Abstract
The chemokine receptor CCR6 is expressed by dendritic cells, B and T cells predominantly within the organized structures of the gut-associated lymphatic tissue. Its ligand CCL20 is synthesized by the follicle-associated epithelium and is crucial for the development of M cells within Peyer's patches. In addition, lineage-negative c-kit positive lymphocytes within cryptopatches (CP) express CCR6. CCR6-deficient mice exhibit an altered intestinal immune system containing increased amounts of intraepithelial lymphocytes and show smaller Peyer's patches, while progression of cryptopatches to mature isolated lymphoid follicles (ILF) is inhibited. In this report, we show that lin- c-kit+ lymphocytes express a variety of different chemokine receptors and that CCR6 identifies those cells located within CP. In contrast, cells found outside CP are positive for CXCR3 and exhibit a different surface marker profile, suggesting that at least two different populations of lin- c-kit+ cells are present. The presence of CCR6 does not influence the expression of Notch molecules on lin- c-kit+ cells, nor does it influence Notch ligand expression on bone marrow-derived dendritic cells. In the human gut, CCR6 identifies clusters of lymphocytes resembling murine CP. CCR6 seems to have an important role for lin- c-kit+ cells inside CP, is expressed in a regulated manner and identifies potential human CP.
Keywords: CCR6, CXCR3, cryptopatch, colitis, GALT
Introduction
In 1996, Kanamori and colleagues [1] initially described small clusters of lymphoid cells inside the murine lamina propria that contain two different cellular subsets: clusters of lymphocytes expressing c-kit but lacking lineage markers resembling T cell precursors [lin- c-kit+ interleukin (IL)-7Rα+ CD44+; CP cells] surrounded by CD11c+ dendritic cells (DCs). Cryptopatches (CP) were not found until day 14 after birth and are distributed throughout the small and large intestine. Studies of variant knock-out mice showed that CP develop independently of T and B cells [present in severe combined immunodeficiency (SCID) and recombinase-activating gene-2 (RAG2−/−) mice] and do not depend upon the non-canonical nuclear factor kappa B (NFκB) pathway but require lymphotoxin signalling [2].
The transfer of these lin- c-kit+ cells into immunodeficient mice reconstitutes specifically αβ and γδ T cell receptor (TCR) intraepithelial lymphocytes (IEL) expressing predominantly the unusual CD8αα co-receptor as well as T cells within mesenteric lymph nodes, but not B cells, suggesting that CP might be a site of extra-intestinal lymphocyte development [3,4]. However, only a low proportion of the precursor cells show T cell commitment by means of CD3-ε, RAG-1 and pre-Tα expression [5].
In contrast, Guy-Grand et al. could not find any RAG activity in CP but identified mesenteric lymph nodes (MLN) and Peyer's patches as a potential site of extrathymic T cell lymphopoiesis [6]. In euthymic mice, the extrathymic developmental pathway was shut off completely and could be unmasked only in severe lymphocytotic depletion (e.g. after radiation). These data suggest that IEL are more likely to be of thymic origin under normal conditions and that CP have a different function. However, this hypothesis was challenged by Nonaka et al. in mouse models depleted of all organized gut-associated lymphoid tissue (GALT) structure except for CP [7]. In conclusion, it cannot be excluded that CP might harbour immature lymphocyte precursor cells that are capable of differentiating into IEL, but this process is unlikely to occur under euthymic conditions. Therefore, a different role of CP has to be postulated under natural conditions.
Recently, Eberl et al. identified CP cells as the adult counterparts of fetal lymphoid tissue inducer (LTi) cells that are among the first haematopoetic cells to colonize developing lymphoid tissue such as Peyer's patch anlagen [8,9]. By tracking the retinoic acid-related orphan receptor gamma T (RORγt), the authors found that this receptor is basically expressed by all lin- c-kit+ lamina propria lymphocytes (LPL). RORγt-deficient mice have an impaired thymic lymphopoiesis and, strikingly, have no CP and no isolated lymphoid follicles. The presence of regular numbers of γδTCR-positive IEL suggests that these cells are not the progeny of CP cells. The authors conclude that CP are more likely to serve as organizers of inducible tertiary lymphoid tissue inside the gut.
In this report, we show that lin- c-kit+ lymphocytes are not restricted to CP inside the gut. Even though this cell population expresses a broad variety of chemokine receptors, the expression of CCR6 identifies specifically those cells located within CP whereas diffusely distributed LTi cells express the chemokine receptor CXCR3, suggesting that CCR6 is a marker for CP cells. In addition, we show that CCR6 positive aggregates are also found within the human lamina propria, suggesting that these organized structures might have a role for inflammatory responses inside the human gut.
Materials and methods
Mice
The gene targeting strategy employed to generate CCR6 enhanced green fluorescent protein (EGFP) knock-in mice has been described previously [10]. The homozygous CCR6-deficient mice used for these studies were back-crossed eight times to C57BL/6. CCR6 knock-out mice and heterozygous CCR6-deficient mice shared the same background. When genotyping of the individual offspring was required, a three-primer polymerase chain reaction (PCR) method was used. Comparisons of CCR6-deficient and knock-out mice were made using heterozygous and homozygous knock-out mice that were typically littermates between 6 and 8 weeks of age.
All experiments including mice were approved by the Institutional Animal Care and Use Committee (authorization no. 9·93·2·10·36·07·081). Experiments using human tissue were approved by the local ethical committee (authorization no. 2007-206-f-S).
Preparation of lamina propria cell suspensions
Lamina propria lymphocytes were prepared by a standard method with minor modifications. Briefly, the small intestine was removed from euthanized mice, followed by identification and resection of Peyer's patches. The remaining small intestinal tissue specimens were opened longitudinally and cut into 0·5-cm pieces, washed four times with cold Ca2+/Mg2+ (CMF) solution [Ca2+ and Mg2+-free Hanks's balanced salt solution (HBSS), 10 mM HEPES, 25 mM NaHCO3, 2% (v/v) fetal bovine serum (FBS), pH 7·2]. The intestinal tissue specimens were transferred into 30 ml of CMF/FBS/ethylenediamine tetraacetic acid (EDTA) solution [Ca2+ and Mg2+-free HBSS, 15 mM HEPES, 5 mM EDTA, 100 µg/ml gentamycin, 10% (v/v) FBS, pH 7·2]. After 30 min of gentle shaking at 37°C, the sample was vortexed for 10 s and cells from the supernatant were collected. This step was repeated three times and all the supernatants representing the epithelial fraction containing IELs were combined. To isolate the LPL further the remaining tissue was incubated with 100 U/ml collagenase D (Roche, Mannheim, Germany) and 5 U/ml DNase (Sigma, St Louis, MO, USA) for 60 min at 37°C in complete RPMI-1640 media. The cells released into the supernatant as well as IEL were purified further by density centrifugation and isolated from the interface of a 44%/66% Percoll (GE Healthcare Europe GmbH, Freiburg, Germany) step gradient centrifuged for 30 min at 600 g and washed in cold PBS. Human tissue samples were prepared in a parallel manner.
Antibodies and flow cytometry
The monoclonal antibodies (mAbs) purchased from BD PharMingen (Heidelberg, Germany) were phycoerythrin (PE)-anti-CD4 (RM4-5), PE-anti-CD8α (53–6·7), PE-anti-CD19 (1D3), PE-anti-CD45Rb (16A), PE-anti-CD25 (PC61), PE-anti-CD44 (IM7), biotin-conjugated lineage marker panel (CD5, CD45R (B220), CD11b, Gr-1 (Ly-6G/C), 7-4 and Ter-119), streptavidin-conjugated peridinin chlorophyll (PerCP), and allophycocyanin (APC)-anti-c-kit (2B8). A rabbit anti-mouse antibody to CXCR3 was obtained from Zytomed (Berlin, Germany) and labelling of positive cells was detected by a secondary fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit Ig (1 µg/ml; Jackson Immunoresearch, Suffolk, UK). PE-anti-mouse-CCR6 (140706) was obtained from R&D Systems (Wiesbaden-Nordenstadt, Germany). PE-anti-CD127 (A7R34) was obtained from eBioscience (Frankfurt, Germany). Antibodies were diluted in PBS containing 0·2% bovine serum albumin (BSA) and 0·02% NaN3 for 30 min on ice. Data on antibody-stained cell suspensions were acquired on a dual laser fluorescence activated cell sorter (FACScan) flow cytometer (Becton Dickinson, Heidelberg, Germany) and the results were analysed using CellQuest version 3·3 (Becton Dickinson). Cell populations were gated on the basis of forward- and side-scatter to allow selection of the viable lymphocytes.
For the study of human LPL the following antibodies were used: biotin anti-human CD11c (3–9), APC anti-human c-kit (YB5.B8), PE anti-human RORγ (AFKJS-9), PE rat IgG2a isotype control and FITC anti-human CCR6 (R6H1) were obtained from eBioscience. PerCP-anti-human CD19 (4G7), PerCP-anti-human CD3 (SK7) and streptavidin–PerCP were obtained from Pharmingen.
Immunofluorescence staining
For detection of in situ EGFP fluorescence, mice were perfused with 3% paraformaldehyde followed by 10% sucrose prior to embedding of the tissue in octreotide (OCT) as described elsewhere. Six-micron frozen sections were cut with a cryostat. CCR6+ cells were detected in heterozygous mice by means of their EGFP expression; control stainings were performed in parallel on tissue from wild-type mice to exclude autofluorescence signals. All controls were negative. 4,6-Diaminido-2-phenylindole (DAPI) (Sigma) was used as nuclear counterstain. Double- and triple-colour fluorescence images were acquired using a Leica microscope. CXCR3 expression was detected on acetone-fixed tissue sections using a polyclonal rabbit anti-mouse antibody to CXCR3 (0·5 µg/ml final concentration; Zytomed) followed by the tyramide signal amplification (TSA) system with peroxidase-conjugated goat anti-rabbit immunoglobulin (Ig) (5 µg/ml; Jackson Immunoresearch) and FITC-tyramide (PerkinElmer Life Sciences, Boston, MA, USA).
Magnetic affinity cell sorting (MACS) isolation procedure
CD117+ lin- precursor-enriched lamina propria mononuclear cells (lamina propria MCs) were finally isolated subsequently using lineage-marker [negative depletion with antibodies to CD5, CD45R (B220), CD11b, Gr-1 (Ly-6G/C), 7-4 and Ter-119] and c-kit microbeads (positive selection) and MACS techniques (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany) according to the manufacturer's instructions.
Isolation of mRNA and reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA of isolated precursor cells and bone marrow-derived dendritic cells (bmDCs) was isolated using TRIzol (Sigma-Aldrich, Hamburg, Germany) according to the manufacturer's recommendations. Reverse transcription into complementary DNA was performed using the Moloney murine leukaemia virus (MMLV) reverse transcriptase (Life Technologies Inc., Carlsbad, CA, USA) method.
Chemokine receptor expression was analysed using two multiplex PCR kits (Maxim Biotech, San Francisco, CA, USA) including CCR1-9 and CX3CR1, according to the manufacturer's instructions.
Notch 1–4 expression by IEL precursors and mature IEL was analysed by RT–PCR as described elsewhere [11]. Notch-ligand expression on bmDC was analysed 24 h after incubation with various concentrations of rmMip3a (R&D Systems) by real-time PCR as described elsewhere [11].
Generation of bmDC
For isolation of bmDC, bone marrow was isolated from femur and tibia and erythrocytes were lysed. The remaining cells were plated at a density of 106 per ml in six-well plates in RPMI-1640 (Hyclone, Logan, UT, USA) supplemented with 10% FBS (Hyclone) and containing 10 ng/ml of murine granulocyte–macrophage colony-stimulating factor (GM-CSF) and 1 ng/ml of murine IL-4 (Peprotech, Rocky Hill, NJ, USA). The cells were incubated at 37°C with 5% CO2. After 2 days of culture the cells were washed gently and replaced with RPMI-10 containing the same concentration of GM-CSF and IL-4 for an additional 5 days and semi-adherent cells were harvested for further experiments. For maturation, bmDC were stimulated further with 1 µg/ml LPS for 24 h and incubated with variable concentrations of rmMip3a (R&D Systems).
Induction of dextran sulphate sodium (DSS) colitis
Colitis was induced by addition of 3% DSS (molecular weight 40 000; ICN Biomedicals, Aurora, OH, USA) to drinking water for 7 days.
Induction of Citrobacter rodenitum colitis
Citrobacter rodentium was grown overnight in Luria–Bertani broth at a concentration of 2·5 × 109/ml. Adult (10-week-old) CCR6 heterozygous mice were infected with 200 µl of the bacterial suspension (5 × 108 bacteria) by oral gavage. To determine bacterial numbers in the stool, fecal pellets were collected from individual mice over a 2- to 3-h period, weighed and homogenized in 2 ml PBS. Serial dilutions of the homogenates were plated onto MacConkey agar (Merck, Darmstadt, Germany), and the number of colony-forming units was determined after overnight incubation at 37°C.
Statistics
Results are generally expressed as the mean ± standard error of the mean (s.e.m.) unless noted otherwise. The statistical significance of differences between groups was evaluated by Student's t-test. A P-value less than 0·05 was considered to be statistically significant.
Results
CCR6 is expressed specifically by lin- c-kit+ cells within CP
Previous studies could show that CCR6 is expressed by lymphocytes within CP. To characterize further the significance of this finding we compared the expression of CCR6 by lin- c-kit+ using immunohistochemistry and flow cytometry. FACS analysis of lin- c-kit+ LPL (Fig. 1a) revealed a significant proportion of CCR6-expressing cells within the lin- c-kit+ LPL cell fraction (approximately 15–20%; analysis of heterozygous EGFP–CCR6 knock-in mice). However, when analysed by immunohistochemistry (Fig. 1b), a significantly higher number of CP cells express this receptor (approximately 75%), indicating that lin- c-kit+ cells must be found outside CP within the lamina propria, and that CCR6 is a marker for localization of these cells within CP.
Fig. 1.
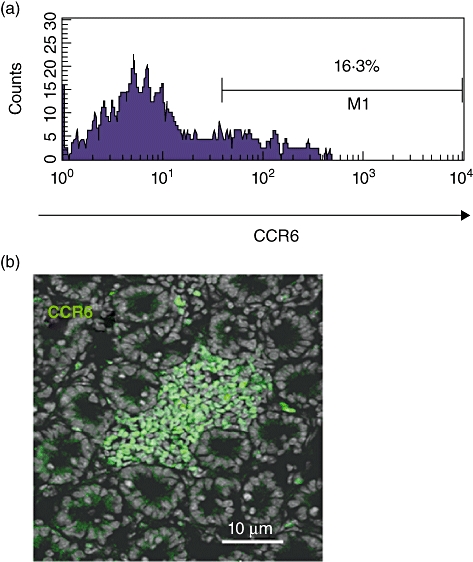
CCR6 characterizes lymphoid tissue inducer (Lti) cells within cryptopatches. Fluorescence activated cell sorter (FACS) analysis of lin- c-kit+ lamina propria cells (a) revealed a significant proportion of CCR6-expressing cells within the lin- c-kit+ cell fraction [approximately 15–20%; analysis of heterozygous enhanced green fluorescent protein (EGFP)–CCR6 knock-in mice, n = 10]. However, when analysed by immunohistochemistry (b), a significantly higher number of cryptopatch cells expressed this receptor (75 ± 8%, n = 10, P < 0001). All control stainings were negative.
Macrophage inflammatory protein-3 (Mip3α)–CCR6 interaction does not influence Notch/Notch-ligand expression
Various data suggest that signals transduced by Notch receptors are important for T cell specification and differentiation of αβversusγδ T lineage decision inside the gut [12]. As CCR6-deficient mice are characterized by an expanded IEL fraction exhibiting a significant expansion of αβTCR IEL with unaltered γδTCR IEL [13–15], we examined the expression of Notch 1–4 by lin- c-kit+ LPL of wild-type and CCR6 knock-out mice supposed to be precursors of intestinal IEL (Fig. 2a). Isolated cells from both types of mice expressed similar levels of Notch-1, -2 and -4, as determined by RT–PCR, whereas no expression of Notch-3 could be found. In addition, we analysed the expression of Notch-ligands by bmDCs expressing high levels of CCR6 (data not shown) after Mip3α stimulation. Again, we were not able to find any significant induction of Jagged-1, Jagged-2 and Delta-4 after Mip3α stimulation (Fig. 2b), suggesting that Notch signalling within CP is unlikely to be involved in the altered IEL development of CCR6 knock-out mice.
Fig. 2.
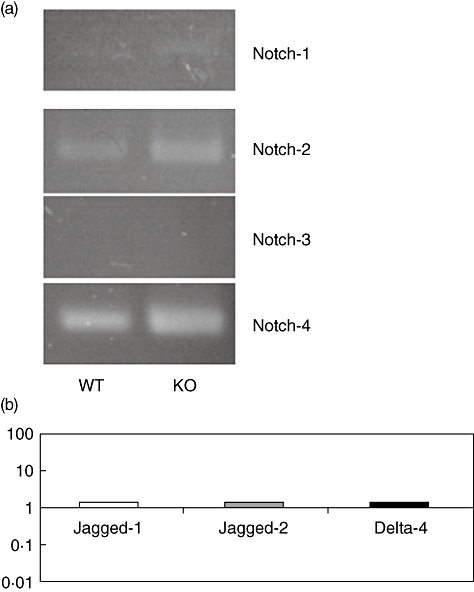
CCR6 does not affect Notch signalling. RNA was isolated from sorted lin- c-kit+ cells from wild-type and CCR6-deficient mice. Reverse transcription–polymerase chain reaction (PT–PCR) analysis revealed a similar expression of Notch receptors by CCR6-deficient lymphoid tissue inducer (Lti) cells (a). In addition, 24 h stimulation of CCR6 expressing bone marrow dendritic cells (bmDC) by Macrophage inflammatory protein 3α (Mip3α) (10 ng/ml) did not induce Notch-ligand expression as measured by real-time PCR (b; CCR6-deficient bmDC served as controls; n = 5 per group).
Chemokine receptor expression by lin- c-kit+ lamina propria lymphocytes
To determine the expression of other chemokine receptors by lin- c-kit+ cells, LPL were isolated from the lamina propria and identified consecutively by staining with antibodies to c-kit and lineage markers (lin). After MACS sorting RNA was isolated from lin- c-kit+ as well as lin+ c-kit+ cells. In parallel, RNA from mature intraepithelial lymphocytes and Peyer's patches were prepared. Chemokine receptor expression was analysed by two different multiplex PCR kits, including primers for amplification of CCR1-9 as well as CX3CR1. As shown in Fig. 3, lin- c-kit+ lymphocytes express a broad variety of different receptors, including CCR1-7 as well as CCR9, while no expression of CCR8 and CX3CR1 was detectable. Corresponding data were obtained from lin+ c-kit+ LPL, and a similar expression profile was found within Peyer's patches that lack a signal for CCR3. In contrast, mature IEL express predominantly CCR9 and CCR5 and limited amounts of CCR2.
Fig. 3.
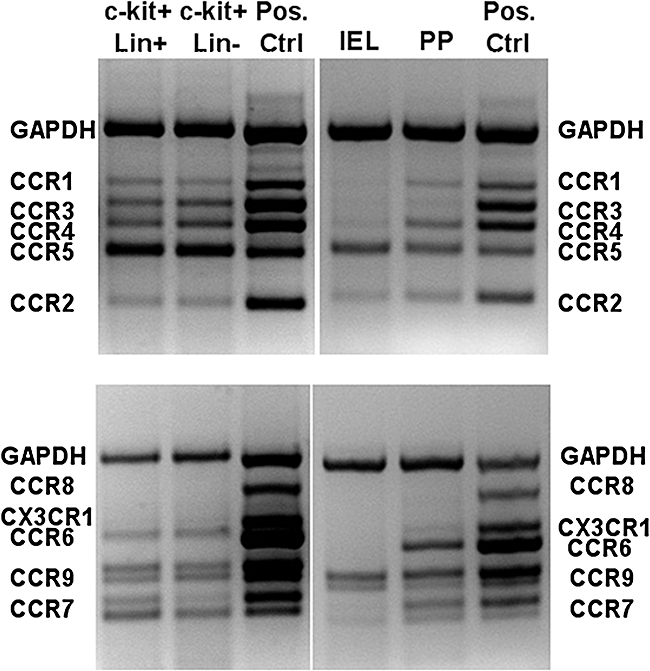
Chemokine receptor expression of lin- c-kit+ cells. Samples of cDNA prepared from sorted lin- c-kit+ and lin+ c-kit+ cells, Percoll gradient-purified intraepithelial lymphocytes (IELs) and Peyer's patches were amplified with multiplex polymerase chain reaction (PCR) primer sets specific for CCR1 to CCR5 (top panels) or CCR6 to CCR9 and CX3CR1 (bottom panels). The location of the specific PCR amplimers in the positive control samples are indicated. Data are representative for three individual experiments. Lin- c-kit+ and lin+ c-kit+ cells were derived from the lamina propria.
Reverse expression of CCR6 and CXCR3 by lin- c-kit+ lamina propria cells
The chemokine receptor CCR6 is expressed by lin- c-kit+ lymphocytes inside CP, while CCR6 expression is absent in lin- c-kit+ cells outside CP as well as in mature IEL. To address this question further we investigated the expression of a chemokine receptor known to be expressed by mature IEL on IEL precursors. To this end, we quadruple-stained LPL cells with antibodies to lineage markers and c-kit as well as CCR6 and CXCR3, and analysed chemokine receptor expression by lin- c-kit+ cells by flow cytometry. As shown in Fig. 4a, CCR6 and CXCR3 are expressed reciprocally by lin- c-kit+ precursors. While only a fraction of about 15–20% stain positive for CCR6, the majority of this population expresses CXCR3. In addition, only a limited fraction of CXCR3-expressing cells stain positive for CCR6. Interestingly, the level of receptor expression clearly decreases while acquiring CXCR3 expression (or vice versa). To confirm further the reciprocal expression of CCR6 and CXCR3, we analysed CXCR3 expression inside CP by immunohistochemistry. As shown in Fig. 4b, CXCR3-expressing cells are found in very limited numbers inside CP, whereas cells outside CP, including intraepithelial lymphocytes, stain positive for CXCR3, suggesting that CCR6 is a specific marker for cells located within CP.
Fig. 4.
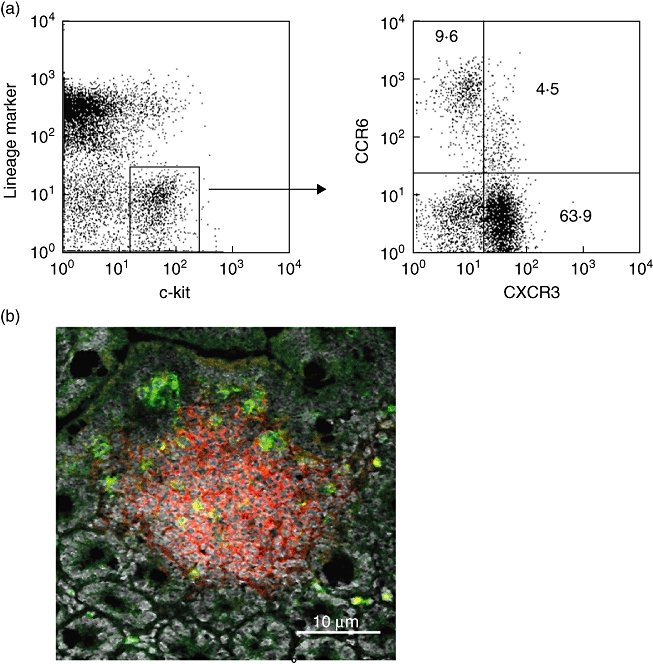
Reciprocal expression of CCR6 and CXCR3 by lin- c-kit+ cells. Percoll-purified lamina proria lymphcytes from wild-type mice were stained with allophycocyanin (APC)-anti-c-kit, biotinylated monoclonal antibodies (mAbs) to lineage markers followed by streptavidin–peridinin chlorophyll (PerCP), phycoerythrin (PE)-anti-CCR6 and rabbit anti-mouse CXCR3 followed by fluorescein isothiocyanate (FITC) goat anti-rabbit immunoglobulin. A dot-plot of CCR6 versus CXCR3 staining is shown for the gated lin- c-kit- population (a). Immunofluorescence staining of a cryptopatch with anti-CXCR3 (green), PE-anti-Thy-1 and Topro-3 as a nuclear counterstain (b). Only rare cells within the cryptopatches are found to be CXCR3+. All control stainings were negative. The data are representative for stainings obtained from five individual wild-type mice.
Surface marker expression on CP cells
To characterize further the different phenotypes of lin- c-kit+ cells located within and outside CP, lymphocytes were isolated from the lamina propria and lin- c-kit+ cells stained for the expression of various surface markers (Fig. 5). While cells outside (CCR6-negative) and inside (CCR6-positive) CP express similar levels of the activation markers CD25 and CD127 as well as CD44, significantly different expression patterns could found for CD45Rb, CD4 and CD8. Corresponding to previous independent immunohistochemical stainings [1], cells within CP are partially CD4+, whereas no CD8 expression is detectable, and a different profile can be found on CCR6- cells. In addition, CP cells express low levels of CD45RB, suggesting that at least two different subtypes of lin- c-kit+ cells are present in the intestine.
Fig. 5.
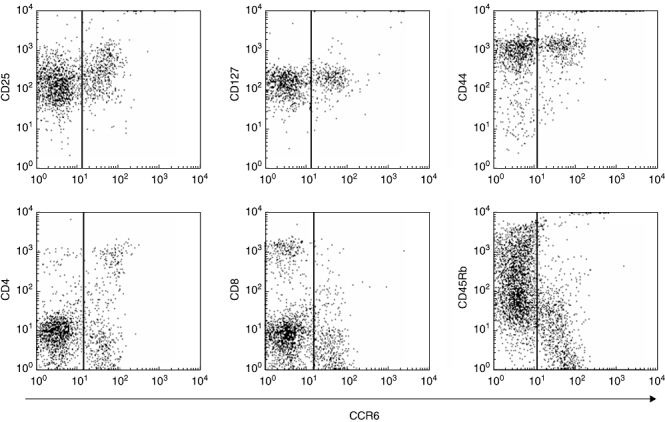
Fluorescence activated cell sorter (FACS) analysis of lymphoid tissue inducer (Lti) cells. Lamina propria cells were isolated and lin- c-kit+ cells (LTi cells) characterized by staining for surface markers. While LTi cells outside (CCR6-) and inside (CCR6+) cryptopatches express similar levels of the activation markers CD25 and CD127, as well as CD44, significantly different expression patterns could be found for CD45Rb, CD4 and CD8, suggesting that at least two different subtypes of LTi cells are present in the intestine. The data are representative for stainings obtained from five individual CCR6 knock-in mice.
Identification of CP-like structures in the human gut
Previous studies have failed to identify CP in the human intestine based on the expression of c-kit. Indeed, staining of human (Fig. 6a) and murine (Fig. 6b) intestinal tissue specimens showed that in contrast to the CP-restricted expression in the murine gut, c-kit+ lymphocytes are found diffusely within the human intestine, suggesting a different expression profile based on this marker. However, small clusters of lymphocytes that include a subset of c-kit+ cells (Fig. 6c) are also found in the human intestine that contains a significant number of CCR6+ lymphocytes (Fig. 6d). These data suggest that these clusters might represent precursors of isolated lymphoid follicles that are developing from CP in the mouse under inflammatory conditions and are known to be present in the human gut. Indeed, multiple expanding clusters of CCR6-expressing cells are found in the mucosa of ulcerative colitis patients (Fig. 6e,f).
Fig. 6.
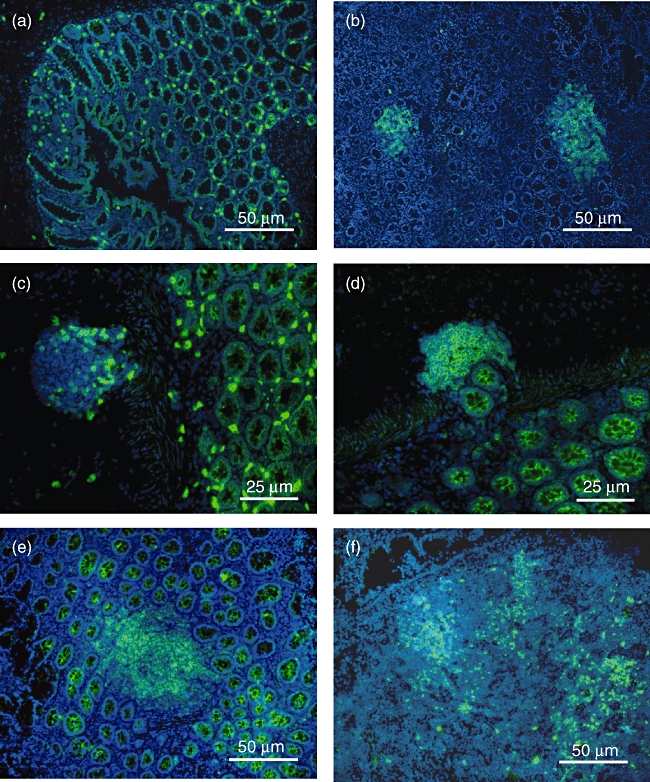
CCR6 and c-kit expression in the gut. Staining of human (a) and murine (b) intestinal tissue specimens showed that in contrast to the cryptopatches-restricted expression in the murine gut, c-kit+ lymphocytes are found diffusely within the human intestine. However, small clusters of lymphocytes partially expressing c-kit (c) are also found in the human intestine containing a significant number of CCR6+ lymphocytes (d). In addition, multiple expanding clusters of CCR6-expressing cells are found in the mucosa of ulcerative colitis patients (e+f). All control stainings were negative.
To confirm further the presence of lin- c-kit+ lymphoid tissue inducer cells within the human intestine we isolated lamina propria leucocytes from full-thickness human small intestinal tissue specimens (4–6 cm2) and stained for the expression of RORγ and CCR6 in CD3-CD11c-CD19- cells (Fig. 7). In contrast to the observations in mice, we could identify an additional cell population expressing high amounts of c-kit in the absence of CD3, CD11c and CD19, but showing a significantly different scatter profile and no RORγ expression. Most probably, these cells represent mast cells known to express c-kit, having high side-scatter (because of granularity) and exhibiting more autofluorescence than most other leucocytes. More importantly, we were also able to find a second CD3-CD11c-CD19- lymphocyte cell population expressing lower amounts of c-kit but which is homogeneously positive for RORγ, suggesting that these cells are the human correlate of murine LTi cells. Like murine LTi cells, approximately 15–20% of these cells express the chemokine receptor CCR6 and represent LTi cells found within CP.
Fig. 7.
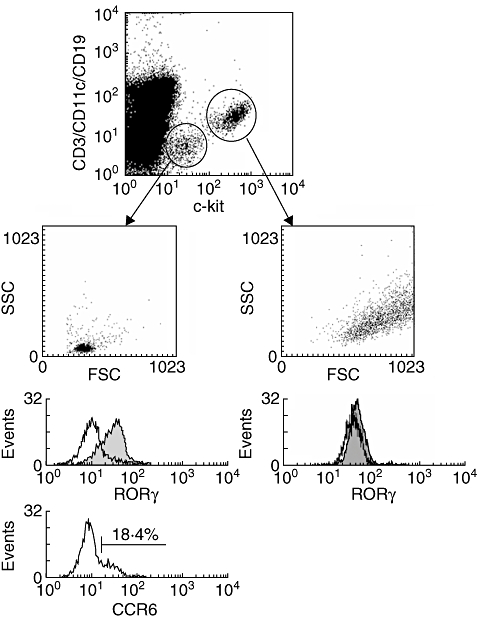
Detection of cryptopatch cells in the human gut. Lamina propria leucocytes were isolated from large intestinal specimens and stained for the expression of CCR6, c-kit and retinoic acid-related orphan receptor gamma (RORγ) in CD3-, CD11c- and CD19- cells. In contrast to the murine intestine, a second population of SSChigh leucocytes expresses high levels of c-kit but does not express RORγ (right panel). In contrast, CD3-CD11c-CD19- c-kitlow cells within the lymphocyte gate express RORγ homogeneously and are partially positive for the chemokine receptor CCR6 (left panel), highly suggestive for the human correlate of murine cryptopatch cells.
Induction of colitis does not expend the number of CP
In order to test whether the number of CP or CP cells increases during the course of colitis we measured the amount of lin- c-kit+ CCR6+ lamina propria by flow cytometry 7 and 14 days after induction of DSS colitis as well 2 and 6 weeks after infection with the pathogen C. rodentium. However, the numbers of CP cells remained constant in both models used, suggesting that CP are not formed de novo under inflammatory conditions (Fig. 8).
Fig. 8.
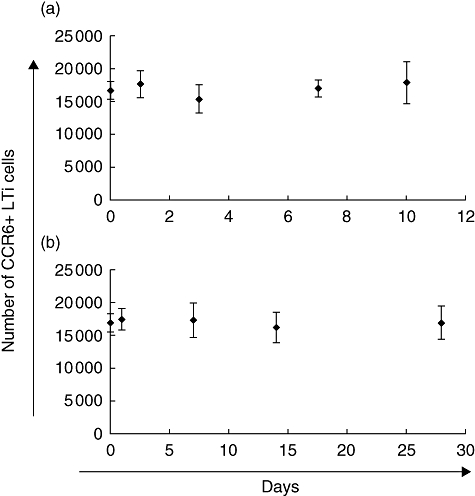
Intestinal inflammation does not extend the number of cryptopatch cells. Large intestinal inflammation was induced by dextran sulphate sodium (DSS) colitis (a) and Citrobacter rodentium colitis (b). The amount of lin- c-kit+ CCR6+ cryptopatch cells within the large intestinal lamina propria was determined at various time-points by flow cytometry giving no evidence for de novo cryptopatch formation over time.
Discussion
The intestinal immune system includes several organized lymphoid structures that constitute an extensive network with other non-organized parts, such as lamina propria and intraepithelial lymphocytes. The majority of the T cells contained in these compartments are the progeny of thymic precursors, but distinct subsets such as CD8αα+ IEL are supposed to develop partially from extrathymic sites [16]. Several years ago CP were identified as the potential site of extrathymic T cell differentiation [1,3,17], but this hypothesis remains controversial, as other data suggest that mesenteric lymph nodes and Peyer's patches are more likely to contribute to T cell differentiation by means of RAG expression, and this process is present only under the setting of significant immunodeficiency [6]. In addition, experiments by Eberl et al. identified lin- c-kit+ cells from the lamina propria, including CP cells, as the adult counterpart of lymphoid tissue inducer cells [9].
CCR6-deficient mice exhibit significantly expanded IEL in multiple independent knock-out constructs [13,14]. We have described previously the expression of CCR6 specifically by lymphoid precursor cells in CP and hypothesized that the predominant expansion of extrathymic IEL in CCR6-deficient mice might be related to this phenomenon [15]. However, mature IEL express no CCR6. In the current study we show clearly that the expression of CCR6 is related specifically to lin- c-kit+ cells inside CP, as cells outside CP lose CCR6 expression and are found positive for an alternate chemokine receptor not present on CP cells, CXCR3. Although lin- c-kit+ cells express various receptors as determined by PCR analysis, suggesting redundancy, CCR6 also seems to have a functional role, as data published by MacDonald et al.[18] suggest that CCR6 is important for the development of mature isolated lymphoid follicles (ILF) from CP. It can be speculated that CCR6 contributes to similar events inside ILF and Peyer's patches development as the latter are size-reduced significantly in the absence of a functional CCR6 receptor, while no change in micro-architecture can be found [19]. Most intriguingly, CCR6 seems to differentiate at least two different subsets of lin- c-kit+ cells that have not been appreciated in other studies, and the majority (>70%) of lin- c-kit+ cells are indeed found outside CP. Recently, Eberl et al. could show that basically all lin- c-kit+ cells express the orphan receptor RORγt. Immunohistochemical studies have identified that these cells are located specifically within CP. The authors concluded that these cells are, rather, organizers of induced organized lymphoid tissue in adults (LTi cells) and do not participate in IEL development. However, our data show that the majority is of these cells is CCR6- (CXCR3+) and therefore found outside CP. It remains to be elucidated if both cell types are the progeny of a common precursor or if, functionally, they constitute different cellular lineages. In addition, it can be speculated that subsets of these cells might contribute to the IEL compartment in specialized settings. However, we were not able to find an influence of CCR6/Mip3α on Notch signalling known to influence αβversusγδ lineage commitment. Strikingly, the flow cytometric phenotype of CCR6+ lin- c-kit+ cells correlates well with earlier data published by Kanamori et al., showing that CP cells are CD8- and partly positive for CD4, while both types express similar levels of CD25, CD44 and CD127 [1].
Previous studies have attempted to identify CP-like structures in humans, but no clusters of c-kit positive cells could be identified. Initial trials by Moghaddami et al. found lymphoid structures with an epithelium resembling follicle-associated epithelium termed ‘lymphocyte-filled villi’[20]. These structures contain different leucocyte subsets such as major histocompatibility complex class II-positive dendritic cells, memory T cells and a variable amount of B cells. The authors concluded that the human gut does not contain CP. In contrast, ILF were appreciated in humans decades ago [21]. However, if CP constitute a precursor structure of ILF, the presence of CP in the human gut has to be postulated in theory. The data presented here show a significant difference of c-kit expression within the murine and human gut. While c-kit specifically stains positive for CP in mice and is rarely found on cells outside CP (e.g. interstitial cells of Cajal) the human gut shows abundant cells distributed diffusely in the lamina propria, which are likely to represent intestinal mast cells which were found to express high levels of c-kit only in humans but not in mice. However, we were also able to find c-kit+ cells negative for B, T and DC markers that express the orphan receptor RORγ homogeneously and which are partially positive for CCR6 in humans. Even though the specific isoform RORγt cannot be differentiated currently from RORγ, as specific antibodies are not available, these data suggest that cryptopatch cells are present in humans and that similar mechanisms of tertiary lymphoid organogenesis are present in the murine and human gut. It is likely that CP and ILF are parts of aggregated lymphoid structures within the small intestine that vary in size and cellular composition. As even in defined mouse models a significant number of these structures do not match with classical definitions of CP and ILF [22], it is likely that the adult human (antigen-exposed) gut rather contains modified variants of CP and ILF.
Recent work in human IBD suggests that colonic ILF hyperplasia is observed in Crohn's disease and ulcerative colitis [23,24]. In fact, it has been reported that the size of ILF may correlate with disease activity of the disease, suggesting that induction of ILF from CP occurs under inflammatory conditions in humans. The induction of tertiary lymphoid structures in the colon has also been appreciated after DSS as well as trinitrobenzesulphonic acid (TNBS) administration [25]. Therefore, the expression of CCR6 in tertiary lymphoid structures in the inflamed human gut suggests that this receptor might represent a valuable target for the treatment of IBD.
Acknowledgments
This study was supported by grants from the Interdisciplinary Center for Clinical Research (grant numbers: IZKF; Kuc2/018/06), Deutsche Forschungsgemeinschaft (DFG LU 816/2-1) and the NIH (DK064730).
Disclosure
None of the authors have conflicts of interest, or any relevant financial interest, in any company or institution that might benefit from this publication.
References
- 1.Kanamori Y, Ishimaru K, Nanno M, et al. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–59. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor RT, Lugering A, Newell KA, Williams IR. Intestinal cryptopatch formation in mice requires lymphotoxin alpha and the lymphotoxin beta receptor. J Immunol. 2004;173:7183–9. doi: 10.4049/jimmunol.173.12.7183. [DOI] [PubMed] [Google Scholar]
- 3.Saito H, Kanamori Y, Takemori T, et al. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–8. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K, Oida T, Hamada H, et al. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity. 2000;13:691–702. doi: 10.1016/s1074-7613(00)00068-6. [DOI] [PubMed] [Google Scholar]
- 5.Lambolez F, Azogui O, Joret AM, et al. Characterization of T cell differentiation in the murine gut. J Exp Med. 2002;195:437–49. doi: 10.1084/jem.20010798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guy-Grand D, Azogui O, Celli S, et al. Extrathymic T cell lymphopoiesis: ontogeny and contribution to gut intraepithelial lymphocytes in athymic and euthymic mice. J Exp Med. 2003;197:333–41. doi: 10.1084/jem.20021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nonaka S, Naito T, Chen H, et al. Intestinal gamma delta T cells develop in mice lacking thymus, all lymph nodes, Peyer's patches, and isolated lymphoid follicles. J Immunol. 2005;174:1906–12. doi: 10.4049/jimmunol.174.4.1906. [DOI] [PubMed] [Google Scholar]
- 8.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 9.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–51. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 10.Kucharzik T, Hudson JT, III, Waikel RL, Martin WD, Williams IR. CCR6 expression distinguishes mouse myeloid and lymphoid dendritic cell subsets: demonstration using a CCR6 EGFP knock-in mouse. Eur J Immunol. 2002;32:104–12. doi: 10.1002/1521-4141(200201)32:1<104::AID-IMMU104>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–26. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 12.Washburn T, Schweighoffer E, Gridley T, et al. Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell. 1997;88:833–43. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 13.Cook DN, Prosser DM, Forster R, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 14.Varona R, Villares R, Carramolino L, et al. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest. 2001;107:R37–45. doi: 10.1172/JCI11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lugering A, Kucharzik T, Soler D, Picarella D, Hudson JT, III, Williams IR. Lymphoid precursors in intestinal cryptopatches express CCR6 and undergo dysregulated development in the absence of CCR6. J Immunol. 2003;171:2208–15. doi: 10.4049/jimmunol.171.5.2208. [DOI] [PubMed] [Google Scholar]
- 16.Lefrancois L, Puddington L. Extrathymic intestinal T-cell development: virtual reality? Immunol Today. 1995;16:16–21. doi: 10.1016/0167-5699(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 17.Oida T, Suzuki K, Nanno M, et al. Role of gut cryptopatches in early extrathymic maturation of intestinal intraepithelial T cells. J Immunol. 2000;164:3616–26. doi: 10.4049/jimmunol.164.7.3616. [DOI] [PubMed] [Google Scholar]
- 18.McDonald KG, McDonough JS, Wang C, Kucharzik T, Williams IR, Newberry RD. CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am J Pathol. 2007;170:1229–40. doi: 10.2353/ajpath.2007.060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lugering A, Floer M, Westphal S, et al. Absence of CCR6 inhibits CD4+ regulatory T-cell development and M-cell formation inside Peyer's patches. Am J Pathol. 2005;166:1647–54. doi: 10.1016/S0002-9440(10)62475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moghaddami M, Cummins A, Mayrhofer G. Lymphocyte-filled villi: comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology. 1998;115:1414–25. doi: 10.1016/s0016-5085(98)70020-4. [DOI] [PubMed] [Google Scholar]
- 21.Morson BC, Dawson IMP. Gastrointestinal pathology. 2nd. Oxford: Blackwell Scientific; 1979. [Google Scholar]
- 22.Pabst O, Herbrand H, Worbs T, et al. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35:98–107. doi: 10.1002/eji.200425432. [DOI] [PubMed] [Google Scholar]
- 23.Kaiserling E. Newly-formed lymph nodes in the submucosa in chronic inflammatory bowel disease. Lymphology. 2001;34:22–9. [PubMed] [Google Scholar]
- 24.Yeung MM, Melgar S, Baranov V, et al. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-gammadelta expression. Gut. 2000;47:215–27. doi: 10.1136/gut.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spahn TW, Herbst H, Rennert PD, et al. Induction of colitis in mice deficient of Peyer's patches and mesenteric lymph nodes is associated with increased disease severity and formation of colonic lymphoid patches. Am J Pathol. 2002;161:2273–82. doi: 10.1016/S0002-9440(10)64503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


