotherapy in patients with severe summer hay fever uncontrolled by antiallergic drugs. Br Med J. 1991;302:265–9. doi: 10.1136/bmj.302.6771.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grammer LC, Shaughnessy MA, Suszko IM, et al. A double-blind histamine placebo-controlled trial of polymerized whole grass for immunotherapy of grass allergy. J Allergy Clin Immunol. 1983;72:448–53. doi: 10.1016/0091-6749(83)90580-8. [DOI] [PubMed] [Google Scholar]
- 45.Zenner HP, Baumgarten C, Rasp G, et al. Short-term immunotherapy: a prospective, randomized, double-blind, placebo-controlled multicenter study of molecular standardized grass and rye allergens in patients with grass pollen-induced allergic rhinitis. J Allergy Clin Immunol. 1997;100:23–9. doi: 10.1016/s0091-6749(97)70190-8. [DOI] [PubMed] [Google Scholar]
- 46.Walker SM, Varney VA, Gaga M, et al. Grass pollen immunotherapy: efficacy and safety during a 4-year follow-up study. Allergy. 1995;50:405–13. doi: 10.1111/j.1398-9995.1995.tb01170.x. [DOI] [PubMed] [Google Scholar]
- 47.Rolinck-Werninghaus C, Hamelmann E, Keil T, et al. The co-seasonal application of anti-IgE after preseasonal specific immunotherapy decreases ocular and nasal symptom scores and rescue medication use in grass pollen allergic children. Allergy. 2004;59:973–9. doi: 10.1111/j.1398-9995.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 48.Tabar AI, Echechipía S, García BE, et al. Double-blind comparative study of cluster and conventional immunotherapy schedules with Dermatophagoides pteronyssinus. J Allergy Clin Immunol. 2005;116:109–18. doi: 10.1016/j.jaci.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Cooke RA, Barnard JH, Hebald S, et al. Serological evidence of immunity with coexisting sensitization in a type of human allergy (hay fever) J Exp Med. 1935;62:733–51. doi: 10.1084/jem.62.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wachholz PA, Soni NK, Till SJ, et al. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112:915–22. doi: 10.1016/s0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- 51.Bousquet J, Maasch H, Martinot B, et al. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. II. Comparison between parameters assessing the efficacy of immunotherapy. J Allergy Clin Immunol. 1988;82:439–46. doi: 10.1016/0091-6749(88)90017-6. [DOI] [PubMed] [Google Scholar]
- 52.Nouri-Aria KT, Wachholz PA, Francis JN, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–9. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 53.Jutel M, Akdis M, Blaser K, et al. Mechanisms of allergen specific immunotherapy – T-cell tolerance and more. Allergy. 2006;61:796–807. doi: 10.1111/j.1398-9995.2006.01175.x. [DOI] [PubMed] [Google Scholar]
- 54.Larché M. Regulatory T cells in allergy and asthma. Chest. 2007;132:1007–14. doi: 10.1378/chest.06-2434. [DOI] [PubMed] [Google Scholar]
- 55.Marcotte GV, Braun CM, Norman PS, et al. Effects of peptide therapy on ex vivo T-cell responses. J Allergy Clin Immunol. 1998;101:506–13. doi: 10.1016/S0091-6749(98)70358-6. [DOI] [PubMed] [Google Scholar]
- 56.Groux H, O'Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 57.Bacchetta R, Passerini L, Gambineri E, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–22. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellinghausen I, Metz G, Enk AH, et al. Insect venom immunotherapy induces interleukin-10 production and a Th2-to-Th1 shift, and changes surface marker expression in venom-allergic subjects. Eur J Immunol. 1997;27:1131–9. doi: 10.1002/eji.1830270513. [DOI] [PubMed] [Google Scholar]
- 59.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 60.Cavani A, Nasorri F, Ottaviani C, et al. Human CD25+ regulatory T cells maintain immune tolerance to nickel in healthy, nonallergic individuals. J Immunol. 2003;171:5760–8. doi: 10.4049/jimmunol.171.11.5760. [DOI] [PubMed] [Google Scholar]
- 61.Hartl D, Koller B, Mehlhorn AT, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119:1258–66. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 62.Pereira-Santos MC, Baptista AP, Melo A, et al. Expansion of circulating Foxp3+)D25bright CD4+ T cells during specific venom immunotherapy. Clin Exp Allergy. 2008;38:291–7. doi: 10.1111/j.1365-2222.2007.02887.x. [DOI] [PubMed] [Google Scholar]
- 63.van Ree R, van Leeuwen WA, Dieges PH, et al. Measurement of IgE antibodies against purified grass pollen allergens (Lol p 1, 2, 3 and 5) during immunotherapy. Clin Exp Allergy. 1997;27:68–74. doi: 10.1046/j.1365-2222.1997.d01-416.x. [DOI] [PubMed] [Google Scholar]
- 64.Fellrath J, Kettner A, Dufour N, et al. Allergen-specific T-cell tolerance induction with allergen-derived long synthetic peptides: results of a phase I trial. J Allergy Clin Immunol. 2003;111:854–61. doi: 10.1067/mai.2003.1337. [DOI] [PubMed] [Google Scholar]


