Abstract
Many cellular stresses and inflammatory stimuli can activate p38 mitogen-activated protein kinase (MAPK), a serine/threonine kinase in the MAPK family. The different stimuli act via different receptors or signalling pathways to induce phosphorylation of the cytosolic protein p47phox, one subunit of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Formyl–methionyl–leucyl–phenylalanine (fMLP) has been shown to induce the p38 MAPK phosphorylation during the respiratory burst in human neutrophils. Here, we show that treatment with S(+)-ketamine or R(-)-ketamine at different concentrations (50, 100, 200, 400 µM) reduced fMLP-induced superoxide anion generation and p47phox phosphorylation in neutrophils in a concentration-dependent manner (y = −0·093x + 93·35 for S(+)-ketamine and y = −0·0982x + 95·603 for R(-)-ketamine, respectively). While treatment with 50 µM ketamine inhibited fMLP-induced superoxide generation by 10%, treatment with 400 µM S(+)-ketamine and R(-)-ketamine reduced fMLP-induced superoxide generation to 60·5 ± 8·3% and 60·0 ± 8·5%, respectively, compared with that in neutrophils treated with fMLP alone. Furthermore, treatment with ketamine down-regulated both fMLP-induced p47phox and isoproterenol-induced p38 MAPK phosphorylation and superoxide production. Interestingly, treatment with SB203580, the p38 MAPK inhibitor, also mitigated fMLP-induced superoxide anion generation and p38 MAPK and p47phox phosphorylation as well as apoptosis in a concentration-dependent fashion in neutrophils. Therefore, ketamine racemes inhibited fMLP-induced superoxide anion generation and p47phox phosphorylation by modulating fMLP-mediated p38 MAPK activation in neutrophils.
Keywords: human neutrophils, ketamine, NADPH, p38 MAPK, superoxide generation
Introduction
Polymorphonuclear neutrophils play a critical role in human innate immunity, defending against bacterial infection [1]. Neutrophils can respond to invading bacteria by chemotaxis, adherence and phagocytosis and by producing free radical species, such as superoxide anion (the product of one-electron reduction of molecular oxygen) [2]. Neutrophils can be activated experimentally by formyl–methionyl–leucyl–phenylalanine (fMLP) or phorbol 12-myristate 13-acetate (PMA), and activated neutrophils can generate superoxide anion and express high levels of adhesion molecules such as CD18 and CD11 [3,4]; this provides an excellent model for the study of neutrophil activation and associated pathophysiological responses. While nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a critical regulator of superoxide anion generation and the process known as the ‘respiratory burst’ or ‘oxidative burst’ in neutrophils, little is known about the signal pathways that regulate inducible superoxide anion generation in neutrophils. Reactive oxygen species (ROS) are responsible for clearance of microorganisms and they also contribute to the pathogenesis of sepsis-related organ failure. Hence, elucidation of the molecular mechanisms underlying the regulation of inducible superoxide generation in neutrophils may have great clinical significance.
Ketamine is a safer anaesthetic alternative to phencyclidine and acts as an antagonist for the N-methyl-D-aspartate (NMDA) receptor [5], as well as binding to some opioid receptors. It is a racemic mixture of S(+)-ketamine and R(-)-ketamine. It has been used for anaesthesia induction, particularly in patients with severe Gram-negative bacterial infection, and for the treatment of pain and depression [6,7]. Recent studies have shown that ketamine inhibits fMLP-induced expression of adhesion molecules on neutrophils, adherence of neutrophils and superoxide anion generation in vitro and mitigates endotoxin-induced neutrophil adhesion and the respiratory burst in vivo[8,9]. Szekely et al.[10,11] investigated the post-ischaemic adherence of neutrophils to the coronary system in isolated guinea pig, and found that S(+)-ketamine inhibited neutrophil adherence to the coronary vasculature after ischaemia, whereas R(-)-ketamine increased coronary vascular fluid leak. However, the mechanisms underlying the effects of ketamine racemes on neutrophil's functions are poorly understood.
fMLP can, through its G protein-coupled receptors, activate neutrophils [11] and the p38 MAPK pathways. Importantly, the p38 MAPK pathway is crucial for fMLP-induced activation of NADPH oxidase [12]. We tested our hypothesis that ketamine may, directly or indirectly, down-regulate the p38 MAPK phosphorylation, which in turn inhibits NADPH oxidase activation and superoxide anion generation in human neutrophils.
Materials and methods
Chemicals and reagents
NADPH, ferricytochrome c (cytochrome c), fMLP, superoxide dismutase (SOD) and SB203580 were purchased from Sigma (St Louis, MO, USA). Ketamine was obtained from Maruishi Pharmaceutical (Osaka, Japan). Stock solutions of ketamine were prepared in KRP (Krebs–Ringer phosphate solution, consisting of 136 mmol/l NaCl, 4·7 mM KCl, 1·25 mM CaCl2 and 1·25 mM MgSO4). The monoclonal antibodies against p47phox, p38 MAPK, phosphor-p47phox, phosphor-p38 MAPK, β-actin and the polyclonal antibody of active caspase-3, peroxidase-conjugated rabbit anti-mouse immunoglobulin (Ig)G antibodies and the ECL Western Blotting Detection System were obtained from Wako (Tokyo, Japan). All other chemical reagents used were of analytical grade and were purchased from Nacalai Tesque (Osaka, Japan), except where indicated otherwise.
Isolation of neutrophils
Human blood polymorphonuclear neutrophils were isolated from the peripheral blood of 10 non-smoking healthy subjects aged 30–40 years, as described previously [13,14]. Briefly, peripheral blood (20 ml) was collected from individual subjects into a heparinized tube and subjected to Ficoll-Hypaque density gradient centrifugation. Subsequently, the neutrophils were washed twice with KRP at pH 7·4. Neutrophils with a purity of greater than 95% were used in this study and suspended in KRP at a concentration of 108 cells/ml. All supplies used in this experiment were either autoclaved or purchased already sterile from the manufacturer. Informed consent was obtained from individual subjects and the experimental protocol was approved by the Institute Research Board of our university.
Superoxide generation assay
The superoxide contents of the supernatants were assayed by measuring the reduction of cytochrome c, as reported previously [14,15]. Briefly, neutrophils (2 × 106) in 2 ml KRP buffer containing 1 mM CaCl2, 20 µM cytochrome c and 10 mM glucose were pretreated with or without S(+)-ketamine or R(-)-ketamine at concentrations of 50, 100, 200 or 400 µM for 3 min and then stimulated with 12·5 nM fMLP at 37°C under continual stirring. The resultant changes in absorbance at 550–540 nm were recorded using a dual-beam spectrophotometer for 4 min. The neutrophils treated with fMLP alone were used as positive controls and the amount of superoxide produced in the experimental groups was expressed as a mean % of the positive control.
Western blot assay
Following treatment with ketamine, SB204580 and/or isoproterenol (1 mM) and/or stimulation with fMLP, neutrophils (108 cells/ml) were pretreated with 2 mM diisopropyl fluorophosphates (DFP) for 10 min on ice and washed three times with cold phosphate-buffered saline glucose (PBSG) containing 1·2 mM MgCl2 and 2 mM NaN3, before being suspended in 500 µl relaxation buffer [100 mM KCl, 3 mM NaCl, 3·5 mM MgCl2, and 10 mM piperazine-N-N′-bis (2-ethanesulphonic acid (PIPES), pH 7·3] for 20 min at 4°C. Subsequently, the cells were sonicated and centrifuged at 500 g for 5 min at 4°C. To obtain the membrane fraction, the supernatants were then ultracentrifuged at 200 000 g for 20 min at 4°C [16]. For immunoblot analysis, the membrane fractions were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% gel) and the proteins were transferred onto Immobilon-P membranes using a semidry blotting apparatus for 90 min at 20 mA/cm2. The transferred proteins were probed with monoclonal antibodies against p38 MAPK, p47phox, phosphorylated p38 MAPK or p47phox, active caspase-3 or β-actin, respectively. The bound antibodies were visualized using peroxidase-conjugated rabbit anti-mouse IgG antibodies and the ECL Western Blotting Detection System. For immunodetection of the caspase-3 protein, the membranes were probed with caspase-3 polyclonal antibody, followed by incubation with alkaline phosphatase-conjugated anti-goat secondary antibody. The relative levels of individual protein to the β-actin were determined by densitometric scanning using EPSONGT 8000 and analysed using NIH Image software [16–18]. The lysates of end-binding protein 1 (EB-1) cells treated with or without fMLP were used as positive or negative controls, respectively.
Statistical analysis
The data shown are representative images or expressed as mean % ± standard deviation (s.d.) of the control, and analysed by Student's t-test for comparisons between groups. The relationship between different values was analysed by Spearman's correlation analysis. A value of P < 0·05 was considered statistically significant.
Results
Effect of ketamine on fMLP-induced generation of superoxide anion in human neutrophils
To test the impact of ketamine on neutrophil activation and associated superoxide anion generation, we first examined the effect of ketamine on fMLP-induced superoxide generation in human neutrophils in vitro. We found that treatment with S(+)-ketamine or R(-)-ketamine inhibited fMLP-induced superoxide generation in human neutrophils in a concentration-dependent manner. While treatment of neutrophils with 50 mM ketamine, a clinically relevant dose, inhibited the generation of superoxide anion by 10%, treatment with 400 µM/l S(+)-ketamine or R(-)-ketamine reduced superoxide anion generation to 60·5 ± 8·3% or 60·0 ± 8·5% of the amount produced by positive controls (both P < 0·01, Fig. 1). Analysis of the relationship between the ketamine concentrations and the superoxide anion levels revealed y = −0·093x + 93·35 for S(+)-ketamine (γ = 0·8797), while y = −0·0982x + 95·603 for R(-)-ketamine (γ = 0·903), respectively. Therefore, ketamine inhibited fMLP-induced superoxide anion generation in human neutrophils in a concentration-dependent manner without stereoselective effects.
Fig. 1.
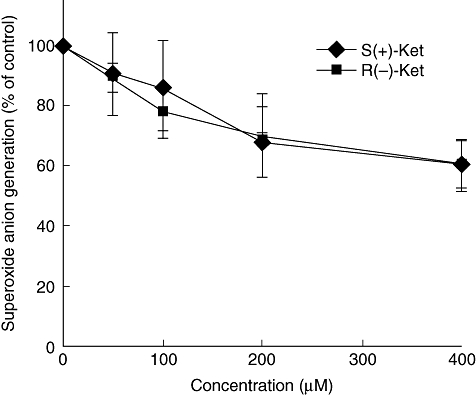
Effect of S(+)-ketamine and R(-)-ketamine on formyl–methionyl–leucyl–phenylalanine (fMLP)-induced superoxide anion generation in human neutrophils. The neutrophils were pretreated with 50, 100, 200 or 400 µM of S(+)-ketamine or R(-)-ketamine for 3 min and then stimulated with 12·5 nM fMLP. The cells without any treatment were used as controls. Very low levels of superoxide anion were detected in control neutrophils. Results are expressed as mean ± standard deviation per group from five independent experiments (n = 5). *P < 0·01 versus the cells treated with fMLP alone.
Effect of ketamine on fMLP-induced phosphorylation of the NADPH oxidase p47phox subunit in neutrophils
Next, we tested whether ketamine could modulate fMLP-induced p47phox phosphorylation of NADPH oxidase in neutrophils. As shown in Fig. 2, while little spontaneous phosphorylation of p47phox was observed in control cells that had not been exposed to fMLP, significantly increased levels of p47phox phosphorylation were detected in fMLP-treated neutrophils. Treatment with 200 mM, but not 50 mM, S(+)-ketamine reduced the levels of phospho-p47phox significantly and treatment with increasing concentrations of S(+)-ketamine enhanced its inhibitory effect. A similar pattern of inhibitory effects of R(-)-ketamine was observed (data not shown). Notably, there was no significant difference in the expression of p47phox among the tested groups of cells. These data indicate that ketamine did not modulate the expression of p47phox, but down-regulated the fMLP-induced activation of neutrophils in our experimental system.
Fig. 2.
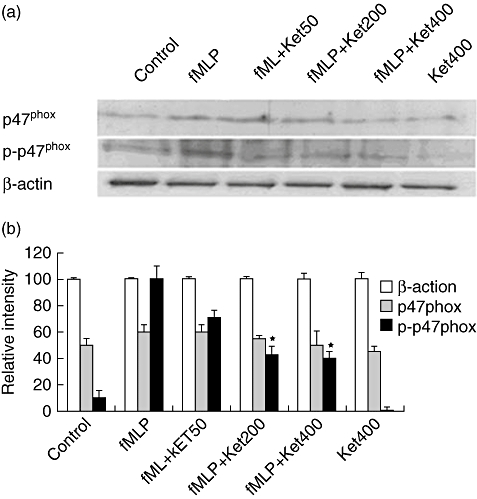
Effect of ketamine on the formyl–methionyl–leucyl–phenylalanine (fMLP)-induced p47phox phosphorylation in neutrophils. Neutrophils from individual subjects were pretreated with S(+)-ketamine or R(-)-ketamine at 50, 200 or 400 µM and exposed to 12·5 nM fMLP. The levels of p47phox phosphorylation were determined by Western blotting assay (a) and the relative levels of p47phox phosphorylation in each group of samples were analysed by densitometric scanning (b). Data shown are representative of each group or expressed as mean ± standard deviation per group from three separated experiments (n = 3). *P < 0·01 versus control cells treated with fMLP alone.
Effect of ketamine on fMLP-induced phosphorylation of p38 MAPK in neutrophils
We further tested our hypothesis that the inhibitory effect of ketamine on the generation of superoxide anion might be mediated by direct or indirect down-regulation of the p38 MAPK phosphorylation by Western blot assays. As shown in Fig. 3a,b, while similar levels of p38 MAPK expression were detected in all groups of the cells, treatment with fMLP elevated levels of phosphorylated p38 MAPK significantly. Interestingly, treatment with 200 mM or 400 mM ketamine mitigated fMLP-induced p38 MAPK phosphorylation significantly. Similarly, treatment with ketamine also blocked p38 MAPK phosphorylation induced by isoproterenol, which is a specific agonist for p38 MAPK (Fig. 3c,d). A similar pattern of inhibitory effect of R(-)-ketamine was observed (data not shown). Hence, ketamine inhibited fMLP-induced and isoproterenol-induced p38 MAPK phosphorylation in neutrophils in vitro.
Fig. 3.
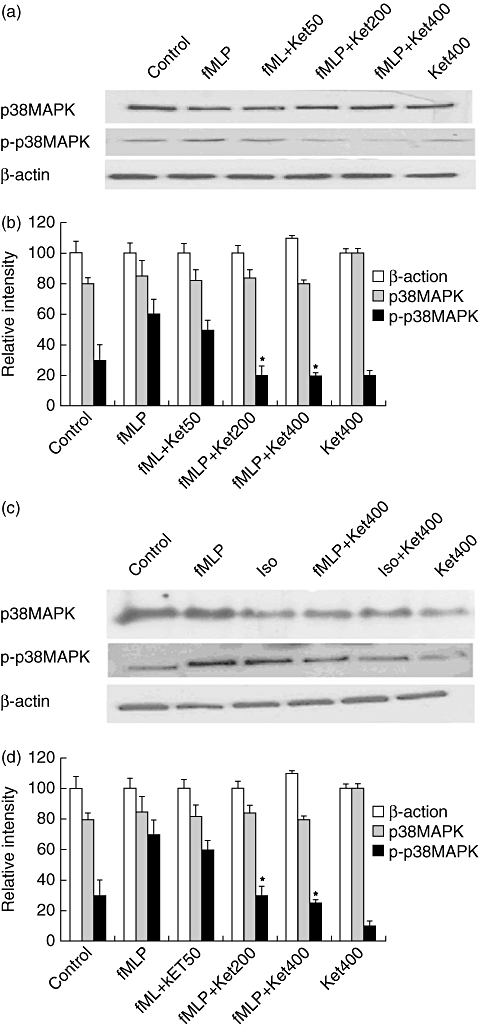
Effect of ketamine on the formyl–methionyl–leucyl–phenylalanine (fMLP)- or isoproterenol-induced p38 MAPK phosphorylation in neutrophils. The neutrophils were pretreated with the indicated concentrations (50, 200 or 400 µM) of S(+)-ketamine or R(-)-ketamine for 3 min and then stimulated with 12·5 nM fMLP. The cells were harvested for analysis of the relative levels of p38 mitogen-activated protein kinase (MAPK) phosphorylation by Western blotting assays (a,c). The levels of p38 MAPK phosphorylation in the cells were determined by densitometric scanning (b,d). A similar pattern of inhibitory effects was observed with R(-)-ketamine (data not shown). Data are representative images or expressed as the means ± standard deviation per group from three independent experiments (n = 3). *P < 0·01 versus the cells treated with fMLP or isoproterenol alone.
Effect of treatment with the p38 MAPK inhibitor on fMLP-induced p38 MAPK and p47phox phosphorylation and active caspase-3 in neutrophils
The p38 MAPK pathway has been shown to be involved in neutrophil-related inflammation; however, little is known about how the activation of p38 MAPK is associated with the phosphorylation of p47phox and superoxide anion generation in neutrophils. We hypothesized that if activation of p38 MAPK is crucial for p47phox phosphorylation and superoxide anion generation, treatment with an inhibitor specific for p38 MAPK, such as SB203580, should have an inhibitory effect. To test this hypothesis, we treated neutrophils with different concentrations of SB203580 prior to determining superoxide anion production induced by fMLP (Fig. 4a).
Fig. 4.
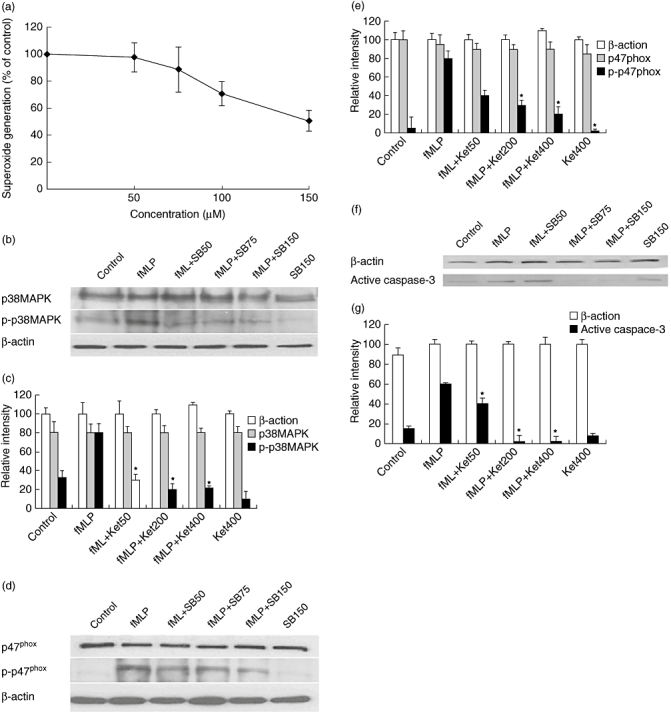
Effect of SB203580 on formyl–methionyl–leucyl–phenylalanine (fMLP)-induced p38 mitogen-activated protein kinase (MAPK) or p47phox phosphorylation and apoptosis in neutrophils. The neutrophils were pretreated with the indicated concentrations (0, 50, 75 or 150 µM) of SB203580 for 3 min and then stimulated with 12·5 nM fMLP. The superoxide anion content of the supernatants was determined (a). The level of p38 MAPK or p47phox phosphorylation and active caspase-3 in the cells was determined by Western blotting assays (b,d,f), followed by densitometric scanning (c,e,g). The superoxide anion is expressed as mean % ± standard deviation (s.d.) of the control for each group (n = 10) from five independent experiments. The levels of p38 MAPK or p47phox phosphorylation in the cells are representative of three separate experiments and are expressed as mean % ± s.d. per group from three experiments (n = 3).*P < 0·01 versus the cells treated with fMLP alone.
Treatment with SB203580 inhibited the generation of fMLP-induced superoxide anion in a dose-dependent fashion. Treatment with 150 mM SB203580 reduced the levels of superoxide anion by 50%. Similarly, treatment with SB203580 reduced the levels of phospho-p38 MAPK and phospho-p47phox in neutrophils and its inhibitory effects were dose-dependent (Fig. 4b–e). The similar inhibitory effects of ketamine and SB203580 suggest that ketamine is likely to inhibit fMLP-induced p38 MAPK activation and down-regulate in turn the phosphorylation of p47phox and the generation of superoxide anion in neutrophils.
Furthermore, we examined the effect of SB203580 on fMLP-induced apoptosis in neutrophils by measuring active caspase-3 using Western blot assays. We found that the relative levels of active caspase-3 in neutrophils treated with 150 mM SB203580 alone were similar to those in unmanipulated control cells. However, the levels of active caspase-3 in neutrophils treated with fMLP and 150 mM SB203580, but not a lower dose, were reduced significantly, compared with those in the cells treated with fMLP alone (Fig. 4f,g). These data indicated that treatment with SB203580 to block the p38MAPK pathway did not affect spontaneous neutrophil apoptosis, but inhibited fMLP-induced neutrophil apoptosis in our experimental model.
Discussion
Neutrophils are an important component of the innate immune response to bacterial infection. Previous studies have shown that ketamine, a commonly used drug in clinical practice, down-regulates stimulus-induced expression of adhesion molecules and inhibits neutrophil activation and associated ROS production [8,9]. In this study, we first tested how S(+)-ketamine and R(-)-ketamine could affect fMLP-induced activation of neutrophils in vitro. We found that both racemes inhibited fMLP-induced generation of superoxide anion in neutrophils in a dose-dependent manner and that their inhibitory effects were undistinguishable, in agreement with a previous report [19]. Given that treatment with a clinical dose of ketamine inhibited fMLP-induced neutrophil activation, the inhibitory effect of ketamine should be clinically achievable and significant.
NADPH oxidase is a crucial regulator of the generation of superoxide anion and the production of the respiratory burst in neutrophils [2]. To understand the molecular mechanism(s) underlying the inhibitory action of ketamine on the activation and function of neutrophils, we determined how treatment with ketamine could affect fMLP-induced phosphorylation of p47phox, a subunit of the NADPH oxidase. We found that treatment with ketamine inhibited fMLP-induced p47phox phosphorylation significantly in neutrophils, and the inhibitory effects of different doses of ketamine were dose-dependent in vitro. These novel findings provide direct evidence that ketamine, directly or indirectly, down-regulates fMLP-induced NADPH oxidase activation in neutrophils. Because the activation of NADPH oxidase is a determinant of superoxide anion generation, the inhibitory effects of ketamine on both fMLP-induced p47phox phosphorylation and superoxide anion generation in neutrophils suggest strongly that ketamine inhibits superoxide anion generation by down-regulating p47phox phosphorylation directly or indirectly.
The p38 MAPK pathway has been associated with the inducible chemotactic activity of neutrophils [19–21] and is crucial for fMLP-induced NADPH oxidase activation in neutrophils [13]. Accordingly, the p38 MAPK pathway may contribute to fMLP-induced superoxide anion generation, a process we have shown to be inhibited by treatment with ketamine. To investigate this possibility, we determined the impact of ketamine on fMLP-induced p38 MAPK phosphorylation in neutrophils and found that ketamine reduced fMLP-induced p38 MAPK phosphorylation significantly in a concentration-dependent manner. These data suggest that the p38 MAPK activation may be crucial for fMLP-induced p47phox phosphorylation, NADPH oxidase activation and superoxide anion generation in neutrophils. Indeed, we found that treatment with ketamine down-regulated the isoproterenol, a p38 MAPK-specific agonist, induced p38 MAPK phosphorylation. These data suggest that ketamine can affect the p38 MAPK activation directly in human neutrophils in vitro. In addition, we found that treatment with SB203580, a specific inhibitor for p38 MAPK, inhibited fMLP-induced p38 MAPK, p47phox phosphorylation and caspase-3 activation in neutrophils.
Our previous study has shown that ketamine can inhibit PMA-induced p47phox phosphorylation and superoxide anion generation in human neutrophils [22]. Given that fMLP has been shown to activate neutrophils through its G-protein receptor-associated signalling, our current data suggest that ketamine may down-regulate the activation of the p38 MAPK pathway directly and/or interfere indirectly with the fMLP-related G-protein receptor-associated signalling, thereby down-regulating the p47phox phosphorylation, mitigating NADPH oxidase activation, and decreasing superoxide anion generation in human neutrophils. However, whether and how ketamine affects fMLP-associated signalling or whether it interferes directly with the p38 MAPK activation in neutrophils remain to be investigated further. Nevertheless, our findings provide new insights into the pharmacological effects of ketamine in humans.
Disclosure
The authors declare no financial or commercial conflicts of interest.
References
- 1.Sjöstedt A, Conlan JW, North RJ. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun. 1994;62:2779–83. doi: 10.1128/iai.62.7.2779-2783.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morel F, Doussiere J, Vignais PV. The superoxide-generating oxidase of phagocytic cells: physiological, molecular and pathological aspects. Eur J Biochem. 1991;201:523–46. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- 3.Tyagi SR, Tamura M, Burnham DN, Lambeth JD. Phorbol myristate acetate (PMA) augments chemoattractant-induced diglyceride generation in human neutrophils but inhibits phosphoinositide hydrolysis. J Biol Chem. 1988;263:13191–8. [PubMed] [Google Scholar]
- 4.Yan SR, Sapru K, Issekutz AC. The CD11/CD18 (β2) integrins modulate neutrophil caspase activation and survival following TNF-α or endotoxin induced transendothelial migration. Immunol Cell Biol. 2004;82:435–6. doi: 10.1111/j.0818-9641.2004.01268.x. [DOI] [PubMed] [Google Scholar]
- 5.Rabben T, Skjelbred P, Øye I. Prolonged analgesic effect of ketamine, an N-methyl-D-aspartate receptor inhibitor, in patients with chronic pain. J Pharmacol Exp Ther. 1999;289:1060–6. [PubMed] [Google Scholar]
- 6.Sarton E, Teppema LJ, Olievier C, et al. The involvement of the µ-opioid receptor in ketamine-induced respiratory depression and antinociception. Anesth Analg. 2001;93:1495–500. doi: 10.1097/00000539-200112000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Julia M, Boris R, Gad S, et al. Involvement of adenosine in the antiinflammatory action of ketamine. Anesthesiology. 2005;102:1174–81. doi: 10.1097/00000542-200506000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Weigand MA, Schmidt H, Zhao Q, Plaschke K, Martin E, Bardenheuer HJ. Ketamine modulates the stimulated adhesion molecule expression on human neutrophils in vitro. Anesth Analg. 2000;90:206–12. doi: 10.1097/00000539-200001000-00041. [DOI] [PubMed] [Google Scholar]
- 9.Zilberstein G, Levy R, Rachinsky M, et al. Ketamine attenuates neutrophil activation after cardiopulmonary bypass. Anesth Analg. 2002;95:531–6. doi: 10.1097/00000539-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Szekely A, Heindl B, Zahler S, Conzen PF, Becker BF. S(+)-ketamine, but not R(-)-ketamine, reduces postischemic adherence of neutrophils in the coronary system of isolated guinea pig hearts. Anesth Analg. 1999;88:1017–24. doi: 10.1097/00000539-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Szekely A, Heindl B, Zahler S, Conzen PF, Becker BF. Nonuniform behavior of intravenous anesthetics on postischemic adhesion of neutrophils in the guinea pig heart. Anesth Analg. 2000;90:1293–300. doi: 10.1097/00000539-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto K, Kuribayashi F, Nakamura M, et al. Involvement of p38 MAP kinase in not only activation of the phagocyte NADPH oxidase induced by formyl–methionyl–leucyl–phenylalanine but also determination of the extent of the activity. J Biochem. 2006;140:739–45. doi: 10.1093/jb/mvj204. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe Y, Sagara Y, Sugahara K, Takeshige K. Iminodipeptides containing proline with C-terminal and N-terminal residues prime the stimulation of human neutrophil superoxide generation by fMLP. Biochem Biophys Res Commun. 1994;205:758–64. doi: 10.1006/bbrc.1994.2730. [DOI] [PubMed] [Google Scholar]
- 15.Lu HW, Sugahara K, Sagara Y, et al. Effect of three flavonoids, 5, 7, 3′, 4′-tetrahydroxy-3-methoxy flavone, luteolin and quercetin, on the stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophil. Arch Biochem Biophys. 2001;393:73–7. doi: 10.1006/abbi.2001.2457. [DOI] [PubMed] [Google Scholar]
- 16.Vauseef WM, Volpp BD, McCormick S, Leidal KG, Clark RA. Assembly of the neutrophils respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J Biol Chem. 1991;266:5911–17. [PubMed] [Google Scholar]
- 17.Wang ZG, Song SJ, Lu HW, et al. Effect of three triterpenoid compounds isolated from root bark of Aralia elata on stimulus-induced superoxide generation and tyrosyl phosphorylation and translocation of p47phox and p67 phox to cell membrane in human neutrophils. Clin Chim Acta. 2003;336:65–72. doi: 10.1016/s0009-8981(03)00326-7. [DOI] [PubMed] [Google Scholar]
- 18.Idvall J, Ahlgren I, Aronsen KR, Stenberg P. Ketamine infusions: pharmacokinetics and clinical effects. Br J Anaesth. 1979;51:1167–73. doi: 10.1093/bja/51.12.1167. [DOI] [PubMed] [Google Scholar]
- 19.Zu YL, Qi J, Gilchrist A, et al. p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNF-alpha or FMLP stimulation. J Immunol. 1998;160:1982–6. [PubMed] [Google Scholar]
- 20.Coffer PJ, Geijsen N, M'Rabet L, et al. Comparison of the roles of mitogen-activated protein kinase and phosphatidylinositol 3-kinase signal transduction in neutrophil effector function. Biochem J. 1998;329:121–30. doi: 10.1042/bj3290121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagata T, Kansha M, Irita K, Takahashi S. Propofol inhibits FMLP-stimulated phosphorylation of p42 mitogen-activated protein kinase and chemotaxis in human neutrophils. Br J Anaesth. 2001;86:853–8. doi: 10.1093/bja/86.6.853. [DOI] [PubMed] [Google Scholar]
- 22.Lu H, Tan W, Wang J. Effect of ketamine on stimulus-induced superoxide generation and intracellular calcium in human neutrophils in vitro. Chin Pharmacol Bull. 2007;23:524–7. [Google Scholar]


