Abstract
Early-life exposure to appropriate microbial flora drives expansion and development of an efficient immune system. Aberrant development results in increased likelihood of allergic disease or increased susceptibility to infection. Thus, factors affecting microbial colonization may also affect the direction of immune responses in later life. There is a need for a manipulable animal model of environmental influences on the development of microbiota and the immune system during early life. We assessed the effects of rearing under low- (farm, sow) and high-hygiene (isolator, milk formula) conditions on intestinal microbiota and immune development in neonatal piglets, because they can be removed from the mother in the first 24 h for rearing under controlled conditions and, due to placental structure, neither antibody nor antigen is transferred in utero. Microbiota in both groups was similar between 2 and 5 days. However, by 12–28 days, piglets reared on the mother had more diverse flora than siblings reared in isolators. Dendritic cells accumulated in the intestinal mucosa in both groups, but more rapidly in isolator piglets. Importantly, the minority of 2–5-day-old farm piglets whose microbiota resembled that of an older (12–28-day-old) pig also accumulated dendritic cells earlier than the other farm-reared piglets. Consistent with dendritic cell control of T cell function, the effects on T cells occurred at later time-points, and mucosal T cells from high-hygiene, isolator pigs made less interleukin (IL)-4 while systemic T cells made more IL-2. Neonatal piglets may be a valuable model for studies of the effects of interaction between microbiota and immune development on allergy.
Keywords: intestinal flora, immune development, neonate, pig
Introduction
Increasing evidence suggests that early-life exposure to microbial flora drives expansion of the immune system, but that development of an efficient immune system depends, at least partially, upon diversity and type of intestinal microbiota [1]. In concordance with this, direct effects of gut flora on immunological development have been demonstrated in humans and in animal models [2–4]. Other variations in early-life care, such as formula feeding and the use of probiotics or antibiotics, have also been shown to modulate the risk of allergic disease [2,5]. Thus, the microbiological milieu into which a neonate is born influences immunological development strongly, and therefore subsequent ability to make appropriate responses. In turn, the process and nature of the microbial colonization itself is affected by environmental factors such as hygiene status and nutrition (e.g. bottle feeding versus breast feeding) [6].
In the intestine, the balance between active immune responses to pathogens and regulation of responses to harmless antigen is particularly important. In addition, studies have demonstrated that immune responses initiated in the intestine may influence the direction of responses outside the gut [7,8].
There is an increasing perception that the pig presents a number of advantages as a pre-clinical, translational model for humans [9–12]. Like humans, the pig is a large, monogastric omnivore with highly comparable intestinal anatomy, physiology and nutritional requirements [13,14]. Additionally, pigs are ideal models for studies of the impact of environmental factors on immunological development: it is possible to remove piglets from the mother in very early life and to rear them in under closely controlled environmental conditions during this time. They are also born immunologically naive: due to placental structure, neither antibody nor antigen is transferred in utero.
Given the evidence for the influence of early-life microbial colonisation on immunological development, we hypothesized that rearing piglets under different conditions – either low-hygiene (allowed to suckle from the sow) or high-hygiene (formula-fed) – would affect the functional development of mucosal immunity. Therefore, we have examined the impact of alternative rearing conditions during the first month of life on intestinal microbiota, antigen-presenting cell (APC) phenotype and T cell function in intestines from neonatal piglets reared under- low and high-hygiene conditions. Further, we have studied the effects of these different rearing conditions on T cell function in the mesenteric lymph nodes and spleen. Our results show differences in the intestinal flora and APC phenotype and, perhaps most importantly, both intestinal and peripheral T cell functions between these two groups of pigs over time. These results demonstrate not only clear differences in immunological development in neonates reared under different environmental conditions; they also indicate that the neonatal pig would be a valuable pre-clinical model for evaluating the effects of early-life environment on immunological development in human infants.
Methods
Animals
Litters of > 10 commercial, outbred healthy piglets were selected from a conventional pig farm. Half of each litter was allocated randomly to remain on the sow without access to creep feed, while the other half was taken to our isolator facilities (see below) at 24 h old, after an initial intake of colostrum. Within each treatment group, newborn piglets were allocated randomly to an age group for future sample collection. Piglets weighing less than 1 kg at birth were excluded from the study and neither group of animals was vaccinated or received antibiotics at any point during the study. All animals were maintained according to institutional guidelines.
Experimental design
At 2, 5, 12, 20 and 28 days of age, samples of spleen (SPL), mesenteric lymph node (MLN), jejunum, ileum and colon were removed under terminal anaesthesia and piglets were killed using intravenous pentobarbitone. This experiment was repeated three times with each replicate a month apart, such that each data point represents a minimum of four and a maximum of six litter-matched animals.
Housing and feeding
The low-hygiene groups were housed under conventional farm conditions throughout the experiment. The piglets suckled from the sow and had no access to solid feed.
At 24 h old, piglets in the high-hygiene group were introduced into our isolator facilities [a specific pathogen-free, positive-pressure unit supplied with a high-efficiency particulate air (HEPA) filter] through a dunk tank containing 1% w/v bactericidal and virucidal disinfectant solution (Virkon; Antec International Ltd, Sudbury, UK). The piglets were housed in individual cages with bedding and a heat lamp and fed a commercial, bovine milk-formula (Piggimilk; Parnutt Feeds, Sleaford, UK).
Analysis of intestinal microbiota
In order to analyse the development and stability of the bacterial community in the intestine, we used the well-established denaturing gradient gel electrophoresis (DGGE) method. This utilizes the sequence diversity among different bacterial 16S rRNA genes to obtain a profile from the total bacterial community rather than focusing upon specific bacterial groups [15–17].
Sample collection and DNA isolation
Luminal samples of gut content from the ileum and the colon were collected and genomic DNA was extracted using the Fast DNA Spin Kit (Qbiogene Inc., Carlsbad, CA, USA), as described previously [16].
Polymerase chain reaction (PCR) amplification
PCR primers S-D-Bact-0968-a-S-GC and S-D-Bact-1401-a-A-17 [18] were used to amplify the V6–V8 region of the 16S rRNA gene with the Taq DNA polymerase kit from Life Technologies (Gaithersburg, MD, USA). The samples were amplified in a T1 Whatman Biometra thermocycler (Göttingen, Germany) for 35 cycles consisting of 94°C for 5 min, and 35 cycles of 94°C for 30 s, 56°C for 20 s, 68°C for 40 s and 68°C for a 7-min final extension. Aliquots of 5 µl were analysed by agarose gel electrophoresis.
DGGE analysis
Amplicons were separated by DGGE. All gels were scanned at 400 dots per inch (dpi) and analysed using the Bionumerics software package version 3·0 (Applied Maths, Kortrijk, Belgium). After normalization, bands were identified for each sample using the program algorithm followed by a manual check to identify bands constituting less than 1% of the total area, which were omitted from further analysis. Clustering was carried out using the Dice similarity coefficient and the unweighted pair-group method with arithmetic mean (upgma) [15]. The stability of bacterial communities was evaluated by a moving window correlation of the samples from the different time-points [17].
Immunohistology
Sample collection
Jejunal samples were mounted in Tissue-Tek OCT (Thermo Fisher Scientific, Waltham, MA, USA), snap-frozen and sectioned, as described previously [19].
Tissue staining
Five-µm sections of tissues were fixed in acetone, and then stained and mounted along with appropriate negative controls, as described previously [19]. The following antibodies were used: porcine major histocompatibility complex class II D-related (MHCII DR) [clone muscle-specific actin-3 (MSA-3; produced in house)][20], CD16 (clone G7; Serotec, Kidlington, UK) [21] and capillary endothelium (clone MIL11; produced in house) [22]. Binding was detected with the following isotype specific anti-mouse anti-sera: goat anti-mouse immunoglobulin G2a (IgG2a) Alexa Fluor 633 (Invitrogen, Paisley, UK), goat anti-mouse IgG1 FITC (Cambridge Bioscience, Cambridge, UK) and biotinylated rat anti-mouse IgE (Cambridge Bioscience) detected with aminomethylcoumarin (AMCA)-avidin D (Vector Laboratories, Peterborough, UK). Non-specific binding was prevented by the use of 5% pig serum, 5% goat serum and 10% rat serum in phosphate-buffered saline (PBS).
Image capture
Images of jejunal lamina propria (LP) were captured using a Leica DMR-A fluorescence microscope fitted with appropriate single-colour filters. Greyscale images were acquired using a Hamamatsu Orca-ER camera (Hamamatsu Photonics UK Ltd, Welwyn Garden City, UK) and Q-fluoro software (Leica Microsystems, Wetzlar, Germany).
Image analysis
Images were analysed as described previously [19] using ImageJ version 1·39u [23]. Briefly, levels of background staining (threshold levels) in all colour channels were obtained from negative control slides using the ImageJ macro multiplecolourbackgrounds[19]. These were then applied to the positively stained images so that all pixels above threshold were set to their appropriate colour and all those below were set to black. We have optimized this technique previously to minimize variation between samples [19]. Thresholds were determined for each sample using negative control slides prepared in conjunction with each stained slide. Areas of positive staining were calculated using the ImageJ macro multiplecolouranalysis[19].
Functional analysis of T cells
Cell isolation
Leucocytes for in vitro culture were isolated from MLN and jejunal LP, as described previously [24]. LP cells were pooled from 25%, 50% and 75% along the length of the jejunum. Care was taken to avoid all contamination with Peyer's patch cells by visual inspection. Spleen cells were isolated by tissue disruption followed by hypotonic lysis (Trizma-buffered ammonium chloride) of erythrocytes.
Cytokine production
Isolated cells were washed and resuspended in RPMI-1640 medium (Dutch modification, Invitrogen). Leucocytes were counted by flow cytometry by the addition of directly conjugated anti-porcine CD45-fluorescein isothiocyanate (FITC) (Serotec) and a known number of calibrated fluorescent beads (Flow-Count™ fluorospheres; Beckman Coulter, High Wycombe, UK).
For culture, cell suspensions were diluted in complete medium to a concentration of 2 × 106 CD45-positive cells per ml and cultured in 24-well plates with and without activation with 5 µg/ml concanavalin A (ConA) at 37°C, 100% relative humidity and 5% CO2. Culture supernatants were harvested after 41 h and stored at −80°C until analysis.
Cytokine assays
Concentrations of interleukin (IL)-2, IL-4, IL-10 and interferon (IFN)-γ in the cell culture supernatants were determined using enzyme-linked immunosorbent assay (ELISA) cytosets (Invitrogen) using the manufacturer's supplied protocol. Each assay was optimized before being used, and all assays for any one cytokine were performed in one batch. Multiple dilutions of sample were assayed and the concentration of each cytokine in a supernatant was determined using dilutions giving readings within the standard curve.
Statistical analysis
For the microbiological analysis, all statistical analyses were performed using the SAS general linear model (GLM) procedure using a two-way analysis of variance (anova).
Effects of age and environment on proportion positive area in the immunohistology images were analysed using SPSS statistical software (version 16·0; SPSS UK Ltd, Working, UK) using multiple regression and Tukey's post-hoc testing with proportion positive area as the dependent variable and age, treatment, gender, litter and replicate as the independent variables.
Cytokine data were analysed using a two-way anova using Stata version 9 (StataCorp, College Station, TX, USA, 2005). Significant main effects were analysed subsequently using one-way anova while significant interaction terms were characterized further using one-way anova and t-tests.
Results
Development of the bacterial community in the ileum and colon
Dice coefficients [standard deviation (s.d.); calculated as shown in Equation 1 and described in [25]] for pairwise comparison of the DGGE profiles were used to construct a dendrogram of similarity for bacterial populations in the ileum (Fig. 1). The majority of samples fell into one of three clusters (1–3). Cluster 1 contained predominantly samples from farm-reared piglets aged 12–28 days. Cluster 2 contained both farm and isolator piglets aged 2 and 5 days, while cluster 3, which was related most closely to cluster 2, contained isolator piglets 12–28 days old. Clusters 4 and 5 contained outlier samples from several sources. The results indicate a high level of similarity between the microbiota of farm and isolator piglets at 2 and 5 days, followed by diversification, which was most marked in the farm pigs. This development reflected divergences in both the position of specific bands and in the number of bands that represented the predominant microbial community.
Fig. 1.
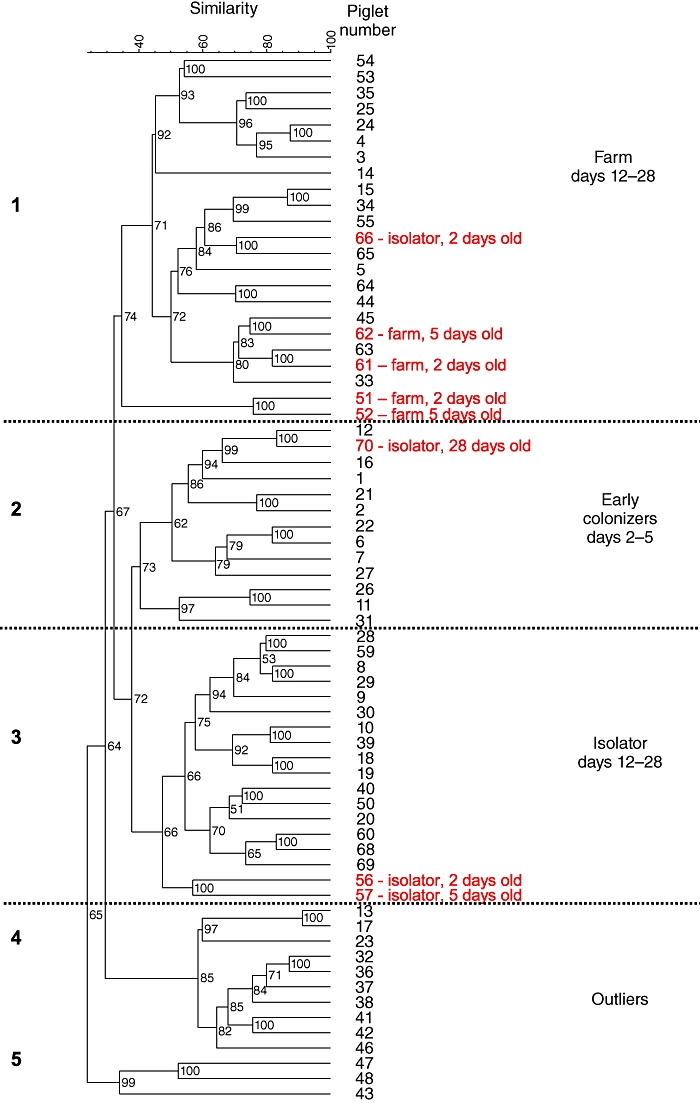
A dendrogram of the mean values of the Dice coefficients for pairwise comparison of the denaturing gradient gel electrophoresis (DGGE) profiles of the ileal flora in piglets of different ages reared under low- and high-hygiene conditions. Outlying piglets (i.e. those that are either of a different age or reared under different conditions to the majority of animals in the indicated group) are listed in red. Dashed lines indicate the borders between the five separate (numbered) clusters.
| (Equation 1) |
where nA = number of DGGE bands in line 1; nB = number of DGGE bands in lane 2; and nAB = number of common DGGE bands.
In order to examine these findings further, we assessed the stability of the microbial community over time (Fig. 2). After 5 days, the ileal flora of the farm pigs was consistently more variable, reflected in a lower similarity index over time. This observation would account for the greater divergence of the flora in the farm group from the early colonizers. Similar results were obtained from colonic samples (data not shown).
Fig. 2.
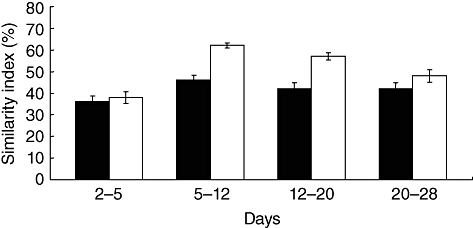
The variability with age of the microbial flora in the ileum of low- and high-hygiene piglets. Black bars: low-hygiene (farm-reared) piglets; open bars: high-hygiene (isolator-reared) piglets. Error bars represent standard error of the mean.
Taken together, these results indicate that rearing environment had a significant effect on the composition and also the stability of the microbiota in the intestine after the first 5 days of life. Given the plasticity of the neonatal immune system, we might expect these differences to impact upon its development.
APC subsets in the jejunal lamina propria
APCs and, in particular, dendritic cells (DCs) are central in detecting changes in the microenvironment and initiating appropriate immune responses. We and others have shown previously that the lamina propria of the pig intestine contains two APC populations expressing MHCII: CD16+ professional, migratory APCs (DCs) [26,27] and non-migratory, stromal APCs (MIL11+ endothelial cells) [26]. Porcine enterocytes do not express MHCII in the normal intestine [26,28]. Given the clear effects of rearing environment on intestinal flora, we were keen to examine the effects on the numbers and type of these two APC populations in the lamina propria of the small intestine (Fig. 3a).
Fig. 3.
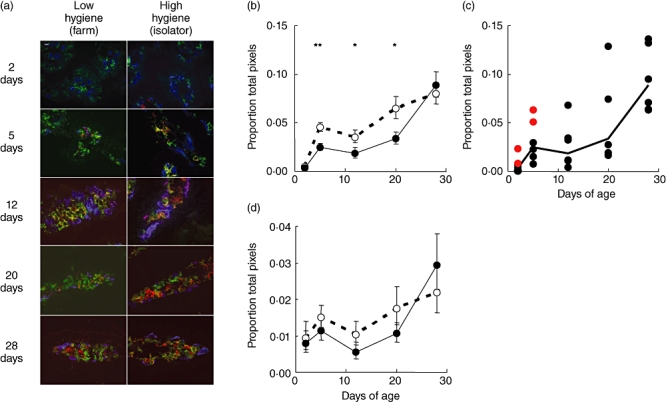
Changes in professional and non-professional antigen-presenting cells (APCs) with age in piglets reared under low- and high-hygiene conditions. (a) Images show major histocompatibility complex class II (MHCII) (red) expression by CD16+ (green) and endothelial (MIL11+; blue) cells in the small intestinal mucosa of low- and high-hygiene pigs at all time-points. Scale bar 10 µm. (b) Changes in CD16+MHCII+[dendritic cell (DC)] staining with age. (c) Changes in CD16+MHCII+ staining with age for individual farm-reared piglets. Each point represents a piglet; for comparison, horizontal bars represent the means for each time-point. The outlying piglets from the microbial flora analysis (Fig. 1) are indicated in red. (d) Changes in MIL11+MHCII+ (capillary endothelial cell) staining with age. Solid line: low-hygiene (farm-reared) piglets; dashed line: high-hygiene (isolator-reared) piglets. *P < 0·05; **P < 0·01. Error bars represent standard error of the mean.
Between the first 2 and 5 days of life, DC (CD16+MHCII+) staining increased significantly in both groups of pigs (Fig. 3b). Interestingly, however, the increase was greater in the high-hygiene group such that, by 5 days of age, there was significantly more CD16+MHCII+ staining in samples from these pigs (P < 0·01). This pattern was repeated between 12 and 20 days of age with DC staining again increasing in both groups (P < 0·05), but more in the high-hygiene piglets. Therefore, between the ages of 5 and 20 days, piglets reared in a high-hygiene environment had significantly more intestinal DC staining than piglets reared in a low-hygiene environment. However, between 20 and 28 days, DC staining increased significantly in low-hygiene pigs (P < 0·01) such that, by 28 days, CD16+MHCII+ staining were at similar levels in the two groups.
The analysis of microbiota identified samples from four 2–5-day-old farm-reared pigs (from the total of 12) that clustered with the 12–28-day-old group rather than with age-matched farm piglets. That is, they developed a flora more typical of an older (12–28-day-old pig) at an early time-point. We hypothesized that, if flora and DC numbers were linked causally, the number of DCs in the intestines of these ‘outlier’ farm pigs should be more similar to DC numbers in 12–28-day-old farm pigs than to the remaining age-matched farm-reared animals. When DC MHCII expression for the individual farm pigs was plotted this was, indeed, the case (Fig. 3c; outlier piglets in red; P = 0·01).
In contrast to the changes observed in DC, there were only marginal effects of age or rearing environment on MHCII expression by capillary endothelium (Fig. 3d). Therefore, during the first month of life, rearing environment appears to have a greater effect on the migratory, professional APC population in the intestine than on the non-migratory, stromal APC population.
Cytokine production by cells from the jejunal lamina propria
Given the effects of rearing environment on DC numbers, we hypothesized that these differences may be reflected in downstream immunological function in the intestine. To examine polarization of immunological cells in the gut, we measured cytokine production by CD45+ cells after stimulation with ConA. Extensive flow cytometric analysis demonstrated no effects of rearing environment on either the relative proportions of T cells and non-T cell leucocytes or between T cell subsets in any tissue (CD2, CD3, CD4, CD8α, CD8β, γδ, CD25, CD45RC, SwC1, SwC9; data not shown). As such, differences in cytokine levels could be attributed to differences in cellular production rather than differences in the relative proportions of leucocyte subsets. Detectable levels of cytokines were not present in supernatants from unstimulated cells. Higher levels of IL-4 were produced by stimulated cells from the farm pigs than from isolator pigs at day 20 (P < 0·01; Fig. 4a). IL-2, IL-10 and IFN-γ production were also measured, but there was no significant effect of age or rearing environment (Fig. 4b and data not shown).
Fig. 4.
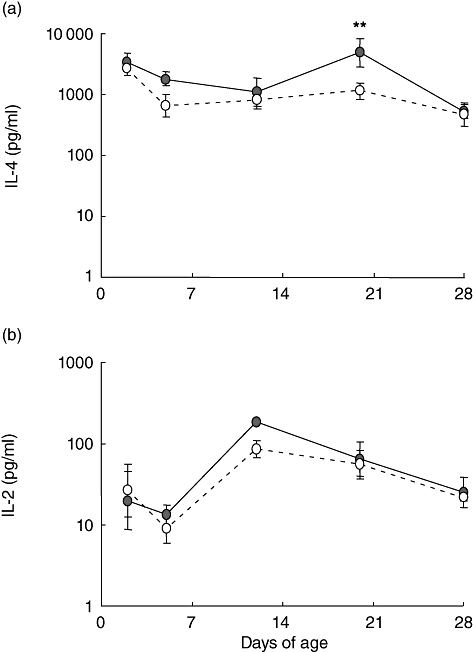
Cytokine production by whole-cell preparations from the small intestine of piglets reared under low- and high-hygiene conditions. (a) Interleukin (IL)-4 production and (b) IL-2 production in the supernatants from whole-cell preparations from the small intestine 41 h after concanavalin A (ConA) stimulation. Solid line: low-hygiene (farm-reared) piglets; dashed line: high-hygiene (isolator-reared) piglets. **P < 0·01 comparing the effect of rearing environment at each time-point.
Cytokine production by whole cell populations from the mesenteric lymph node and spleen
Epidemiological studies in human infants have identified links between environmental factors and systemic immunological development. In particular, these studies have examined the effects of environmental factors on the polarisation of systemic immune responses [29,30]. Because rearing environment had an effect on several components of the intestinal immune system, we examined the cytokine production by cells from the mesenteric lymph node and spleen at all time-points. Detectable levels of cytokines were not present in supernatants from unstimulated cells. In both tissues, rearing environment resulted in higher levels of IL-2 production by stimulated cells from isolator than from farm pigs at 20 and 28 days of age (P < 0·05). There was no effect of environment on IL-4 production by stimulated cells (Fig. 5b) or on IL-10 and IFN-γ production (data not shown).
Fig. 5.
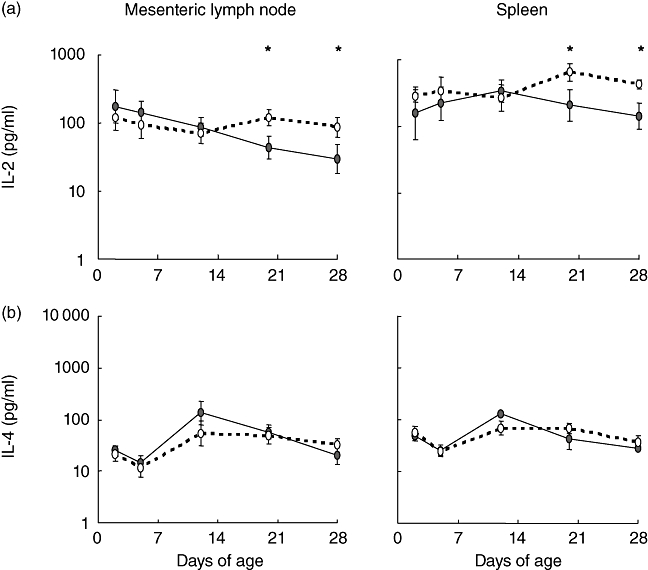
Cytokine production by whole-cell preparations from the small intestine of piglets reared under low- and high-hygiene conditions. (a) Interleukin (IL)-2 production and (b) IL-4 production in the supernatants from whole-cell preparations from the mesenteric lymph node and spleen 41 h after concanavalin A (ConA) stimulation. Solid line: low-hygiene (farm-reared) piglets; dashed line: high-hygiene (isolator-reared) piglets. *P < 0·05 comparing the effect of rearing environment at each time-point.
Discussion
These studies describe a pig model of the impact of early-life environmental factors on immunological development. While the data presented do not examine directly the effects of environment on the development of allergy, they do demonstrate clear effects on intestinal flora, DC phenotype and T cell function and therefore provide a sound basis for subsequent studies.
As in humans, the immune system of the piglet undergoes rapid change during the neonatal period [31,32] and studies have suggested that, during this time, the immune response is highly plastic depending on conditions at the time of antigen exposure [33]. This is supported by the study presented here, where two groups of animals, exposed to different types and quantities of microbial and food antigens associated with environments comparable to breast feeding and formula feeding in human infants, show different immunological development. These differences were apparent by day 5 for DC but not until day 20 for mucosal and systemic T cells. Thus, the initial impact of the different environments appeared to be on DC recruitment and/or differentiation, such that mucosal DC numbers were higher in isolator pigs. With the data collected in this study, it is not currently possible to separate the effects of microbial and food-derived antigen, or the effects of differences in other environmental factors such as the availability of maternal IgA, stress associated with removal from the sow and possible viral infection. These factors are also present in other studies using specific pathogen-free (SPF) and germ-free piglets [31,34–36], and are important components of the different rearing environments for human infants. The potential importance of these factors is highlighted by the group of four farm piglets whose flora at 2 and 5 days was comparable to older animals, and whose mucosal DCs were also increased significantly. This indicates clearly a correlation between microbiota and DC recruitment, linked to a variable not directly controlled in this study. Consistent with this, previous studies in human infants have identified differences in immunological development in children with different intestinal flora [1] and also between breast and bottle-fed infants [5], in concordance with the data presented here. The pig model described provides a system in which the influence of these factors can be tested.
As discussed, the observed differences in immunological development in the low- and high-hygiene groups do not give an indication of the propensity to develop allergic disease. However, given the central role of the DC in the generation and direction of immune responses [37,38], the difference in MHCII+ DC numbers in the low- and high-hygiene groups is an important observation. The functional significance of the increased DC in the high-hygiene piglets is not clear: in particular, the extent to which this early increase in DC was linked to the later decrease in the ability of mucosal T cells to make IL-4, and increased ability of systemic cells to secrete IL-2. The increased levels of IL-4 production at 20 days by intestinal cells from the farm piglets initially seems counterintuitive, as IL-4 is recognized as a T helper type 2 (Th2) cytokine [39] and is secreted preferentially by cord-blood leucocytes from human neonates who develop allergies subsequently [40]. Increasingly, however, the role of the Th1 Th2 paradigm in allergic disease is seen as an over-simplification of the in vivo situation [39,41]. In addition, it is important to note that the difference in IL-4 production occurred at one time-point (day 20) and therefore is unlikely to indicate the type of long-term ‘skewing’ towards an atopic phenotype of the kind reported in allergic individuals. That is not to imply that the difference in IL-4 production is not functionally significant. It may be, for example, that it coincided with increased immunoglobulin class-switching within the gut in response to the more dramatic changes in the flora in farm-reared piglets. Clearly, this point warrants further investigation. It is interesting to note that the differences in cytokine production in the two treatment groups persisted at 28 days in the periphery but not in the intestine. Similarly, the significance of the difference in IL-2 secretion is not clear, as IL-2 has been identified both as a Th0 or Th1 cytokine [42]. In either case, the persistence of differences in cytokine production outside the intestine indicates a longer-term effect of rearing environment on the peripheral immune response than the intestinal mucosal response, consistent with studies in humans [43].
In concordance with our results, previous studies in germ-free pigs have demonstrated clear effects of colonization on the intestinal immune system [31,34–36]. These studies are clearly vital in identifying the basic mechanisms involved in immunological development after colonization with specific bacterial species. The less-controlled ‘isolator’ model described in our work has already been used to good effect to examine the influence of total parenteral nutrition on neonatal intestinal immunity [44,45]. However, the focus of our study has been on developing this system as a model for the effects of rearing environment on intestinal microbiota and neonatal immunological development. Clearly, further studies are required to clarify these effects and to dissect the relative importance of the various environmental factors on this development. However, we would suggest that this model represents a useful tool for addressing important questions regarding the influence of environmental factors on immunological development.
Acknowledgments
This work was funded by EU Framework V.
Disclosure
None.
References
- 1.Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108:516–20. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 2.Rautava S, Kalliomaki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002;109:119–21. doi: 10.1067/mai.2002.120273. [DOI] [PubMed] [Google Scholar]
- 3.Smits HH, Engering A, van der Kleij D, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–7. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 4.Sudo N, Yu XN, Aiba Y, et al. An oral introduction of intestinal bacteria prevents the development of a long-term Th2-skewed immunological memory induced by neonatal antibiotic treatment in mice. Clin Exp Allergy. 2002;32:1112–16. doi: 10.1046/j.1365-2222.2002.01430.x. [DOI] [PubMed] [Google Scholar]
- 5.van Odijk J, Kull I, Borres MP, et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy. 2003;58:833–43. doi: 10.1034/j.1398-9995.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 6.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–45S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 7.Gibbons DL, Haque SFY, Copestake SL, et al. Suppression of airway inflammation by a natural acute infection of the intestinal epithelium. Mucosal Immunol. 2009;2:144–55. doi: 10.1038/mi.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. J Immunol. 2006;177:1628–35. doi: 10.4049/jimmunol.177.3.1628. [DOI] [PubMed] [Google Scholar]
- 9.Helm RM, Furuta GT, Stanley JS, et al. A neonatal swine model for peanut allergy. J Allergy Clin Immunol. 2002;109:136–42. doi: 10.1067/mai.2002.120551. [DOI] [PubMed] [Google Scholar]
- 10.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 11.Rupa P, Hamilton K, Cirinna M, Wilkie BN. A neonatal swine model of allergy induced by the major food allergen chicken ovomucoid (Gal d 1) Int Arch Allergy Immunol. 2008;146:11–18. doi: 10.1159/000112498. [DOI] [PubMed] [Google Scholar]
- 12.Barker E, Haverson K, Stokes CR, Birchall M, Bailey M. The larynx as an immunological organ: immunological architecture in the pig as a large animal model. Clin Exp Immunol. 2006;143:6–14. doi: 10.1111/j.1365-2249.2005.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YS, Li JS, Jiang JW, et al. Glycyl-glutamine-enriched long-term total parenteral nutrition attenuates bacterial translocation following small bowel transplantation in the pig. J Surg Res. 1999;82:106–11. doi: 10.1006/jsre.1998.5525. [DOI] [PubMed] [Google Scholar]
- 14.Rothkotter HJ. Anatomical particularities of the porcine immune system – a physician's view. Dev Comp Immunol. 2009;33:267–72. doi: 10.1016/j.dci.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara R, Watanabe J, Sonoyama K. Assessing changes in composition of intestinal microbiota in neonatal BALB/c mice through cluster analysis of molecular markers. Br J Nutr. 2008;99:1174–7. doi: 10.1017/S0007114507862349. [DOI] [PubMed] [Google Scholar]
- 16.Konstantinov SR, Awati AA, Williams BA, et al. Post-natal development of the porcine microbiota composition and activities. Environ Microbiol. 2006;8:1191–9. doi: 10.1111/j.1462-2920.2006.01009.x. [DOI] [PubMed] [Google Scholar]
- 17.Possemiers S, Verthe K, Uyttendaele S, Verstraete W. PCR-DGGE-based quantification of stability of the microbial community in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol. 2004;49:495–507. doi: 10.1016/j.femsec.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Nuebel U, Engelen B, Felske A, et al. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol. 1996;178:5636–43. doi: 10.1128/jb.178.19.5636-5643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inman CF, Rees LEN, Barker E, Haverson K, Stokes CR, Bailey M. Validation of computer-assisted, pixel-based analysis of multiple-colour immunofluorescence histology. J Immunol Methods. 2005;302:156–67. doi: 10.1016/j.jim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Sinkora J, Rehakova Z, Haverson K, Sinkora M, Dominguez J, Huang CA. Monoclonal antibodies putatively recognising activation and differentiation antigens. Vet Immunol Immunopathol. 2001;80:143–64. doi: 10.1016/s0165-2427(01)00283-5. [DOI] [PubMed] [Google Scholar]
- 21.Sweeney SE, Halloran PJ, Kim YB. Identification of a unique porcine Fc gamma RIIIA alpha molecular complex. Cell Immunol. 1996;172:92–9. doi: 10.1006/cimm.1996.0219. [DOI] [PubMed] [Google Scholar]
- 22.Wilson AD, Haverson K, Southgate K, Bland PW, Stokes CR, Bailey M. Expression of major histocompatibility complex class II antigens on normal porcine intestinal endothelium (vol. 88, p. 98, 1996) Immunology. 1996;88:658–658. doi: 10.1046/j.1365-2567.1996.d01-640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasband WS. ImageJ. Bethesda, MD: US National Institutes of Health; 1996. –present. [Google Scholar]
- 24.Haverson K, Bailey M, Stokes CR. T-cell populations in the pig intestinal lamina propria: memory cells with unusual phenotypic characteristics. Immunology. 1999;96:66–73. doi: 10.1046/j.1365-2567.1999.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konstantinov SR, Zhu WY, Williams BA, Tamminga S, de Vos WM, Akkermans ADL. Effect of fermentable carbohydrates on piglet faecal bacterial communities as revealed by denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA. FEMS Microbiol Ecol. 2003;43:225–35. doi: 10.1111/j.1574-6941.2003.tb01062.x. [DOI] [PubMed] [Google Scholar]
- 26.Haverson K, Singha S, Stokes CR, Bailey M. Professional and non-professional antigen-presenting cells in the porcine small intestine. Immunology. 2000;101:492–500. doi: 10.1046/j.1365-2567.2000.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bimczok D, Post A, Tschernig T, Rothkotter HJ. Phenotype and distribution of dendritic cells in the porcine small intestinal and tracheal mucosa and their spatial relationship to epithelial cells. Cell Tissue Res. 2006;325:461–8. doi: 10.1007/s00441-006-0195-3. [DOI] [PubMed] [Google Scholar]
- 28.Dvorak P, Hruban V, Horak V. The distribution of class II molecules in the pig intestine. Folia Morphol (Prague) 1987;35:396–9. [PubMed] [Google Scholar]
- 29.Halonen M, Lohman IC, Stern DA, et al. Th1/Th2 patterns and balance in cytokine production in the parents and infants of a large birth cohort. J Immunol. 2009;182:3285–93. doi: 10.4049/jimmunol.0711996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holt PG, Macaubas C, Prescott SL, Sly PD. Microbial stimulation as an aetiologic factor in atopic disease. Allergy. 1999;54:12–16. doi: 10.1111/j.1398-9995.1999.tb04382.x. [DOI] [PubMed] [Google Scholar]
- 31.Butler JE, Sun J, Weber P, Navarro P, Francis D. Antibody repertoire development in fetal and newborn piglets, III. Colonization of the gastrointestinal tract selectively diversifies the preimmune repertoire in mucosal lymphoid tissues. Immunology. 2000;100:119–30. doi: 10.1046/j.1365-2567.2000.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothkotter HJ, Mollhoff S, Pabst R. The influence of age and breeding conditions on the number and proliferation of intraepithelial lymphocytes in pigs. Scand J Immunol. 1999;50:31–8. doi: 10.1046/j.1365-3083.1999.00557.x. [DOI] [PubMed] [Google Scholar]
- 33.Adkins B, Jones M, Bu YR, Levy RB. Neonatal tolerance revisited again: specific CTL priming in mouse neonates exposed to small numbers of semi- or fully allogeneic spleen cells. Eur J Immunol. 2004;34:1901–9. doi: 10.1002/eji.200324271. [DOI] [PubMed] [Google Scholar]
- 34.Pabst R, Geist M, Rothkotter HJ, Fritz FJ. Postnatal development and lymphocyte production of jejunal and ileal Peyer's patches in normal and gnotobiotic pigs. Immunology. 1988;64:539–44. [PMC free article] [PubMed] [Google Scholar]
- 35.Shirkey TW, Siggers RH, Goldade BG, et al. Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp Biol Med. 2006;231:1333–45. doi: 10.1177/153537020623100807. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Wen K, Azevedo MSP, et al. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet Immunol Immunopathol. 2008;121:222–31. doi: 10.1016/j.vetimm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 38.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu JF, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piccinni MP, Beloni L, Giannarini L, et al. Abnormal production of T helper 2 cytokines interleukin-4 and interleukin-5 by T-cells from newborns with atopic parents. Eur J Immunol. 1996;26:2293–8. doi: 10.1002/eji.1830261004. [DOI] [PubMed] [Google Scholar]
- 41.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy. Chem Immunol Allergy. 2006;91:159–73. doi: 10.1159/000090279. [DOI] [PubMed] [Google Scholar]
- 42.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 43.Holt PG, Okeeffe P, Holt BJ, et al. T-cell priming against environmental allergens in human neonates – sequential deletion of food antigen reactivity during infancy with concomitant expansion of responses to ubiquitous inhalant allergens. Pediatr Allergy Immunol. 1995;6:85–90. doi: 10.1111/j.1399-3038.1995.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 44.Ganessunker D, Gaskins HR, Zuckermann FA, Donovan SM. Total parenteral nutrition alters molecular and cellular indices of intestinal inflammation in neonatal piglets. J Parenter Enteral Nutr. 1999;23:337–44. doi: 10.1177/0148607199023006337. [DOI] [PubMed] [Google Scholar]
- 45.Park YK, Monaco MM, Donovan SM. Delivery of total parenteral nutrition (TPN) via umbilical catheterization: development of a piglet model to investigate therapies to improve gastrointestinal structure and enzyme activity during TPN. Biol Neonate. 1998;73:295–305. doi: 10.1159/000013988. [DOI] [PubMed] [Google Scholar]


