Abstract
AIM: To evaluate the results of segmental duodenectomy (SD) and pancreaticoduodenectomy (PD) for duodenal gastrointestinal stromal tumor (GIST) and help clinicians with surgical management.
METHODS: All patients who underwent surgery for non-metastatic GIST of the duodenum in a single institution since 2000 were prospectively followed up. Seven patients (median age 51 years, range: 41-73 years) were enrolled: five underwent SD and two underwent PD.
RESULTS: All the patients had a complete resection (R0), with no postoperative morbidity and mortality. Among the SD group, GIST was classified as low risk in two patients, intermediate risk in two, and high risk in one, according to the Fletcher scale, (vs two high risk patients in the PD group). With a median follow-up of 41 (18-85) mo, disease-free survival (DFS) rates were 100% after SD and 0% after PD (P < 0.05). The median DFS was 13 mo in the PD group.
CONCLUSION: Whenever associated with clear surgical margins, SD is a reliable and curative option for most duodenal GISTs, and is compatible with long-term DFS.
Keywords: Gastrointestinal stromal tumor, Duodenal neoplasms, Segmental duodenectomy, Pancreaticoduodenectomy
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the digestive tract, with an estimated annual incidence between 10 and 20/106 people[1,2]. Although GISTs are encountered all along the digestive tract, the most frequent sites of occurrence are the stomach (50%) and small bowel (30%). Duodenal GISTs are less frequent and account for < 5% of cases, but still represent approximately 30% of primary duodenal tumors[3].
Surgery is still the only curative approach for GIST[4-6], but the optimal surgical procedure for duodenal GIST remains to be established. A number of authors have reported various procedures including pancreaticoduodenectomy (PD), pancreas-sparing duodenectomy, segmental duodenectomy (SD), or wedge local resection, but few have correlated the different options with oncological results[7-16].
Two major tumor characteristics have to be considered for surgical resection of duodenal GIST, which differs from duodenal adenocarcinoma[17]. First, GIST spreads specifically hematogenously and is rarely, if ever, associated with lymphatic invasion, as in other sarcomas[18]. Secondly, GISTs are well encapsulated tumors that rarely have a tendency to local invasion[4]. For these reasons, radical lymphadenectomy or extended resection of adjacent organs should not confer a survival advantage in non-metastatic duodenal GIST[6,19,20].
Therefore, this study was undertaken to audit the oncological results of segmental duodenal resection in comparison with more extensive procedure such as PD for primary non-metastatic duodenal GIST.
MATERIALS AND METHODS
This was a prospective cohort study of all surgical patients treated in our department for primary non-metastatic duodenal GIST from 2000 to 2008. Inclusion criteria were patients presenting with suspicion of non-metastatic duodenal GIST in a single hospital (Figures 1 and 2). Exclusion criteria were a clearly metastatic disease, poor health condition that precluded laparotomy (severe pulmonary disease, non-treatable coagulation abnormality), and patient’s refusal to participate in this study. All patients had complete surgical resection (R0). Seven cases were included. Median age was 51 years (range: 41-73 years).
Figure 1.
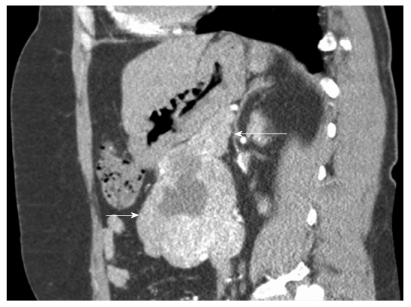
Gastrointestinal stromal tumor (GIST) located in the third part of the duodenum with typical computed tomography (CT) appearance. Note the typical CT appearance of GIST with an area of necrosis (central cavitations with surrounding highly vascular tissue) (white arrow: pancreas; black arrow: GIST).
Figure 2.
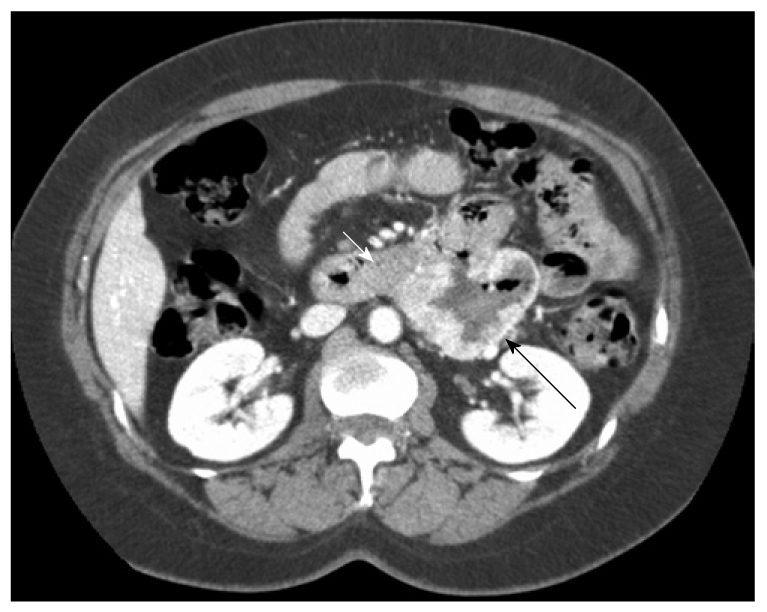
GIST located ion the horizontal duodenum (patient 5, Table 1). Note the close relation between the GIST (short arrow) and the pancreas (long arrow), which was easily dissected during surgery.
Five patients had an SD and two a cephalic PD. PD was the operation chosen for relatively large and difficult-to-reach tumors (first and second part of the duodenum). Among the SD group, one patient had a duodenal patch resection of a small D1 GIST with direct closure (case 3, Table 1), the others had a complete SD with latero-lateral duodeno-jejunal reconstruction (Figure 3).
Table 1.
Patients characteristics and follow-up
| Cases | Localization | Size (cm) | Mitosis/50 HPF | Fletcher grade | Surgery | Follow-up | DFS | Second-line therapy |
| 1 | D2 | 6.5 | 34 | HR | PD | 60 | 13 | Imatinib and hepatectomy |
| 2 | D1 | 7 | 19 | HR | PD | 56 | 12 | Imatinib |
| 3 | D1 | 2.5 | 3 | LR | SD | 85 | 85 | - |
| 4 | D2 | 2 | 2 | LR | Atypical duodenectomy | 40 | 40 | - |
| 5 | D2-3 | 10 | 10 | HR | SD | 37 | 37 | - |
| 6 | D3 | 7 | 2 | IR | SD | 29 | 29 | - |
| 7 | D4 | 5.5 | 5 | IR | SD | 18 | 18 | - |
D1: First part of the duodenum; D2: Second part of the duodenum; D3: Third part of the duodenum; D4: Forth part of the duodenum; HR: High risk; IR: Intermediate risk; LR: Low risk GIST according to Fletcher scale; HPF: High-power field; DFS: Disease free survival.
Figure 3.
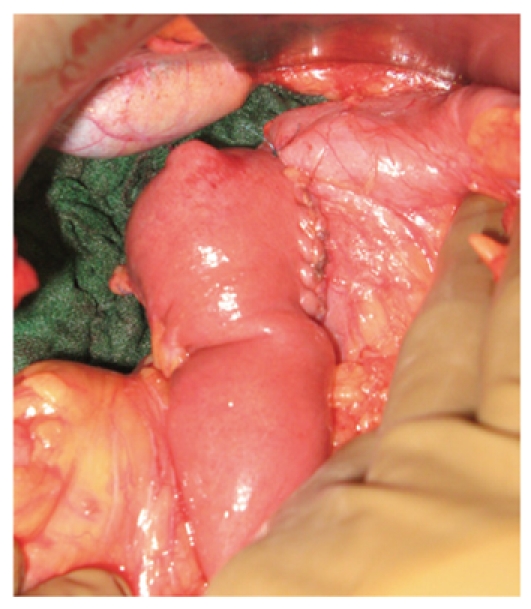
Duodeno-jejunal anastomosis. Latero-lateral duodeno-jejunal anastomosis between the second part of the duodenum and the first jejunal loop was easily performed after distal duodenectomy and Kocher maneuver.
Pathological diagnosis of GIST was confirmed for all according to histological and immunohistochemical work-up. All tumors were c-kit positive. GIST was classified according to the Fletcher scale[21] and our scale[4].
The primary endpoint for this analysis was disease-free survival (DFS), which was defined as time from surgery to GIST recurrence. Follow-up was available for all patients at the date set for collecting data, November 2008. Follow-up was carried out through routine visits at our Outpatient Oncological Clinic. Clinical assessment was made every 3 mo during the first 2 years after surgery and every 6 mo thereafter, with detection of recurrence as soon as possible to allow adjuvant therapy with imatinib. Yearly chest X-rays and abdominal computed tomography (CT) were routinely performed in all patients and additional imaging was requested when clinical suspicion of GIST recurrence occurred. The median follow-up was 41 (18-85) mo.
Statistical analysis
Statistical analysis was performed using GraphPad InStat (GraphPad Software, San Diego, CA, USA). When appropriate, data were analyzed using two-sided Fischer’s test or two-sided t test. P < 0.05 was considered statistically significant.
RESULTS
Study population and pathological data
Seven patients were included in the analysis. Four patients presented with upper digestive tract hemorrhage, the others with abdominal discomfort. In all patients, the diagnosis of duodenal GIST was made through CT after it was suspected by endoscopy in those with digestive bleeding. No preoperative biopsies were performed.
All GISTs were localized without metastases or peritoneal dissemination. According to the Fletcher classification, the GISTs were considered as high risk in the two patients in the PD group, and in the SD group as high risk in one, intermediate risk in two, and low risk in two patients (P > 0.05). According to our classification, the two GISTs in the PD were classified as malignant, as was one of the five GISTs in the SD group (P < 0.05).
Postoperative outcomes
No postoperative morbidity and mortality were recorded. All patients had complete surgical (R0) resection of their duodenal GIST. Five patients underwent SD and two PD (Table 1). GIST-free surgical margins along the duodenum ranged from 0.5 cm to 3 cm.
Follow-up results
The median follow-up was 41 mo (range: 18-85 mo). Median follow-up was 58 mo (range: 56-60 mo) and 37 mo (range: 18-85 mo) for the PD and SD group, respectively. Two patients in the PD group demonstrated recurrence with a median disease-free interval of 13 mo, whereas no recurrence was observed in the SD group (P < 0.05). All patients are alive and disease free in the SD group with a median DFS of 37 mo. The two patients with recurrence after PD presented with liver metastases, which were treated with imatinib mesylate, and one was also treated with partial hepatectomy. A statistically significant difference was detected between the PD and SD group for DFS (P = 0.048), however, this should be balanced by a higher rate of malignant GIST and longer median follow-up in the PD group.
DISCUSSION
The optimal surgical procedure for GISTs of the duodenum remains poorly defined in terms of oncological results. This study was undertaken to compare oncological results of SD and the more radical PD. According to our data, duodenal GIST prognosis is dependent on tumor malignant potential when clear surgical margins can be achieved and not on size of surgical margins or lymphatic dissection. The data presented herein demonstrate that SD is associated with prolonged DFS.
Surgical resection is still the only curative therapy for GIST[1,6,19]. GISTs are known to be resistant to chemotherapy and radiotherapy, and the recently developed molecular targeted therapies (imatinib mesylate and sunitinib), while being highly effective in disease control, are not curative[1,5,6]. The optimal surgical procedure for duodenal GIST remains poorly defined in terms of oncologic results. The reports in the literature addressing surgical procedures for duodenal GIST demonstrate the feasibility of various surgical procedures: PD, pancreas-sparing duodenectomy, SD, or wedge local resection[8,9,11-17,22-25]. These papers can help us a little to determine which surgical procedure is optimal in terms of short- and long-term oncological results. The largest series of duodenal GISTs (n = 156) evaluated prognosis according to tumor grade[3]. In this pathological review, around 60% of patients underwent pancreas-preserving duodenectomy and 11% had PD, but due to the retrospective nature of this analysis, no correlation between type of operation and oncological results were reported. Very recently, Tien et al[26] have reported their experience, in which they compared nine PD with 16 limited operations (11 wedge resections, and five SDs) for duodenal GIST. They have shown that the type of operation is not correlated to operative risk or disease recurrence. They have concluded that limited procedures, like SD, should be attempted for duodenal GIST without involvement of the papilla of Vater. Others have reported similar results[10].
The choice of surgical procedure for duodenal GIST can be guided by the size and exact location of the tumor[6,11,17,25]. However, some principles of GIST surgical treatment have to be considered by a visceral surgeon when approaching duodenal GIST[1,4,6,10,17]. First, GISTs are mesenchymal tumors that behave as other sarcomas and not like adenocarcinomas[4,18]. GIST spreads specifically hematogenously and is rarely, if ever, associated with lymphatic invasion, as in other sarcomas[4,6,18]. Therefore, lymphadenectomy is not recommended[4,11,19]. This seems true for duodenal GIST as local lymph node invasion has never been described, even after PD[3,11-13,17]. Secondly, GISTs are well encapsulated tumors that rarely show a tendency to local invasion even for high risk tumors[4,6,20]. They should be approached with the intention of performing complete en bloc removal (R0 resection) of the tumor and surrounding digestive tract tissue[1,4,6,7,10,19]. The size of surgical margins along the segment of digestive tract involved are not formally defined, however there is little submucosal spread in GIST and clear margins of 1 or 2 cm are recommended[4,6,19,20]. When extracapsular GIST mobilization is possible, there is no need for extensive surgical margins on adjacent organs and peri-tumoral resection with an intact capsule is sufficient[4,6,19,20].
Segmental or atypical duodenectomy for duodenal GIST is in accordance with these principles and could be beneficial for patients because it does not involve the excessive resection and morbidity associated with PD. After complete surgical resection, duodenal GIST prognosis seems not to be influenced by the pancreatic margins, according to the present study and the sparse literature on the subject. Prognosis is mainly dependent on malignant status, which is determined by size and mitotic rate (Fletcher scale). This has been clearly shown for duodenal GIST in the study by Miettinen et al[3] and was true in our small series. However, some authors have advocated the need for PD as pancreatic invasion cannot be ruled out on preoperative studies[17,27]. Although close contact between the GIST capsule and pancreas is usually the rule for large duodenal GISTs on CT (Figures 1 and 2), this is rarely correlated with pancreatic tumoral invasion, which allows treatment with pancreas-sparing duodenectomy[12,28,29]. As a result of these considerations, we think that segmental or atypical duodenectomy is the optimal procedure for duodenal GIST, as previously proposed by others[10-13,15,22-25,30]. The exact type of duodenectomy to perform might be influenced by GIST size and location, ranging from wedge resection with primary closure for small proximal duodenal GIST to SD with duodeno-jejunal anastomosis for large distal duodenal GIST[11,15,30-32]. One exception to this might be periampullary or ampullary GIST, which can present with jaundice, for which pancreas-preserving duodenectomy can be challenging compared to cephalic PD, when the ampulla needs to be resected to obtain clear surgical margins[8,27].
The present study has several limitations. First, it could be argued that a higher rate of malignant GIST is present in the PD group, because in part, PD was chosen for larger and more difficult-to-reach lesions. Furthermore, a longer median follow-up was available for the PD group. These two points could counter-balance the results. Finally, the sample was small, but duodenal GIST remains a rare tumor. Previous studies published in the literature have not reported large numbers of patients, and most of the time, only case reports[8-17,19,22-27,30].
In conclusion, pancreas-preserving segmental or atypical duodenectomy seems to be a reliable and curative option in duodenal GIST. Despite being limited in their extent, these methods of resection, when performed with negative margins, are compatible with long-term DFS, and should be preferred, whenever possible, to PD. This is related to the tumoral characteristics of GIST, which is generally well encapsulated, even when highly malignant and with extremely rare lymphatic spread. When clear surgical margins are achieved, prognosis depends on tumoral malignant potential and not on the extent of the surgical margins, especially the pancreatic margin, for duodenal GIST. However, PD remains a good alternative for tumors in the vicinity of the ampulla of Vater.
COMMENTS
Background
Duodenal gastrointestinal stromal tumors (GISTs) are rare primary duodenal tumors, and there are few guidelines to help the clinician in their surgical management. Surgery is still the only curative approach for GIST, but the optimal procedure remains to be established. Although, numerous authors have reported various surgical procedures, few have correlated their results with oncological outcomes.
Research frontiers
This study was designed to assess the optimal surgical procedure for duodenal GIST, and to compare segmental resection with more extensive pancreaticoduodenectomy (PD).
Innovations and breakthroughs
The authors reported good oncological outcomes with long-term disease-free survival (DFS) in the segmental duodenectomy (SD) group. Thus, whenever associated with clear surgical margins, SD is a reliable and curative option for most duodenal GISTs. However, PD remains a good alternative for tumors in the vicinity of the ampulla of Vater.
Applications
Segmental resection should be preferred, when possible, to more extensive procedures for duodenal GIST. However, for tumors located in the vicinity of the ampulla of Vater, PD remains a good option.
Peer review
The authors evaluated the results of SD (five cases) and PD (two cases) for duodenal GIST. The very low number of patients is certainly a weakness of this study; on the other hand, duodenal GIST is very rare. The authors obtained good DFS following limited resection (SD) with clear margins.
Footnotes
Peer reviewer: Ahmet Tekin, MD, Department of General Surgery, IMC Hospital, Istiklal Cad no:198, Mersin 33100, Turkey
S- Editor Wang JL L- Editor Kerr C E- Editor Zheng XM
References
- 1.Bucher P, Villiger P, Egger JF, Buhler LH, Morel P. Management of gastrointestinal stromal tumors: from diagnosis to treatment. Swiss Med Wkly. 2004;134:145–153. doi: 10.4414/smw.2004.10530. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 3.Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625–641. doi: 10.1097/00000478-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bucher P, Egger JF, Gervaz P, Ris F, Weintraub D, Villiger P, Buhler LH, Morel P. An audit of surgical management of gastrointestinal stromal tumours (GIST) Eur J Surg Oncol. 2006;32:310–314. doi: 10.1016/j.ejso.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466–477. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 6.Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006;244:176–184. doi: 10.1097/01.sla.0000218080.94145.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aparicio T, Boige V, Sabourin JC, Crenn P, Ducreux M, Le Cesne A, Bonvalot S. Prognostic factors after surgery of primary resectable gastrointestinal stromal tumours. Eur J Surg Oncol. 2004;30:1098–1103. doi: 10.1016/j.ejso.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Cavallini M, Cecera A, Ciardi A, Caterino S, Ziparo V. Small periampullary duodenal gastrointestinal stromal tumor treated by local excision: report of a case. Tumori. 2005;91:264–266. doi: 10.1177/030089160509100311. [DOI] [PubMed] [Google Scholar]
- 9.Chiarugi M, Galatioto C, Lippolis P, Zocco G, Seccia M. Gastrointestinal stromal tumour of the duodenum in childhood: a rare case report. BMC Cancer. 2007;7:79. doi: 10.1186/1471-2407-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goh BK, Chow PK, Kesavan S, Yap WM, Wong WK. Outcome after surgical treatment of suspected gastrointestinal stromal tumors involving the duodenum: is limited resection appropriate? J Surg Oncol. 2008;97:388–391. doi: 10.1002/jso.20954. [DOI] [PubMed] [Google Scholar]
- 11.Goh BK, Chow PK, Ong HS, Wong WK. Gastrointestinal stromal tumor involving the second and third portion of the duodenum: treatment by partial duodenectomy and Roux-en-Y duodenojejunostomy. J Surg Oncol. 2005;91:273–275. doi: 10.1002/jso.20311. [DOI] [PubMed] [Google Scholar]
- 12.Kwon SH, Cha HJ, Jung SW, Kim BC, Park JS, Jeong ID, Lee JH, Nah YW, Bang SJ, Shin JW, et al. A gastrointestinal stromal tumor of the duodenum masquerading as a pancreatic head tumor. World J Gastroenterol. 2007;13:3396–3399. doi: 10.3748/wjg.v13.i24.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanuke K, Bathe OF, Mack LA. Local excision of duodenal gastrointestinal stromal tumor. J Surg Oncol. 2007;95:267–269. doi: 10.1002/jso.20618. [DOI] [PubMed] [Google Scholar]
- 14.Stratopoulos C, Soonawalla Z, Piris J, Friend PJ. Hepatopancreatoduodenectomy for metastatic duodenal gastrointestinal stromal tumor. Hepatobiliary Pancreat Dis Int. 2006;5:147–150. [PubMed] [Google Scholar]
- 15.Takeda A, Watanabe Y, Uehara T, Maruyama T, Tanaka H, Matsuzaki H, Arima H, Natsune T, Kudo H, Sakama A, et al. Successful surgical resection of a huge gastrointestinal stromal tumor of the third portion of the duodenum. J Gastroenterol Hepatol. 2007;22:283–284. doi: 10.1111/j.1440-1746.2006.04484.x. [DOI] [PubMed] [Google Scholar]
- 16.Yildirgan MI, Başoglu M, Atamanalp SS, Albayrak Y, Gürsan N, Onbaş O. Duodenal stromal tumor: report of a case. Surg Today. 2007;37:426–429. doi: 10.1007/s00595-004-3400-6. [DOI] [PubMed] [Google Scholar]
- 17.Winfield RD, Hochwald SN, Vogel SB, Hemming AW, Liu C, Cance WG, Grobmyer SR. Presentation and management of gastrointestinal stromal tumors of the duodenum. Am Surg. 2006;72:719–722; discussion 722-723. [PubMed] [Google Scholar]
- 18.Woodall CE, Scoggins CR. Retroperitoneal and visceral sarcomas: issues for the general surgeon. Am Surg. 2007;73:631–635. [PubMed] [Google Scholar]
- 19.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655–664. doi: 10.1016/s1470-2045(02)00899-9. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 22.De Marco G, Roviello F, Marrelli D, De Stefano A, Neri A, Rossi S, Corso G, Rampone B, Nastri G, Pinto E. A clinical case of duodenal gastrointestinal stromal tumor with a peculiarity in the surgical approach. Tumori. 2005;91:261–263. [PubMed] [Google Scholar]
- 23.De Nicola P, Di Bartolomeo N, Francomano F, D'Aulerio A, Innocenti P. Segmental resection of the third and fourth portions of the duodenum after intestinal derotation for a GIST: a case report. Suppl Tumori. 2005;4:S108–S110. [PubMed] [Google Scholar]
- 24.Kurihara N, Kikuchi K, Tanabe M, Kumamoto Y, Tsuyuki A, Fujishiro Y, Otani Y, Kubota T, Kumai K, Kitajima M. Partial resection of the second portion of the duodenum for gastrointestinal stromal tumor after effective transarterial embolization. Int J Clin Oncol. 2005;10:433–437. doi: 10.1007/s10147-005-0503-z. [DOI] [PubMed] [Google Scholar]
- 25.Sun YH, Wang XF, Hou YY, Qin XY. [Clinical characteristics and surgical treatment of 18 cases of duodenal gastrointestinal stromal tumors] Zhonghua Weichang Waike Zazhi. 2007;10:26–28. [PubMed] [Google Scholar]
- 26.Tien YW, Lee CY, Huang CC, Hu RH, Lee PH. Surgery for gastrointestinal stromal tumors of the duodenum. Ann Surg Oncol. 2010;17:109–114. doi: 10.1245/s10434-009-0761-5. [DOI] [PubMed] [Google Scholar]
- 27.Filippou DK, Pashalidis N, Skandalakis P, Rizos S. Malignant gastrointestinal stromal tumor of the ampulla of Vater presenting with obstructive jaundice. J Postgrad Med. 2006;52:204–206. [PubMed] [Google Scholar]
- 28.Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Charnsangavej C. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 29.Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C. Gastrointestinal stromal tumor: role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinib. Radiographics. 2006;26:481–495. doi: 10.1148/rg.262055097. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto Y, Yamamoto J, Takahashi H, Kokudo N, Yamaguchi T, Muto T, Makuuchi M. Segmental resection of the third portion of the duodenum for a gastrointestinal stromal tumor: a case report. Jpn J Clin Oncol. 2003;33:364–366. doi: 10.1093/jjco/hyg063. [DOI] [PubMed] [Google Scholar]
- 31.Nauta RJ. Duodenojejunostomy as an alternative to anastomosis of the small intestine at the ligament of Treitz. Surg Gynecol Obstet. 1990;170:172–174. [PubMed] [Google Scholar]
- 32.Sarmiento JM, Thompson GB, Nagorney DM, Donohue JH, Farnell MB. Pancreas-sparing duodenectomy for duodenal polyposis. Arch Surg. 2002;137:557–562; discussion 562-563. doi: 10.1001/archsurg.137.5.557. [DOI] [PubMed] [Google Scholar]