Abstract
Patients with chronic granulomatous disease (CGD), an inherited disorder of phagocytic cells, often contract recurrent life-threatening bacterial and fungal infections. CGD is considered to arise from a functional defect of the O2-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in phagocytes. To determine whether or not NADPH oxidase is crucial to the host defence against Mycobacterium avium, we investigated the response against M. avium using CGD model mice (gp91-phox-) of C57BL/6 strain. A tracheal injection of 1 × 107 colony-forming units (CFU)/head of M. avium strain FN into the CGD mice resulted in a pulmonary infection, while also increasing the mortality rate. In contrast, normal C57BL/6 mice injected with same dose of the organisms did not develop severe pulmonary infection and were able to survive through 2 months of observation. The macrophages obtained from the CGD mice were observed to have a higher burden of the bacterial growth than macrophages from normal C57BL/6 mice. These results suggest that the defect of the NADPH oxidase function impairs the host defence against M. avium infection.
Keywords: animal study, innate immunity, mycobacteriosis, NADPH oxidase, opportunistic infection
Introduction
Mycobacterium avium is a bacterium pathogenic for humans which proliferate intracellularly in phagocytes. The bacterium causes chronic progressive respiratory infection as well as disseminated diseases in immunocompromised hosts, such as human immunodeficiency virus (HIV) patients [1,2]. It has been reported that a clarithromycin-containing regimen demonstrated a 59–92% response rate to the infection [3–5]; however, relapses after medical therapy are common [6,7]. Investigation of the host defence mechanism against M. avium infection could lead to the development of a new remedy against M. avium infection.
Chronic granulomatous disease (CGD), an inherited disorder of phagocytic cells, results from an inability of phagocytes to kill certain types of bacteria, leading to recurrent life-threatening bacterial and fungal infections. CGD is due to a functional defect of the O2-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase of phagocytes. Generally, CGD patients are susceptible to catalase-positive, hydrogen peroxide-negative bacteria [8,9]. Phagocytes from CGD patients showed normal bacterial killing ability against non-catalase-producing bacteria, because phagocytes can generate hypochlorous acid (HOCl) using hydrogen peroxide produced by bacteria in phagozomes. Although M. avium produces catalase, there have so far been few clinical case reports of M. avium infection associated with CGD. The relationship between CGD, dysfunctional NADPH oxidase and mycobacterial infection therefore remains uncertain.
We used a mouse strain developed by Pollock et al. that has been established as a mouse model for an X-linked (gp91-phox-) form of CGD [10]. In this study, we injected M. avium into mice with CGD (CGD mice) and their wild-type C57BL/6 counterparts and then compared their susceptibility to the pathogen. We report herein that NADPH oxidase dysfunction results in an impaired host defence against M. avium infection.
Materials and methods
Bacteria
Strain FN of clinically isolated M. avium from our hospital was used in this study. All strains were grown in Middlebrook 7H9 broth with Middlebrook albumin dextrose catalase (ADC) enrichment (Becton, Dickinson and Company, Sparks, MD, USA) at 37°C with shaking or on Middlebrook 7H10 agar with Middlebrook oleic acid albumin dextrose catalase (OADC) enrichment (Becton, Dickinson and Company) at 37°C for 14 days. The plates were incubated at 37°C in 90% humidity and the colonies were counted after 14 days [11]. The M. avium strains produced catalase, but not hydrogen peroxide, as confirmed by our new agar to detect hydrogen peroxide-producing bacteria based on Prussian blue-forming reaction [12].
Mice
X-linked CGD mice, which were produced by a knock-out of gp91-phox from C57BL/6 mice, were donated by M. C. Dinauer (Riley Children's Hospital, Indianapolis, IN, USA) and A. Kume (Division of Genetic Therapeutics, Center for Molecular Medicine, Jichi Medical School, Tochigi, Japan). These mice were provided with sterile food and water in an environmentally controlled room. Eight-week-old X-linked CGD mice were used in the experiment; C57BL/6 mice of the same age were used as the controls. The mice were given food and water ad libitum throughout the experiments.
Animal model of M. avium infection
A clinical strain FN of M. avium isolated from a patient in our hospital was used in this study. The intratracheal administration of M. avium[1 × 107 colony-forming units (CFU)/head] in 50 µl of sterile saline was performed via a tracheotomy to the mice under anaesthesia. As a control, 50 µl of saline was injected [13]. The survival of the mice was noted daily for 60 days after inoculation. Mice were killed on day 21 after infection. To measure the mycobacterial burden after inoculation, the left lungs of the mice were incised and homogenized using a stainless mesh. Viable counts of mycobacterium in the homogenate were determined by inoculation on Middlebrook 7H10 agar plates to count the number of colonies. After death, the right lungs were fixed with 10% formalin for 24 h and embedded in paraffin. Sections were obtained subsequently and stained with haematoxylin and eosin (H&E) and Ziehl–Neelsen (Z-N) staining.
This study was approved by our Institutional Animal Care and Use Committee.
Macrophage isolation and infection by M. avium
Bacterial proliferation in macrophages was assayed by methods described previously [14,15], with some modifications. Briefly, the peritoneal macrophages were obtained at 3 days after inoculation in 2 ml of 3% thioglycollate medium (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Macrophages were incubated in RPMI-1640 medium with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich Japan, Tokyo, Japan) without antibiotics using 12-well cell culture plates (Becton, Dickinson and Company, Tokyo, Japan). Approximately 1 × 106 cells were incubated per well. M. avium was added to culture medium at multiplicity of infection (MOI) 10. Macrophages were collected and washed twice by phosphate-buffered saline (PBS) and lysed by the addition of sterilized water. The lysed medium was inoculated into Middlebrook 7H10 agar plates and the bacterial number was counted after 2 weeks of incubation at 37°C.
Statistics
Data were expressed as the mean ± standard error (s.e.). The Mann–Whitney test was used to compare the two groups. A value of P < 0·05 was considered to indicate a significant difference. Statistical analyses were performed using a computer program (StatView version 5·0).
Results
To examine the influence of a defect in NADPH oxidase function on the susceptibility of mice to M. avium, we evaluated the differences in mortality rate, the histological features of the lung and bacterial burden in organs between C57BL/6 and CGD mice after intratracheal inoculation of the lung. Inoculation of 1 × 107 CFU of the clinically isolated strain resulted in the death of nearly half the CGD mice. In contrast, all C57BL/6 mice survived for more than 60 days of observation (Fig. 1). The right lungs were tested for lung pathology and the left lungs were tested for bacterial growth. In comparison to the wild-type mice, the lung pathology of the CGD mice demonstrated severe lung damage involving the infiltration of inflammatory cells and a large burden of lymphocytes and macrophages, as well as a small amount of neutrophils. In addition, destruction of the lung structures was observed (Fig. 2a). The wild-type mice demonstrated a small degree of inflammation in a very confined area of development at day 21. Inflammatory cells were mainly lymphocytes. To examine the relationship between lung pathological changes and the bacterial burden in the lungs of C57BL/6 and CGD mice, we determined the number of bacteria in the lung tissue (Fig. 2b). There was a greater number of bacteria in the lungs of the CGD mice in comparison to the lungs of the C57BL/6 mice at 21 days after infection. These results correlated well with the lung histology data. Although a defect in NADPH oxidase function impairs certain aspects of neutrophil activity, the proliferation of M. avium is considered to be related to the macrophage function. We therefore analysed the effect of NADPH oxidase impairment on the anti-mycobacterial activity of macrophages. Peritoneal macrophages from C57BL/6 and CGD mice were infected with M. avium and the intracellular growth of the pathogen in the macrophages was determined after 7 days of infection. There was a greater proliferation of M. avium in macrophages from the CGD mice than in those from the C57BL/6 mice (Fig. 3).
Fig. 1.
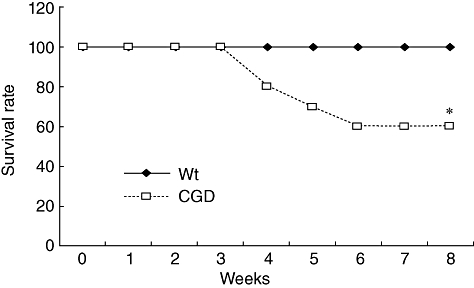
The survival rate of mice infected intratracheally with Mycobacteriun avium. M. avium strain FN was injected intratracheally at dose of 1 × 107 colony-forming units/head. WT: wild-type mice of C57BL/6; CGD: X-linked chronic granulomatous disease (CGD) mice. Each group consisted of 10 mice. *Significant difference (P < 0·05).
Fig. 2.
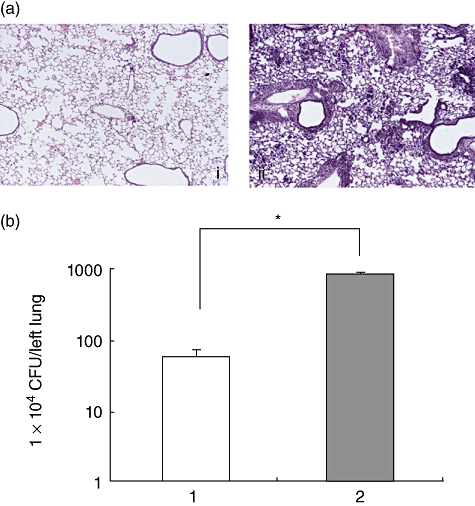
The lung histology of the mice after Mycobacterium avium administration. (a) Lung histology was obtained 21 days after M. avium administration. (i) Wild-type mice that received M. avium; (ii) chronic granulomatous disease (CGD) mice that received M. avium. Note the marked inflammatory cell infiltrate in the lungs from CGD mice. All panels are haematoxylin and eosin-stained and viewed at the same magnification. Original magnification: 40×. We examined five mice in each group and all mice showed similar results. The figure represented the findings for one mouse. (b) Bacterial growth in the lungs was measured at 21 days after M. avium inoculation. Bacterial growth is shown by a log scale. Each group consisted of five mice. *Significant difference (P < 0·05).
Fig. 3.
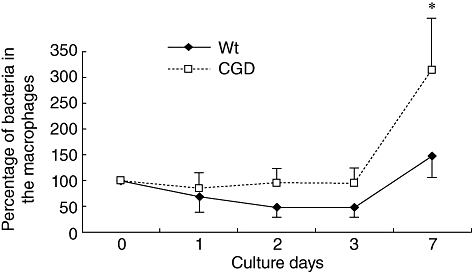
Bacterial growth in the lungs and macrophages. Macrophages were obtained from either chronic granulomatous disease (CGD) mice or wild-type mice and infected with Mycobacterium avium at the dose of multiplicity of infection (MOI) 10. The bacterial growth was quantified and is shown by a log scale. Each group consisted of five mice. *Significant differences (P < 0·05). WT: wild-type mice of C57BL/6; CGD: CGD mice.
Discussion
Human defence mechanisms include both innate and adaptive immunity. Innate immunity involves phagocytosis and intracellular killing by neutrophils and macrophages that act during the early stages of infection, while adaptive immunity involves T cell stimulation, macrophage activation and antibody production [16]. Neutrophils and macrophages kill microorganisms by generating microbicidal reactive oxygen species, such as superoxide anions, hydrogen peroxide, hydroxyl anions and hypochlorous acid [17].
CGD patients have a susceptibility to microorganisms that does not usually cause disease in people with normal immune systems. Bacteria are among the most common organisms that cause disease in CGD patients, particularly catalase-positive, hydrogen peroxide-negative bacteria such as Staphylococcus aureus, Serratia marcescens and Aspergillus species. However, susceptibility to mycobacteria remains controversial. In humans, mycobacterial infection has not been considered to be associated with CGD. For example, CGD neutrophils are as effective as normal neutrophils in destroying M. tuberculosis[18]. Recently, however, Lee et al. documented the cases of 17 patients with CGD living in a region endemic for tuberculosis [19]. In addition, Ohga published a case report of CGD complicated with M. avium[20]. Kusuhara et al. also reported bacillus Calmette–Guérin (BCG) lymphadenitis in CGD patients [21]. These recent clinical reports suggested that the oxidative burst may be an important aspect of host defence against mycobacterial infections. To confirm the role of NADPH oxidase in host defence against M. avium, we performed an experiment using CGD model mice (gp91-phox-) infected with M. avium. We demonstrated that CGD mice (gp91-phox-) are susceptible to M. avium based on mortality, bacterial number, lung histology and proliferation in macrophages. These results suggest that a defect in the NADPH oxidase function causes lethal respiratory insufficiency; therefore, superoxide anion and its metabolites produced by phagocytes play an important role in host protection against M. avium infection.
The M. avium strain used in this study was catalase-positive, hydrogen peroxide-negative. Another clinically isolated strain of M. avium from our university was also catalase-positive, hydrogen peroxide-negative. These strains are presumably virulent to CGD hosts. Segal et al. reported that the histopathology and cytokine expression data were similar between p47phox−/− and wild-type mice, thus indicating that NADPH-derived reactive oxidants probably do not modulate the pulmonary inflammatory response to M. avium[22]. Furthermore, there was no significant difference in the spleen and lung M. avium burden between p47phox−/− and wild-type mice. They concluded that NADPH oxidase-derived oxidants do not alter significantly the host response to M. avium. We do not understand fully the discrepancy between their data and our data. This discrepancy could be due to the difference in mouse strains, bacterial strains or route of infection. Adams et al. injected M. tuberculosis intravenously to CGD mice and observed that M. tuberculosis growth was enhanced markedly in the lungs of X-CGD mice compared with B6 mice, but was controlled in the spleen and liver [23].
In conclusion, our findings suggest that NADPH oxidase plays an important role in the innate immunity against M. avium in mice. In future, the susceptibility to mycobacteria with regard to human subjects should be investigated.
Acknowledgments
We appreciate the assistance of Dr Brian Quinn for editing the English usage.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Medical Section of the American Lung Association. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997;156:S1–25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- 2.Griffith DE, Aksamit T, Brown-Elliott BA, et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 3.Wallace RJ, Jr, Brown BA, Griffith DE, Girard WM, Murphy DT. Clarithromycin regimens for pulmonary Mycobacterium avium complex: the first 50 patients. Am J Respir Crit Care Med. 1996;153:1766–72. doi: 10.1164/ajrccm.153.6.8665032. [DOI] [PubMed] [Google Scholar]
- 4.Griffith DE, Brown BA, Girard WM, Griffith BE, Couch LA, Wallace RJ., Jr Azithromycin-containing regimens for treatment of Mycobacterium avium complex lung disease. Clin Infect Dis. 2001;32:1547–53. doi: 10.1086/320512. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka E, Kimoto T, Tsuyuguchi K, et al. Effect of clarithromycin regimen for Mycobacteriuim avium complex pulmonary disease. Am J Respir Crit Care Med. 1999;160:866–72. doi: 10.1164/ajrccm.160.3.9811086. [DOI] [PubMed] [Google Scholar]
- 6.Corpe RF. Surgical management of pulmonary disease due to Mycobacterium avium-intracellulare. Rev Infect Dis. 1981;3:1064–7. doi: 10.1093/clinids/3.5.1064. [DOI] [PubMed] [Google Scholar]
- 7.Moran JF, Alexander LG, Stauh EW, Young WG, Sealy WC. Long-term results of pulmonary resection for atypical mycobacterial disease. Am Thorac Surg. 1983;35:597–604. doi: 10.1016/s0003-4975(10)61069-7. [DOI] [PubMed] [Google Scholar]
- 8.Lehrer RI. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975;55:338–46. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Washburn RG, Gallin JI, Bennett JE. Oxidative killing of Aspergillus fumigatus proceeds by parallel myeloperoxidase-dependent and -independent pathways. Infect Immun. 1987;55:2088–92. doi: 10.1128/iai.55.9.2088-2092.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollock JD, Williams DA, Gifford MA, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–9. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 11.Fratazzi C, Manjunath N, Arbeit RD, et al. A macrophage invasion mechanism for mycobacteria implicating the extracellular domain of CD43. J Exp Med. 2000;192:183–92. doi: 10.1084/jem.192.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito M, Seki M, Iida K, Nakayama H, Yoshida S. A novel agar medium to detect hydrogen peroxide-producing bacteria based on the prussian blue-forming reaction. Microbiol Immunol. 2007;51:889–92. doi: 10.1111/j.1348-0421.2007.tb03971.x. [DOI] [PubMed] [Google Scholar]
- 13.Fujita M, Ye Q, Ouchi H, et al. Doxycycline attenuated pulmonary fibrosis induced by bleomycin in mice. Antimicrob Agents Chemother. 2006;50:739–43. doi: 10.1128/AAC.50.2.739-743.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida S, Goto Y, Mizuguchi Y, Nomoto K, Skamene E. Genetic control of natural resistance in mouse macrophages regulating intracellular Legionella pneumophila multiplication in vitro. Infect Immun. 1991;59:428–32. doi: 10.1128/iai.59.1.428-432.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita M, Ikegame S, Harada E, et al. TNF receptor 1 and 2 contribute in different ways to resistance to Legionella pneumophila-induced mortality in mice. Cytokine. 2008;44:298–303. doi: 10.1016/j.cyto.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Salyers AA. A molecular approach. In: Whitt DD, editor. Bacterial pathogenesis. 2nd. Washington, DC: ASM Press; 2002. pp. 53–100. [Google Scholar]
- 17.Rossi F, Della Bianca V, de Togni P. Mechanisms and functions of the oxygen radicals producing respiration of phagocytes. Comp Immunol Microbiol Infect Dis. 1985;8:187–204. doi: 10.1016/0147-9571(85)90044-x. [DOI] [PubMed] [Google Scholar]
- 18.Jones GS, Amirault HJ, Andersen BR. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J Infect Dis. 1990;162:700–4. doi: 10.1093/infdis/162.3.700. [DOI] [PubMed] [Google Scholar]
- 19.Lee PP, Chan KW, Jiang L, et al. Susceptibility to mycobacterial infections in children with X-linked chronic granulomatous disease: a review of 17 patients living in a region endemic for tuberculosis. Pediatr Infect Dis J. 2008;27:224–30. doi: 10.1097/INF.0b013e31815b494c. [DOI] [PubMed] [Google Scholar]
- 20.Ohga S, Ikeuchi K, Kadoya R, et al. Intrapulmonary Mycobacterium avium infection as the first manifestation of chronic granulomatous disease. J Infect. 1997;34:147–50. doi: 10.1016/s0163-4453(97)92509-3. [DOI] [PubMed] [Google Scholar]
- 21.Kusuhara K, Ohga S, Hoshina T, et al. Disseminated bacillus Calmette–Guérin lymphadenitis in a patient with gp91phox – chronic granulomatous disease 25 years after vaccination. Eur J Pediatr. 2009;168:745–7. doi: 10.1007/s00431-008-0824-9. [DOI] [PubMed] [Google Scholar]
- 22.Segal BH, Doherty TM, Wynn TA, Cheever AW, Sher A, Holland SM. The p47(phox−/−) mouse model of chronic granulomatous disease has normal granuloma formation and cytokine responses to Mycobacterium avium and Schistosoma mansoni eggs. Infect Immun. 1999;67:1659–65. doi: 10.1128/iai.67.4.1659-1665.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams LB, Dinauer MC, Morgenstern DE, Krahenbuhl JL. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber Lung Dis. 1997;78:237–46. doi: 10.1016/s0962-8479(97)90004-6. [DOI] [PubMed] [Google Scholar]


