Abstract
AIM: To assess whether the use of fibrin sealant shortens the closure time of postoperative enterocutaneous fistulas (ECFs).
METHODS: The prospective case-control study included 70 patients with postoperative ECFs with an output of < 500 mL/d, a fistulous tract of > 2 cm and without any local complication. They were divided into study (n = 23) and control groups (n = 47). Esophageal, gastric and colocutaneous fistulas were monitored under endoscopic visualization, which also allowed fibrin glue application directly through the external hole. Outcome variables included closure time, time to resume oral feeding and morbidity related to nutritional support.
RESULTS: There were no differences in mean age, fistula output, and follow-up. Closure-time for all patients of the study group was 12.5 ± 14.2 d and 32.5 ± 17.9 d for the control group (P < 0.001), and morbidity related to nutritional support was 8.6% and 42.5%, respectively (P < 0.01). In patients with colonic fistulas, complete closure occurred 23.5 ± 19.5 d after the first application of fibrin glue, and spontaneous closure was observed after 36.2 ± 22.8 d in the control group (P = 0.36). Recurrences were observed in 2 patients because of residual disease. One patient of each group died during follow-up as a consequence of septic complications related to parenteral nutrition.
CONCLUSION: Closure time was significantly reduced with the use of fibrin sealant, and oral feeding was resumed faster. We suggest the use of fibrin sealant for the management of stable enterocutaneous fistulas.
Keywords: Enterocutaneous fistulas, Fibrin sealant, Spontaneous closure
INTRODUCTION
The formation of enterocutaneous fistulas (ECFs) can be either spontaneous or as a consequence of intra-abdominal surgery. The incidence of spontaneous fistulas is around 15%-25%[1,2]. These can be secondary to Crohn’s disease, malignancy, infectious diseases such as tuberculosis and deep mycosis, diverticulitis, vascular failure, radiation exposure and ischemia of the mesentery[3]. Postoperative fistulas account for 75%-85% out of all fistulas in the digestive tract and they arise from unintentional enterotomy, dehiscence of an anastomosis because of tension in the suture line, a foreign body located close to the anastomosis, inadequate suture techniques, distal obstructions, hematomas, abscess formation at the site of the anastomosis, or tumors[3-5]. Morbidity and mortality associated with postoperative fistulas are substantial as they are highly associated with nutritional deficits, septic complications and concomitant diseases that may appear during prolonged hospital stays[5-7]. The conventional treatment for postoperative fistulas includes intestinal rest, correction of electrolytic disturbances, parenteral nutrition, protection of the skin surrounding the fistula, and treatment and prophylaxis of any related local or systemic septic complications[5,6]. Spontaneous closure of ECFs occurs after 6-8 wk in 60%-70% of the cases after specific medical management[8]. Fistulas that do not close with conservative medical treatment require surgery[2,7].
Biological fibrin glues have long been used extensively in many surgical and speciality fields[9,10]. Fibrin glue contains high concentrations of human fibrinogen and thrombin and it has been widely applied clinically as a biological adhesive system for tissue adhesion or hemostasis[11-13]. Additionally, in comparison with other adhesives, this compound has several advantages in terms of biocompatibility, biodegradation and hemostasis[14-16]. Its use has also been extended for the management of untreatable fistulas that have not responded to conservative treatment[11,12]. The efficacy of fibrin varies depending on a number of features, such as the output volume of the fistula, the location of the fistula, and the presence of a fistulous tract long enough (greater than 2 cm) to allow fixation of the patch[17]. Following the placement of the fibrin patch, it is replaced by collagen after about 4 wk, leading to cessation of drainage and promotion of closure of the fistula[9,16] with avoidance of inflammatory processes, finally resulting in improved healing[18].
The aim of this study was to determine the outcomes and advantages of treating low-output-volume ECFs with the addition of fibrin glue and compare them with conservative management without the use of adjuvant application of fibrin glue into the fistulous tract.
MATERIALS AND METHODS
Over an 8-year period, 70 male and female adults with stable, non-complicated, low-output ECFs were included in this non-randomized prospective case-control study. During the same period, a total of 218 patients were evaluated for postoperative enterocutaneous fistulas. One hundred and forty-eight patients were not considered for the study because of high output volume (> 500 mL/24 h), abdominal infection, fistulous tract < 2 cm, entero-atmospheric fistulas and residual intestinal or peritoneal disease. Patients were divided into 2 groups, a study group (n = 23) in which patients received variable doses of fibrin glue through the external opening of the ECFs, and a control group (n = 47) of patients treated without fibrin glue. Both groups were assessed to compare endpoint variables. Patients belonging to the control group were selected according to the origin of the fistula, using 1 case per 2 controls. Both groups were treated with the same conservative management, known to promote spontaneous closure. Patients with any concomitant condition that impeded spontaneous closure were excluded from the study.
Evaluation of the external fistula and medical treatment
Patients with a fistula output volume of less than 500 mL/24 h for at least 3 consecutive days were chosen to participate in the study. We evaluated the characteristics of the fistulas with contrast fistulography, computed tomography (CT) scans and proximal or distal endoscopy to confirm the absence of any condition that might impede spontaneous closure of the fistula, such as complex tracts, associated abscesses, intra-abdominal sepsis, residual disease, foreign bodies or distal obstruction, and to determine the length of the fistulous tract. Those cases with purulent collections were treated with percutaneous drainage before the application of the fibrin glue. Fistulous tracts measuring less than 2 cm were not included in the study because it was not possible to produce an adequate adhesion area for the patch into the fistulous tract. Pure pancreatic fistulas were not included.
Conservative treatment consisted of fasting, suppression of gastric secretion with oral or intravenous omeprazole or ranitidine, subcutaneous octreotide and nutritional support, either parenteral or enteral according to the anatomic location of the fistula. All patients received skin protection with barriers to prevent burns. The most important endpoint variables were the changes in the output volume, the time required to reduce the output of the fistula to zero for 2 consecutive days, the time required to resume normal oral intake, and fistula reopening. In addition, we studied complications related to the use of nutritional support.
Fibrin glue application
To allow the adhesion of the fibrin glue patch, all fistulous tracts were debrided with an endoscopic brush to produce a smooth surface. The adhesive was composed of fibrinogen at a concentration of 80 mg/mL and 1000 IU/mL of thrombin (both of human extraction) combined with tranexamic acid and calcium chloride (Quixil®; Omrix Biopharmaceuticals Ltd., Tel-Hashomer, Israel). Prior to application, the adhesive was defrosted and components were placed separately in a double-syringe system with distal mixing device. The application of the glue through the external opening of the fistula was controlled endoscopically to assure total occlusion of the internal hole, in those patients with esophageal, gastric and colocutaneous fistulas. Patients with fistulas arising from the duodenum (after gastrectomy and gastrojejunostomy), jejunum and ileum, the control of the glue application was performed with fistulography to establish the distance between the internal and external holes, fistulous tract diameter and estimated volume of fibrin glue to administrate into the fistulous tract. We considered therapeutic failure when the fistula output persisted after the third application of the adhesive without a limit time.
Ethical considerations
This study was conducted according to the declaration of Helsinki of 1989 and Mexican Health Guidelines. The protocol was approved by the Ethical Committee of the Western National Medical Center at the Mexican Institute of Social Security. Full written informed consent was obtained from all patients before their inclusion to this study.
Statistical analysis
Results are described as percentages and central tendency and dispersion measures. Qualitative variables were analyzed using χ2 or Fisher’s exact test, and for quantitative variables the Mann Whitney U test or Student’s t test was used according to the resulting distribution. All P values less than 0.05 were considered statistically significant.
RESULTS
Between January of 2000 and December of 2007, 70 patients were included. The study group consisted of 23 patients (14 male, 9 female) with a mean age of 51.95 ± 11.67 years, and a control group of 47 patients (30 male, 17 female) with a mean age of 51.17 ± 13.78 years. In both groups, all external fistulas were classified according to the anatomical origin (Table 1).
Table 1.
General characteristics of the patients with postoperative enterocutaneous fistulas and final results (mean ± SD)
| Type of fistula | Sex (Male/female) | Age (yr) | Time of evolution (d) | Fistula output (mL/24 h) | Fistula closure time (d) | Restart oral intake (d) |
| Esophago-gastrocutaneous | ||||||
| Study group (n = 3) | 3/0 | 48.6 ± 12.2 | 23.3 ± 7 | 70 ± 42.7 | 8 ± 4 | 12 ± 3.6 |
| Control group (n = 7) | 7/0 | 49.1 ± 10.5 | 18.8 ± 4.4 | 88.1 ± 19 | 19.1 ± 6 | 24 ± 8.6 |
| 1P value | P = 0.85 | P = 0.24 | P = 0.15 | P < 0.05 | P < 0.05 | |
| Gastrocutaneous | ||||||
| Study group (n = 5) | 4/1 | 58.6 ± 9.4 | 93.8 ± 85.1 | 151.4 ± 146.1 | 7 ± 3.1 | 9.8 ± 2.4 |
| Control group (n = 10) | 6/4 | 47.4 ± 16.7 | 86.6 ± 69.8 | 131.4 ± 28.8 | 35.2 ± 18.7 | 39.7 ± 20.1 |
| 1P value | P = 0.19 | P = 0.86 | P = 0.67 | P < 0.01 | P < 0.01 | |
| Duodeno-jejunocutaneuos | ||||||
| Study group (n = 5) | 2/3 | 38.2 ± 10.4 | 25.6 ± 4.9 | 111.6 ± 75.9 | 7.6 ± 2.6 | 11.6 ± 4.3 |
| Control group (n = 10) | 6/4 | 44.1 ± 11.2 | 22.8 ± 4.2 | 123.6 ± 19.9 | 30 ± 17.1 | 34.9 ± 17.2 |
| 1P value | P = 0.34 | P = 0.27 | P = 0.63 | P < 0.01 | P < 0.01 | |
| Ileocutaneous | ||||||
| Study group (n = 6) | 2/4 | 53.1 ± 5 | 28.1 ± 9.6 | 132.8 ± 47.6 | 16.1 ± 21.6 | 18.3 ± 20.6 |
| Control group (n =12) | 6/6 | 55.4 ± 13.1 | 23 ± 4.7 | 143 ± 36 | 37.8 ± 17.2 | 42.5 ± 17.7 |
| 1P value | P = 0.69 | P = 0.13 | P = 0.61 | P < 0.05 | P < 0.05 | |
| Colocutaneous | ||||||
| Study group (n = 4) | 3/1 | 61.5 ± 8.3 | 40.2 ± 17 | 87.7 ± 40.8 | 23.5 ± 19.5 | 25 ± 17.8 |
| Control group (n = 8) | 5/3 | 60.1 ± 11.9 | 33.1 ± 13.2 | 106.1 ± 16.5 | 36.2 ± 22.8 | 40 ± 23.1 |
| 1P value | P = 0.84 | P = 0.44 | P = 0.28 | P = 0.36 | P = 0.25 | |
| All types of enterocutaneous fistulas | ||||||
| Study group (n = 23) | 14/9 | 51.9 ± 11.6 | 43.3 ± 46.3 | 116.2 ± 81.5 | 12.5 ± 14.2 | 15.3 ± 13.3 |
| Control group (n = 70) | 30/17 | 51.1 ± 13.7 | 37.5 ± 40.9 | 121.9 ± 31.5 | 32.5 ± 17.9 | 37.2 ± 18.4 |
| 2P value | P = 0.81 | P = 0.59 | P = 0.74 | P = 0.000 | P = 0.000 |
P value from the Mann Whitney U test;
P value from the Student’s t test.
Esophago-gastrocutaneous fistulas
We included 10 patients with esophago-gastrocutaneous fistulas secondary to esophageal resection of distal carcinoma, caustic burns or high-grade dysplasia associated with Barrett’s metaplasia. In all cases, the esophagus was replaced by gastric transposition through the posterior mediastinum. There were 3 cases in the study group and 7 in the control group. There was no difference in mean age between groups (P = 0.85). The average output of the fistulas was 70 ± 42.7 mL and 88.1 ± 19 mL, respectively (P = 0.15). The fistulous tract measured 3.6 ± 0.36 cm and 3.65 ± 0.48 cm, respectively (P = 0.86). The mean quantity of fibrin glue applied into the external opening was 9 mL (range 7-11 mL). The time to obtain complete closure following the initial application was 8 ± 4 d in the study group. Spontaneous closure was observed after 19.1 ± 6 d of medical treatment in the control group (P < 0.05).
Gastrocutaneous fistulas
Five patients with gastrocutaneous fistulas were included in the study group and 10 in the control group. Gender distribution was similar in both groups and there were no differences in age (P = 0.19). Average fistula output was 151.4 ± 146.1 mL and 131.4 ± 28.8 mL (P = 0.67). The majority originated from endoscopic or surgical gastrostomies. One case in the control group had a fistula that originated from a partial dehiscence of a gastro-jejunostomy. The fistulous tract measured 4.3 ± 0.77 cm and 4.1 ± 0.61 cm (P = 0.76) in study and control groups, respectively. The adhesive application was performed under endoscopic visual control to ensure complete occlusion of the internal hole (Figure 1). The mean quantity of fibrin glue was 18 mL (range 6-30 mL). In the study group, complete closure was observed 7.0 ± 3.1 d after the application. In the control group, spontaneous closure occurred after 35.2 ± 18.7 d (P < 0.01).
Figure 1.
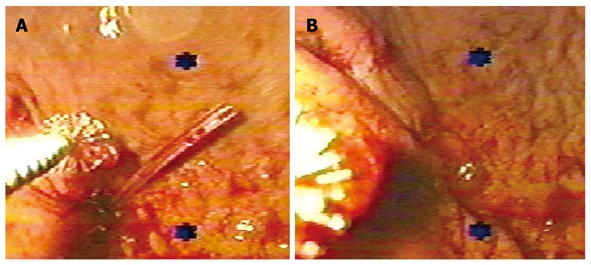
Endoscopic control of the application of fibrin glue in a patient with a gastrocutaneous fistula. A: The sealant was instilled externally; B: For better results, a complete occlusion of the internal hole of the fistulous tract is required.
Duodenum and jejunocutaneous fistulas
Fifteen patients, 8 male and 7 female, were included in this group. One patient in the study group and 2 patients in the control group had pure duodenal fistulas after total or partial gastric resections. The remaining 12 cases had external jejunocutaneous fistulas, 4 in the study group and 8 in the control group, all of which resulted from partial dehiscence of jejunorrhaphy or anastomoses after resection. There were no differences in age between groups (P = 0.34). The mean fistula output was 111.6 ± 75.9 mL in the study group and 123.6 ± 19.9 mL in the control group (P = 0.63). The fistulous tract measured 6.5 ± 0.56 cm and 6.9 ± 0.68 cm (P = 0.26), respectively. The mean quantity of the fibrin glue applied into the external opening was 12.5 mL (range 10-15 mL). Complete closure was observed 7.6 ± 2.6 d after the application of the adhesive in the study group and at 30.0 ± 17.1 d in the control group (P < 0.01).
Ileocutaneous fistulas
Eighteen patients, 8 male and 10 female were included in this group, 6 in the study group and 12 in the control group. All fistulas were secondary to partial dehiscence of end-to-end anastomoses or enterorrhaphy. There was no age difference between the groups (P = 0.69). The average fistula output was 132.8 ± 47.6 mL vs 143.0 ± 36.6 mL in study and control groups, respectively (P = 0.61). The fistulous tract measured 10.4 ± 1.8 cm and 10.6 ± 1.9 cm, respectively (P = 0.85). The mean quantity of fibrin glue applied into the external opening was 14.5 mL (range, 10-19 mL). For the study group, complete closure was observed 16.1 ± 21.6 d after the first application and 37.8 ± 17.2 d in the control group (P < 0.05). In one of the patients the fibrin glue was applied endoscopically because the fistula originated from dehiscence of an ileocolic anastomosis 70 cm from the anal margin.
Colocutaneous fistulas
This group had 12 patients, 8 male and 4 female, 4 in the study group and 8 in the control group. There was no age difference between groups (P = 0.84). The average of the fistula output was 87.7 ± 40.8 mL in the study group and 106.1 ± 16.5 mL in the control group (P = 0.28). The fistulous tract measured 15.4 ± 2.1 cm and 14.1 ± 1.5 cm (P = 0.24). All fistulas were secondary to left colon resection, with manually constructed end-to-end anastomosis or primary closure after trauma. Diagnoses made before the resection were diverticular disease, sigmoid or proximal rectal cancer, and trauma with incidental perforation. The mean quantity of fibrin glue applied into the external opening was 14.5 mL (range 12-26 mL). In the study group, complete closure occurred 23.5 ± 19.5 d after the first application, and spontaneous closure was observed after 36.2 ± 22.8 d in the control group (P = 0.36).
Fistula recurrence, complications related to nutritional support and mortality
During an 18-mo follow-up period, reopening of fistulous tracts was observed in 2 patients with colocutaneous fistulas. Both patients belonged to the study group. One patient suffered incidental perforation of the left colon during a necrosectomy with primary closure. After the reopening of the fistulous tract, endoscopic evaluation showed sigmoid stenosis, and a CT scan demonstrated a retroperitoneal inflammatory process probably related to a previous episode of acute pancreatitis, and after 8 mo he experienced spontaneous closure. The second case was a patient with residual diverticular disease of the left colon that was not identified during the initial evaluation before fibrin glue application. This patient underwent a segmental colon resection with subsequent resolution of the complication.
Morbidity related to nutritional support was significantly different between groups. In the study group, there was one minor and one major complication (8.6%). One patient required a change of jejunostomy tube because of obstruction, and the other patient, who had short bowel syndrome and was receiving long-term total parenteral nutrition, developed septic shock secondary to Candida albicans infection 11 mo after the resolution of the fistulous complication. This patient eventually died of multiple organ failure. In the control group, morbidity was observed in 20 patients (42.5%). This difference was statistically significant (P < 0.01). Minor complications such as tube obstruction, enteral nutrition-associated diarrhea, and central venous catheter obstruction were observed in 8 cases (17%). The remaining 12 patients developed major complications, such as fever related to a central venous catheter in 11 cases: 4 with negative cultures which were resolved with a new access; 7 patients with positive cultures, 6 with Gram-positive cocci (Staphylococcus aureus and Streptococcus epidermidis); and the remaining patient died of septic complications caused by a systemic C. albicans infection. There were 2 additional deaths during the follow-up from non-related causes, including one patient from each group (acute myocardial infarction and diffuse abdominal carcinomatosis). There were no complications related to the application of the fibrin glue. Finally, when we considered all patients stratified only by the interventional maneuver, we observed highly significant results. The time to achieve total fistula closure was 12.5 ± 14.2 d in the study group (fibrin glue) and 32.5 ± 17.9 d in the control group (P < 0.001).
DISCUSSION
Fibrin sealant, also referred to as fibrin glue or fibrin tissue adhesive, is a surgical hemostatic agent derived from plasma coagulation proteins[19]. Fibrin sealants are widely used for many surgical procedures in all fields of surgery[20]. They can be used for hemostasis, wound closure, and tissue sealing, and in contrast to synthetic adhesives, they have the advantage of being biocompatible and biodegradable, and they are not associated with inflammatory processes, foreign body reactions, tissue necrosis, or extensive fibrosis. With normal wound healing, fibrin absorption occurs within days to weeks of application, depending on the type of surgery, the proteolytic activity at the treated site, and the amount of sealant used[21].
Commercial concentrates containing human fibrinogen, factor XIII and bovine thrombin became available in Europe in the late 1970s and have been extensively used since then for hemostasis and other indications[22]. All these products contained antifibrinolytic agents. However, when first introduced, fibrin sealants were excluded from use in the United States based on the risk of transmission of infection[21,22]. The risk of viral transmission was reduced through careful donor selection followed by heat treatment of the human fibrinogen component. Virally inactivated human thrombin has replaced bovine thrombin in most European products[23]. A number of researchers have described the postoperative development of antibodies to bovine thrombin in some patients[24].
The first new generation fibrin sealants used in the United States, with removed or inactivated viruses, was Tisseel (Baxter Healthcare), which was approved by the US Food and Drug Administration (FDA) in 1998[25]. Tisseel was used in the majority of the studies performed to assess the use of this adhesive. In the present study, we used a fibrin sealant known as a second-generation sealant, which received FDA approval in March 2003 and is known as Crosseal (American Red Cross)[26]. Both Tisseel and Quixil are composed of human fibrinogen and human thrombin, which are combined at the time of use. The respective formula differs from each other because Tisseel contains bovine aprotinin (BA), while Quixil contains tranexamic acid, both known as antifibrinolytic agents[26].
Adverse events associated with the use of fibrin sealant over the least 25 years have been inflammatory processes, allergic reactions and viral infections secondary to the bovine component[27].
Hino et al[28] recently reported 3 cases of iatrogenic parvovirus B19 infection after the use of a fibrin sealant. This viral transmission was attributed to the use of dry-heat viral inactivation, which is not effective against non-enveloped viruses. Thus far, there are no reports of human immunodeficiency virus seroconversion or hepatitis B or C infection after the use of fibrin sealant[29]. However, the risk of virus transmission by fibrin sealants is still a subject of debate[19]. Three cases of anaphylaxis have been reported in Japanese patients, one of which was linked to the bovine aprotinin component of the sealant[30].
Fibrin adhesives have been used for several decades to close or obliterate fistulous tracts[31] and their effectiveness varies depending on the output volume of the fistula, its location, and the presence of a sufficiently long fistulous tract (greater than 2 cm) to allow fixation of the patch[32]. Postoperative fistulas of the digestive tract are relatively frequent in surgical practice. Serious technical difficulties such as variability in the location of the fistulas, quantification of drainage, time of evolution of the fistula, patient status and underlying pathology, have hindered scientific research comparing various therapeutic options and treatments[32]. This is one of the reasons why there are just a few randomized controlled clinical trials with the use of fibrin sealants[19].
In 1996, Hwang et al[32], published a small randomized clinical trial in patients with very low-output enterocutaneous fistulas (less than 20 mL). They used fibrin glue extracted from a blood bank and activated with bovine thrombin. The anatomic origin of the fistulas was varied and included upper and lower digestive tract fistulas. In 6 patients treated with external application of the adhesive, the time needed to close the fistulous tract was reduced significantly (2 ± 0.4 d), as was the hospital stay. Control patients (n = 7) received conventional treatment with total parenteral nutrition. In this group, spontaneous closure was observed after 13 ± 2 d (P < 0.01). The authors concluded that the use of fibrin glue is safe and effective for patients with stable and low-output enterocutaneous fistulas.
Waag et al[33] performed the first endoscopic application of an adhesive in 1979, for the treatment of a tracheo-esophageal fistula. Subsequently, with the introduction of different types of biological tissue adhesives and the development of dual and triple lumen catheters in 1984, fibrin glues have been used in the endoscopic treatment of fistulas of the digestive tract[34]. However, only proximal and distal digestive tract fistulas are accessible for endoscopic evaluation and management. In 1990, Nakagawa et al[35] reported the use of a small-diameter endoscope for the evaluation and treatment of fistulous tracts with fibrin glue. Fifteen patients were included in this study, and they were submitted to endoscopic examination, the clinical significance of which was assessed, and it was concluded that fistuloscopy is a safe and easy technique that results in less stress for the patients and is considered to be effective for the examination and treatment of fistulas[35].
Lange et al[36] investigated whether the endoscopic procedure could be used as an adjuvant technique for the sealing of gastrointestinal fistulas in 17 enterocutaneous fistulas. The success rate was 64.5%, and they described complementary treatment for abscesses associated with the fistulous tract. Some patients developed complications because of the high pressure of the fibrin glue application, and one patient died of pulmonary air embolism[36].
As reported by Shand et al[37] in 1997, some adjuvants have been purported to favor the early closure of fistulous tracts. They described a case of a patient with a non-healing low-output gastrocutaneous fistula closed by endoscopic means with fibrin glue and surgical packs. The fistula reopened 3 d after the procedure, and closure of the fistula was reattempted by direct endoscopic application of surgical packs and adhesive. Complete closure was obtained 11 d after direct endoscopic application of the fibrin glue[37].
Recently, Lomis et al[38] performed a study in which they evaluated 7 patients with persistent fistula, treating them with collagen plugs. Under fluoroscopic guidance and using direct-catheter techniques, collagen plugs were applied into the fistulas. The success rate was 85.7%; 6 of the 7 patients had resolution of the fistula with no evidence of fistula recurrence 30-180 d after the closure.
In 1990, Eleftheriadis et al[39] reported 7 cases of proximal digestive fistulas with high-volume output (700 mL in 24 h minimum) that were closed with fibrin gel by endoscopic means, with a 100% success rate. In 2002, the same authors reported a study in 14 patients; 7 with low-volume-output fistulas (discharging 20-50 mL/d) and 7 with high-volume-output fistulas (200-1000 mL/d), using fistuloscopy to evaluate the fistulous tracts and to measure the length of the fistula, with removal of necrotic material and non-absorbable sutures. Fibrin sealant was applied under direct observation. The fistulas healed within 2-17 d (mean 9.2 ± 5.1 d) in all patients except one with peritoneal carcinomatosis. The authors concluded that fistuloscopy could be used as both a diagnostic and a therapeutic tool in low- and high-output postoperative fistulas resistant to conservative treatment[40]. More recently, in 2004, the same author reported the endoscopic application of fibrin sealant for the treatment of gastrocutaneous fistula after bariatric surgery in three morbidly obese patients[41].
Kurokawa et al[42], reported an 85% success rate after the selective occlusion of complex digestive fistulas with fibrin glue application under fistuloscopy in patients in whom fistula closure had not occurred after 3 wk of conservative postoperative treatment.
Ramón Rábago et al[43], recently reported the largest series of postoperative digestive fistulas treated with transendoscopic application of fibrin glue. They included 30 patients with proximal, distal and internal fistulas of low- and high-output. The medium time of conservative treatment was 95 ± 199 d for all types of digestive fistulas. The success rate was 80% and 25% respectively and 55% for internal fistulas. They recommended that conservative treatment should not be prolonged beyond 2-4 wk, since most postoperative fistulas required 14 d to stabilize, and that endoscopic treatment should be performed at that stage. After follow-up periods ranging from 6 mo to more than 6 years, only one of the sealed fistulas reopened requiring a new endoscopic resealing. Rabago and his colleagues concluded that endoscopic treatment achieves a very high success rate, without complications and at a lower cost[43].
The first Mexican experience with fibrin sealants was published in 1997 by Justo-Janeiro et al[44]. They evaluated the application of concentrated human fibrinogen activated with bovine thrombin, fundamentally for hemostatic purposes, protection of high-risk anastomosis, seroma prevention and closure of enterocutaneous fistulas (4 patients). The success rate in the early closure of fistulous tracts was limited (50%), and failures were a consequence of distal obstruction to the fistula origin and intense inflammatory reactions[44].
Since 1998 commercially-prepared fibrin glue became available in our country and in 1999 our group designed protocols to evaluate the efficacy of the fibrin sealant in different surgical indications, including general surgery. In this study, we used human fibrin glue exclusively in low-output digestive fistulas, and we made comparisons with patients who received the same medical treatment but without the use of the adhesive. Our results are relevant as the closure times achieved were significantly reduced, and the time needed to resume oral intake was also decreased. Application through the external opening is very useful when the characteristics of the fistulous tract are well known. Recently, Murakami et al[45], demonstrated adequate closure of complex postoperative digestive fistulas identifying the characteristics of the fistulous tract with an injection of contrast medium through the drainage tube. Eighteen patients were included in the study, with an 88% success rate (16 patients) with the applications of 1 to 9 treatment sessions of diluted solution of thrombin and fibrinogen 80 mg/mL. They studied the effect of thrombin dilution in saline solution and found the optimal concentration at 8 mg/mL of thrombin with a coagulation time of 45 ± 5 s. The delayed coagulation time permitted injection of the glue through all the complex fistulous tracts reaching a high success rate despite 60% of patients having abscesses associated with the fistulous tracts. The treatment was offered between 31 and 168 (66 ± 40.4) d after the surgical procedures.
In the series presented here, endoscopic control of the applications was performed in 13 cases with proximal or distal fistulas. The persistence of inflammation and inadvertent diverticular disease induced the recurrence of 2 colonic fistulas which eventually resolved with medical and surgical treatment. We attribute the success rate, even without performing a fistuloscopy or transendoscopic application, to the meticulous instillation of the fibrin glue, the low-volume output of the enterocutaneous fistulas, and the absence of complex fistulous tracts or any other condition that impeded spontaneous closure of the fistulas, such as associated abscesses, intra-abdominal sepsis, residual disease and foreign bodies or distal obstruction. Morbidity related to nutritional support was higher in the control group due to the prolonged time to feeding, and mortality was secondary to catheter-related sepsis in chronic parenteral nutrition. Since accelerated closure was not the purpose of this study, we proposed to treat patients as soon as the fistulas became stable and low-output without any associated obstruction, infection or residual disease, as other authors suggested in treatment of this complication[32,43,45].
In summary, we recommend the use of fibrin glue in those patients who do not experience spontaneous closure, to reduce the time needed to complete the resolution of the fistula and to minimize the rate of complications related to the secretions of the fistulas. The sealant should be applied as soon as the fistula becomes stable with the lowest output to assure closure after one or more applications of the glue.
Endoscopic control of the fibrin glue application should be performed whenever possible to ensure complete occlusion of the internal hole. The technique employed in this study has the advantage of allowing the removal of necrotic tissue and non-absorbable sutures from the fistulous tracts. Radiological visualization of the fistulous tracts, as studied here, requires less technological support, and we successfully treated digestive fistulas originating from the small bowel, including the duodenum, with external application of the adhesive. Patients did not present any adverse reactions, and recurrences were attributed to residual or untreated disease, particularly in those patients with colonic fistulas.
COMMENTS
Background
The formation of enterocutaneous fistulas can be either spontaneous or as a consequence of intra-abdominal surgery. The incidence of spontaneous fistulas is around 15%-25%, and postoperative fistulas account for 75%-85% of all fistulas of the digestive tract. Morbidity and mortality associated with postoperative fistulas are substantial as they are highly associated with nutritional deficits, septic complications and concomitant diseases that may appear during prolonged hospital stays. The conventional treatment for postoperative fistulas includes intestinal rest, correction of electrolytic disturbances, parenteral nutrition, and protection of the skin surrounding the fistula, and treatment and prophylaxis of any related local or systemic septic complications. Spontaneous closure occurs after 6-8 wk in 60%-70% of cases after specific medical management.
Research frontiers
Biological fibrin glues have long been used extensively in many surgical and specialty fields. Its use has also been extended for the management of untreatable fistulas that have not responded to conservative therapy. The efficacy of fibrin varies depending on the features of the fistula, such as output volume, location, and the presence of a fistulous tract long enough (> 2 cm) to allow fixation of the patch. Following the placement of the fibrin patch, it is replaced by collagen, leading to cessation of drainage and closure of the fistula with avoidance of inflammatory processes, finally resulting in improved healing.
Innovations and breakthroughs
This study determined that the application of fibrin glue through the external opening of stable enterocutaneous fistula reduced the closure time and the morbidity associated with nutritional support.
Applications
Instillation of variable quantities of fibrin glue (6 to 30 mL) according to the fistulous tract in one to 3 treatment sessions. The application of the glue was controlled endoscopically to assure total occlusion of the internal hole in proximal and distal fistulas.
Peer review
This is a good paper which targets a difficult and important topic.
Footnotes
Supported by A Grant from the National Council of Science and Technology, No. Conacyt-SiMorelos 2000-0302003
Peer reviewer: Vamsi R Velchuru, MRCS, FRCSEd, FRCS (Gen Surg), James Paget University Hospital, Great Yarmouth, 6 Pickwick Drive, Off Market Lane, Blundeston, NR32 5BX, United Kingdom
S- Editor Wang YR L- Editor Cant MR E- Editor Ma WH
References
- 1.Berry SM, Fischer JE. Classification and pathophysiology of enterocutaneous fistulas. Surg Clin North Am. 1996;76:1009–1018. doi: 10.1016/s0039-6109(05)70495-3. [DOI] [PubMed] [Google Scholar]
- 2.Arenas-Marquez H, Anaya-Prado R, Hurtado H, Juarez F, Fernandez J, Galindo-Mendoza L, Terrazas-Espitia F, Aiello V, Mondragón R, Gudiño-Lever I, et al. Mexican consensus on the integral management of digestive tract fistulas. Ixtapa-Zihuatanejo, Mexico, August 21-23, 1997. Nutrition. 1999;15:235–238. doi: 10.1016/s0899-9007(98)00193-2. [DOI] [PubMed] [Google Scholar]
- 3.Metcalf C. Enterocutaneous fistulae. J Wound Care. 1999;8:141–142. doi: 10.12968/jowc.1999.8.3.25854. [DOI] [PubMed] [Google Scholar]
- 4.Berry SM, Fischer JE. Enterocutaneous fistulas. Curr Probl Surg. 1994;31:469–566. doi: 10.1016/0011-3840(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 5.Evenson AR, Fischer JE. Current management of enterocutaneous fistula. J Gastrointest Surg. 2006;10:455–464. doi: 10.1016/j.gassur.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre PB, Ritchie JK, Hawley PR, Bartram CI, Lennard-Jones JE. Management of enterocutaneous fistulas: a review of 132 cases. Br J Surg. 1984;71:293–296. doi: 10.1002/bjs.1800710416. [DOI] [PubMed] [Google Scholar]
- 7.Rubelowsky J, Machiedo GW. Reoperative versus conservative management for gastrointestinal fistulas. Surg Clin North Am. 1991;71:147–157. doi: 10.1016/s0039-6109(16)45339-9. [DOI] [PubMed] [Google Scholar]
- 8.Dorta G. Role of octreotide and somatostatin in the treatment of intestinal fistulae. Digestion. 1999;60 Suppl 2:53–56. doi: 10.1159/000051481. [DOI] [PubMed] [Google Scholar]
- 9.Jung M, Manegold BC, Brands W. Endoscopic therapy of gastrointestinal fistulae with fibrin tissue sealant. In: Waclawiczek HW, editor. Progress in fibrin sealing. Berlin: Springer Verlag; 1989. p. 43–52. [Google Scholar]
- 10.Redl H, Schlag G. Properties of different tissue sealants with special emphasis on fibrinogen-based preparations. Fibrin sealant in operative medicine, otohinolaryngology. Vol 1. Berlin, Heidelberg: Springer-Verlag; 1986. pp. 27–38. [Google Scholar]
- 11.Venkatesh KS, Ramanujam P. Fibrin glue application in the treatment of recurrent anorectal fistulas. Dis Colon Rectum. 1999;42:1136–1139. doi: 10.1007/BF02238564. [DOI] [PubMed] [Google Scholar]
- 12.Willetts IE, Dudley NE, Tam PK. Endoscopic treatment of recurrent tracheo-oesophageal fistulae: long-term results. Pediatr Surg Int. 1998;13:256–258. doi: 10.1007/s003830050310. [DOI] [PubMed] [Google Scholar]
- 13.Jessen C, Sharma P. Use of fibrin glue in thoracic surgery. Ann Thorac Surg. 1985;39:521–524. doi: 10.1016/s0003-4975(10)61991-1. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy PM. Fibrin glue in cardiothoracic surgery. Transfus Med Rev. 1993;7:173–179. doi: 10.1016/s0887-7963(93)70136-4. [DOI] [PubMed] [Google Scholar]
- 15.Stricker RB, Lane PK, Leffert JD, Rodgers GM, Shuman MA, Corash L. Development of antithrombin antibodies following surgery in patients with prosthetic cardiac valves. Blood. 1988;72:1375–1380. [PubMed] [Google Scholar]
- 16.Schlag G, Redl H. Fibrin sealant: Efficacy, quality and safety. In Waclawiczek HW, editor. Progress in fibrin sealant. Heidelberg: Springer; 1989. pp. 3–17. [Google Scholar]
- 17.Lange V, Maiwald G, Souvatzi T, Meyer G. Endoscopic approaches for occlusion of fistulas. En Schlang G, Wayand W, editors. Fibrin sealing in surgical and nonsurgical fields: endoscopy. Berlin: Springer; 1995. pp. 58–64. [Google Scholar]
- 18.Sheppard BB, De Virgilio C, Bleiweis M, Milliken JC, Robertson JM. Inhibition of intra-abdominal adhesions: fibrin glue in a long term model. Am Surg. 1993;59:786–790. [PubMed] [Google Scholar]
- 19.Jackson MR. Fibrin sealants in surgical practice: An overview. Am J Surg. 2001;182:1S–7S. doi: 10.1016/s0002-9610(01)00770-x. [DOI] [PubMed] [Google Scholar]
- 20.Spotnitz WD. Commercial fibrin sealants in surgical care. Am J Surg. 2001;182:8S–14S. doi: 10.1016/s0002-9610(01)00771-1. [DOI] [PubMed] [Google Scholar]
- 21.Reece TB, Maxey TS, Kron IL. A prospectus on tissue adhesives. Am J Surg. 2001;182:40S–44S. doi: 10.1016/s0002-9610(01)00742-5. [DOI] [PubMed] [Google Scholar]
- 22.Martinowitz U, Saltz R. Fibrin sealant. Curr Opin Hematol. 1996;3:395–402. doi: 10.1097/00062752-199603050-00011. [DOI] [PubMed] [Google Scholar]
- 23.Jackson MR, MacPhee MJ, Drohan WN, Alving BM. Fibrin sealant: current and potential clinical applications. Blood Coagul Fibrinolysis. 1996;7:737–746. [PubMed] [Google Scholar]
- 24.Stricker RB, Lane PK, Leffert JD, Rodgers GM, Shuman MA, Corash L. Development of antithrombin antibodies following surgery in patients with prosthetic cardiac valves. Blood. 1988;72:1375–1380. [PubMed] [Google Scholar]
- 25.Tisseel VH. [package insert] Glendale, Calif: Baxter Healthcare Corp; 2000. [Google Scholar]
- 26.Crosseal [package insert]. Washington, DC: American Red Cross, 2003 [Google Scholar]
- 27.Albala DM, Lawson JH. Recent clinical and investigational applications of fibrin sealant in selected surgical specialties. J Am Coll Surg. 2006;202:685–697. doi: 10.1016/j.jamcollsurg.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Hino M, Ishiko O, Honda KI, Yamane T, Ohta K, Takubo T, Tatsumi N. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br J Haematol. 2000;108:194–195. doi: 10.1046/j.1365-2141.2000.01818.x. [DOI] [PubMed] [Google Scholar]
- 29.Spotnitz WD, Prabhu R. Fibrin sealant tissue adhesive--review and update. J Long Term Eff Med Implants. 2005;15:245–270. doi: 10.1615/jlongtermeffmedimplants.v15.i3.20. [DOI] [PubMed] [Google Scholar]
- 30.Mitsuhata H, Horiguchi Y, Saitoh J, Saitoh K, Fukuda H, Hirabayasi Y, Togashi H, Shimizu R. An anaphylactic reaction to topical fibrin glue. Anesthesiology. 1994;81:1074–1077. doi: 10.1097/00000542-199410000-00034. [DOI] [PubMed] [Google Scholar]
- 31.Hedelin H, Nilson AE, Teger-Nilsson AC, Thorsen G. Fibrin occlusion of fistulas postoperatively. Surg Gynecol Obstet. 1982;154:366–368. [PubMed] [Google Scholar]
- 32.Hwang TL, Chen MF. Randomized trial of fibrin tissue glue for low output enterocutaneous fistula. Br J Surg. 1996;83:112. doi: 10.1002/bjs.1800830135. [DOI] [PubMed] [Google Scholar]
- 33.Waag KL, Joppich I, Manegold BC. Endoscopic closure of tracheoesophageal fistula. Z Kinderchir. 1979;27(supl):93–95. [Google Scholar]
- 34.Groitl H, Scheele J. Initial experience with the endoscopic application of fibrin tissue adhesive in the upper gastrointestinal tract. Surg Endosc. 1987;1:93–97. doi: 10.1007/BF00312692. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa K, Momono S, Sasaki Y, Furusawa A, Ujiie K. Endoscopic examination for fistula. Endoscopy. 1990;22:208–210. doi: 10.1055/s-2007-1012849. [DOI] [PubMed] [Google Scholar]
- 36.Lange V, Meyer G, Wenk H, Schildberg FW. Fistuloscopy--an adjuvant technique for sealing gastrointestinal fistulae. Surg Endosc. 1990;4:212–216. doi: 10.1007/BF00316795. [DOI] [PubMed] [Google Scholar]
- 37.Shand A, Pendlebury J, Reading S, Papachrysostomou M, Ghosh S. Endoscopic fibrin sealant injection: a novel method of closing a refractory gastrocutaneous fistula. Gastrointest Endosc. 1997;46:357–358. doi: 10.1016/s0016-5107(97)70125-1. [DOI] [PubMed] [Google Scholar]
- 38.Lomis NN, Miller FJ, Loftus TJ, Whiting JH, Giuliano AW, Yoon HC. Refractory abdominal-cutaneous fistulas or leaks: percutaneous management with a collagen plug. J Am Coll Surg. 2000;190:588–592. doi: 10.1016/s1072-7515(00)00237-4. [DOI] [PubMed] [Google Scholar]
- 39.Eleftheriadis E, Tzartinoglou E, Kotzampassi K, Aletras H. Early endoscopic fibrin sealing of high-output postoperative enterocutaneous fistulas. Acta Chir Scand. 1990;156:625–628. [PubMed] [Google Scholar]
- 40.Eleftheriadis E, Kotzampassi K. Therapeutic fistuloscopy: an alternative approach in the management of postoperative fistulas. Dig Surg. 2002;19:230–235; discussion 236. doi: 10.1159/000064218. [DOI] [PubMed] [Google Scholar]
- 41.Papavramidis ST, Eleftheriadis EE, Papavramidis TS, Kotzampassi KE, Gamvros OG. Endoscopic management of gastrocutaneous fistula after bariatric surgery by using a fibrin sealant. Gastrointest Endosc. 2004;59:296–300. doi: 10.1016/s0016-5107(03)02545-8. [DOI] [PubMed] [Google Scholar]
- 42.Kurokawa T, Okushiba S, Kadoya M, Miyamoto D, Kurashima Y, Kitagami H, Ikeda J, Sunaga M, Shinzato Y, Ozawa T, et al. Selective occlusion with fibrin glue under fistuloscopy: seven cases of postoperative management for intractable complex fistulas. Endoscopy. 2002;34:220–222. doi: 10.1055/s-2002-20294. [DOI] [PubMed] [Google Scholar]
- 43.Ramón Rábago L, Moral I, Delgado M, Guerra I, Quintanilla E, Castro JL, Llorente R, Martínez Veiga JL, Gea F. [Endoscopic treatment of gastrointestinal fistulas with biological fibrin glue] Gastroenterol Hepatol. 2006;29:390–396. doi: 10.1157/13091451. [DOI] [PubMed] [Google Scholar]
- 44.Justo-Janeiro JM, Pavon-Vargas A. First clinical experience in Mexico with the use of fibrine glue. Cir Gen. 1997;19:103–108. [Google Scholar]
- 45.Murakami M, Tono T, Okada K, Yano H, Monden T. Fibrin glue injection method with diluted thrombin for refractory postoperative digestive fistula. Am J Surg. 2009;198:715–719. doi: 10.1016/j.amjsurg.2008.10.026. [DOI] [PubMed] [Google Scholar]


