Abstract
Objective
To investigate the clinical and genetic variables at initial presentation that predict survival in the Genetics versus Environment in Scleroderma Outcome Study (GENISOS) cohort.
Methods
GENISOS is a prospective, observational study of a multiethnic early systemic sclerosis (SSc) cohort. To date, a total of 250 patients have been enrolled. In addition to clinical and laboratory data, electrocardiograms (EKGs), chest radiographs, and pulmonary function tests have been obtained from each patient. A modified Rodnan skin thickness score, HLA class II genotyping, and a Medsger Damage Index also have been collected. We performed multivariable analyses utilizing the Cox regression following a purposeful model building strategy.
Results
The study analyzed 122 white, 47 African American, and 71 Hispanic SSc patients with an average disease duration of 2.6 years at enrollment. At the time of analysis, 52 (20.8%) of the 250 patients had died. In the final multivariable model excluding HLA genes, 7 variables emerged as significant predictors of mortality: age ≥65 years at enrollment, forced vital capacity <50% predicted, clinically significant arrhythmia on EKG, absence of anticentromere antibodies, hypertension, chest radiograph suggestive of pulmonary fibrosis, and low body mass index (BMI). In separate modeling that included HLA genes, HLA alleles DRB1*0802 and DQA1*0501 were significant predictors of mortality in addition to the predictors mentioned above.
Conclusion
A limited number of variables collected at presentation, including BMI, are able to predict mortality in patients with early SSc. In addition, some of the HLA genes associated with SSc susceptibility are useful for predicting SSc outcome.
INTRODUCTION
Systemic sclerosis (SSc; scleroderma) is a chronic disease characterized by widespread fibrosis of the skin and internal organs, a small-vessel vasculopathy, and evidence of immune dysregulation with production of autoantibodies. It is a clinically heterogeneous disease ranging from a milder form with less extensive involvement of the internal organs to a more severe type with widespread internal organ involvement, characterized by rapid progression and resulting in disability and death within several years.
Although survival in SSc has improved over the past several decades, SSc is still associated with a considerable reduction in survival compared with that in age- and sex-matched populations (1–4). Survival studies continue to be published and are important for the very reason that they help to document any change in the natural history of the disease and, more importantly, the effects of new treatment. A recent study detailed the changes in organ system causes of mortality over 25 years at a single university center. This study showed that the lung, including both pulmonary fibrosis and pulmonary hypertension, has be come the primary cause of SSc-related deaths, replacing SSc renal crisis, which was formerly the leading cause of mortality before the widespread use of angiotensin-converting enzyme (ACE) inhibitors (5).
Multiple studies have reported that increased mortality in SSc is associated with older age at presentation, male sex, poor socioeconomic status, and widespread skin disease, as well as severe organ involvement of the lung, heart, and kidneys. Laboratory values such as elevated erythrocyte sedimentation rate (ESR), anemia, and proteinuria have also been linked to poorer survival (6–16). None of the studies to date have been conducted in a prospective multiethnic cohort that included a considerable number of Hispanic patients.
Previous HLA studies in SSc have suggested that major histocompatibility complex (MHC) genes exert their influence through autoantibody expression (17,18). However, only 1 study has investigated the association of the MHC genes with survival in SSc. This study reported that the presence of HLA–DRw6, now designated as HLA–DRB1*13 and DRB1*14, was associated with significantly increased risk of mortality in a cohort of 126 patients with SSc (19). However, the study did not characterize these genetic risks in the context of ethnicity.
A prior study investigating the differences among 3 ethnic groups at baseline in the Genetics versus Environment in Scleroderma Outcome Study (GENISOS) cohort reported that African Americans and Hispanics were more likely to have diffuse skin involvement, skin pigmentary changes, digital ulcers, and pulmonary hypertension, although this last variable was seen in the African American group only (18). A more recent study examining early pulmonary involvement and its associations in the GENISOS cohort reported that African Americans were more likely to have pulmonary fibrosis at initial presentation than the white or Hispanic groups, and that African American patients were more likely to have lower forced vital capacity (FVC), forced expiratory volume in 1 second, and diffusing capacity for carbon monoxide (DLco) values as compared with the other groups (20). Although baseline differences among the ethnic groups upon enrollment into the GENISOS cohort have been described, factors associated with survival have not been investigated.
Reported here are the results from an investigation of clinical and genetic variables (including ethnicity) at entry to the GENISOS cohort, either alone or in combination, to predict survival over an average of 6.2 years of followup after enrollment.
PATIENTS AND METHODS
GENISOS is a prospective outcomes study and is designed as a collaboration among the University of Texas-Houston Health Science Center, the University of Texas Medical Branch at Galveston, and the University of Texas-San Antonio Health Science Center. Institutional Review Board approval was obtained at each study site, and written informed consent was obtained from all study participants.
Patient selection
Details of patient selection and recruitment have been previously published by Reveille et al (18). Inclusion criteria for GENISOS were the following: 1) age ≥18 years, 2) diagnosis according to the American College of Rheumatology (formerly the American Rheumatism Association) 1980 preliminary classification criteria of SSc (21), 3) disease onset (defined as the first non-Raynaud’s symptom) within the previous 5 years, and 4) defined ethnicity with all 4 grandparents from the same ethnic group. Exclusion criteria include patients having SSc-like illnesses associated with environmental, ingested, or injected agents. Recruitment started in 1998 and continues through the present. All the enrolled patients at the above-mentioned 3 study sites at the time of analysis were included in the current study. For analysis purposes, patients were assigned to the following ethnic groups: white, African American, Hispanic, and other. Ten patients (1 Native American and 9 Asian) were assigned to the other group.
Data collection
For each patient, a standardized clinical manifestation form was completed at baseline and then with each regularly scheduled visit thereafter (18). In addition, laboratory tests, electrocardiograms (EKGs), chest radiographs, and pulmonary function tests were obtained at baseline and then annually. A modified Rodnan skin thickness score (MRSS) (22) and a previously validated SSc severity scale, the Medsger Damage Index (23), were also calculated at each visit. At the baseline visit, plasma and serum samples were analyzed for complete blood count with differential and platelet count, complete metabolic panel, creatine phosphokinase, and urinalysis with microscopic examination. Sera samples were also analyzed for the presence of autoantibodies. Antinuclear antibodies and anticentromere antibodies (ACAs) were detected by indirect immunofluorescence using HEp-2 cell substrates (Antibodies, Davis, CA). Anti–topoisomerase I antibody testing was performed by passive immunodiffusion against calf thymus extract (Inova Diagnostics, San Diego, CA). Anti–RNA polymerase III antibodies were determined by enzyme-linked immunosorbent assay (MBL, Nagoya, Japan). HLA class II genotyping was performed on extracted and purified genomic DNA amplified by standard laboratory procedures. All of the investigated independent variables for this study were ascertained at the enrollment visit. The vital status of each patient was determined as of December 31, 2005 by review of the National Death Index. Additionally, on April 11, 2008, the Social Security Death Index was reviewed to determine any additional deaths of patients in this cohort. The cause of death was determined based on data obtained from the National Death Index.
Statistical analysis
All enrolled patients were included in our analysis. Systolic and diastolic blood pressure (24), body mass index (BMI) in kg/m2 (25), FVC % predicted, and DLco % predicted (26) were subcategorized according to clinically established cut points. Univariate differences in baseline measurements across the 3 ethnic groups were assessed using chi-square tests for categorical variables and analysis of variance for continuous variables. A Bonferroni correction was utilized for the subsequent pairwise comparisons if the overall comparison was significant. The date of enrollment into the study was used as the starting point for our time-to-event analysis. An initial bivariate (two-at-a-time) analysis included age with each remaining individual variable as a candidate independent risk factor in Cox proportional hazards regression models. Subsequently, a multivariable model was constructed following a purposeful variable selection method (27). First, purposeful selection was initiated by including all variables considered clinically important along with all variables achieving a 0.20 significance level in the univariate Cox models. To reduce the initial saturated model, successive models eliminated one-at-a-time covariates with the highest P value greater than 0.05. Reduced models were compared with each previous model to assess the potential for confounding before eliminating a nonsignificant covariate. Nonsignificant covariates whose exclusion changed the coefficients of the remaining covariates by >20% were retained as potentially important confounders. Covariates excluded from interim models were added back to the final model (one-at-a-time) to confirm their lack of both statistical significance and importance as a potential con-founder. First-order interaction terms were tested for significance on all two-at-a-time combinations of variables retained in the final model. The proportional hazards model assumption was tested using Schoenfeld residuals. Additional post-estimation diagnostics for the fit of the final models included the examination of Cox-Snell residual plots and the Harrell’s C concordance statistic. Analyses were conducted with NCSS2006 (NCSS, Kaysville, UT) and Stata 2008 (StataCorp, College Station, TX) statistical software.
RESULTS
Overall, 250 patients were included in our study, consisting of 122 white (48.8%), 47 African American (18.8%), and 71 Hispanic (28.4%) patients. The mean ± SD age at enrollment (baseline) was 48.85 ± 13.17 years, and the majority of patients (84%) were women. The mean ± SD disease duration at enrollment was 2.6 ± 1.64 years, and 80% of the enrolled patients had a disease duration of <3 years. The mean ± SD time from Raynaud’s symptom onset to enrollment was longer (4.53 ± 5.49 years). A total of 143 patients (57.4%) had diffuse cutaneous disease, whereas 106 patients (42.6%) had limited cutaneous involvement (28). The mean ± SD MRSS was 11.25 ± 11.70 in the overall population. As expected, the MRSS was higher in patients with diffuse cutaneous disease (mean ± SD 21.55 ± 11.21) than in patients with limited skin involvement (mean ± SD 6.72 ± 5.2). The mean ± SD disease durations were 2.58 ± 1.73 years and 2.63 ± 1.52 years in the patients with diffuse and limited cutaneous involvement, respectively. The time between Raynaud’s symptom onset and enrollment in the diffuse and limited disease groups was 4.06 ± 5.23 years and 5.19 ± 5.81 years, respectively. The demographic and clinical features of the investigated subjects in each ethnic category and the overall population are shown in Table 1. Across the ethnic groups, there were significant differences in FVC % predicted (P = 0.048) and frequency of increased reticular markings on chest radiograph consistent with pulmonary fibrosis (P = 0.0129). In the pairwise comparisons, the FVC was significantly lower in the African American patients than in whites (P = 0.011 after Bonferroni adjustment). Other demographic, serologic, and clinical variables including average time of followup did not differ significantly among the ethnic groups.
Table 1.
Baseline characteristics*
| White (n = 122) |
African American (n = 47) |
Hispanic (n = 71) |
Other (n = 10) |
Total (n = 250) |
|
|---|---|---|---|---|---|
| Age at enrollment, mean ± SD years | 51.19 ± 12.68 | 46.73 ± 13.83 | 46.47 ± 13.67 | 47.31 ± 7.71 | 48.85 ± 13.17 |
| Women | 98 (80.3) | 41 (87.2) | 62 (87.3) | 9 (9) | 210 (84) |
| Disease duration, mean ± SD years | 2.41 ± 1.65 | 2.89 ± 1.62 | 2.75 ± 1.58 | 2.50 ± 1.94 | 2.60 ± 1.64 |
| Follow up time mean ± SD, years† | 6.56 ± 3.05 | 5.34 ± 3.67 | 6.15 ± 3.58 | 6.09 ± 3.19 | 6.2 ± 3.34 |
| Diffuse disease | 66 (54.1) | 30 (65.2) | 39 (54.9) | 8 (80) | 143 (57.4) |
| Skin score, mean ± SD | 14.88 ± 11.52 | 16.36 ± 13.09 | 15.22 ± 11.28 | 14.9 ± 11.38 | 15.25 ± 11.70 |
| BMI, mean ± SD kg/m2 | 25.89 ± 5.04 | 26.3 ± 5.25 | 26.35 ± 5.98 | 25.63 ± 11.17 | 26.08 ± 5.69 |
| ANA | 115 (94.3) | 46 (97.9) | 69 (97.2) | 8 (88.9) | 238 (95.6) |
| ACA | 19 (15.6) | 2 (4.3) | 7 (9.9) | 1 (11.1) | 29 (11.7) |
| ATA | 21 (17.2) | 10 (21.3) | 12 (16.9) | 4 (44.4) | 47 (18.9) |
| Anti-RNA polymerase III | 27 (22.1) | 9 (19.2) | 19 (26.8) | 2 (22.2) | 57 (22.9) |
| DLco, mean ± SD | 75.36 ± 24.76 | 69 ± 21.44 | 79.54 ± 27.19 | 75.50 ± 23.05 | 75.41 ± 24.95 |
| FVC, mean ± SD† | 88.08 ± 19.1 | 75.84 ± 23.98 | 83.01 ± 23.73 | 78 ± 28.71 | 84.05 ± 22.15 |
| PF on chest radiograph‡ | 15 (12.3) | 12 (25.5) | 14 (19.7) | 4 (40) | 45 (18) |
| Arrhythmia | 17 (14.4) | 6 (14.6) | 10 (15.4) | 3 (30) | 36 (15.4) |
Values are the number (percentage) of patients unless otherwise indicated. Time variable in years used for the Cox regression analysis, which consists of the average followup time for all patients. BMI = body mass index; ANA = antinuclear antigen; ACA = anticentromere antibodies; ATA = antitopoisomerase; DLco = diffusing capacity for carbon monoxide; FVC = forced vital capacity; PF = pulmonary fibrosis.
P = 0.0129.
P = 0.048.
A total of 52 deaths occurred in our study population. Among the ethnic groups, 20 (16.4%) of 122 white, 11 (23.4%) of 47 African American, 18 (25.4%) of 71 Hispanic, and 3 (30%) of 10 other patients died over the followup period. According to the data obtained from the National Death Index, 29 deaths (55.8%) were related to SSc, 12 (23.1%) were unrelated to SSc, and the cause of death could not be determined in 11 cases (21.1%). Of the 29 SSc-related deaths, 10 were caused by pulmonary involvement, whereas cardiac, gastrointestinal, and muscular complications were responsible for 4, 2, and 1 deaths, respectively. The SSc organ-specific cause of death could not be determined in the remaining 12 cases.
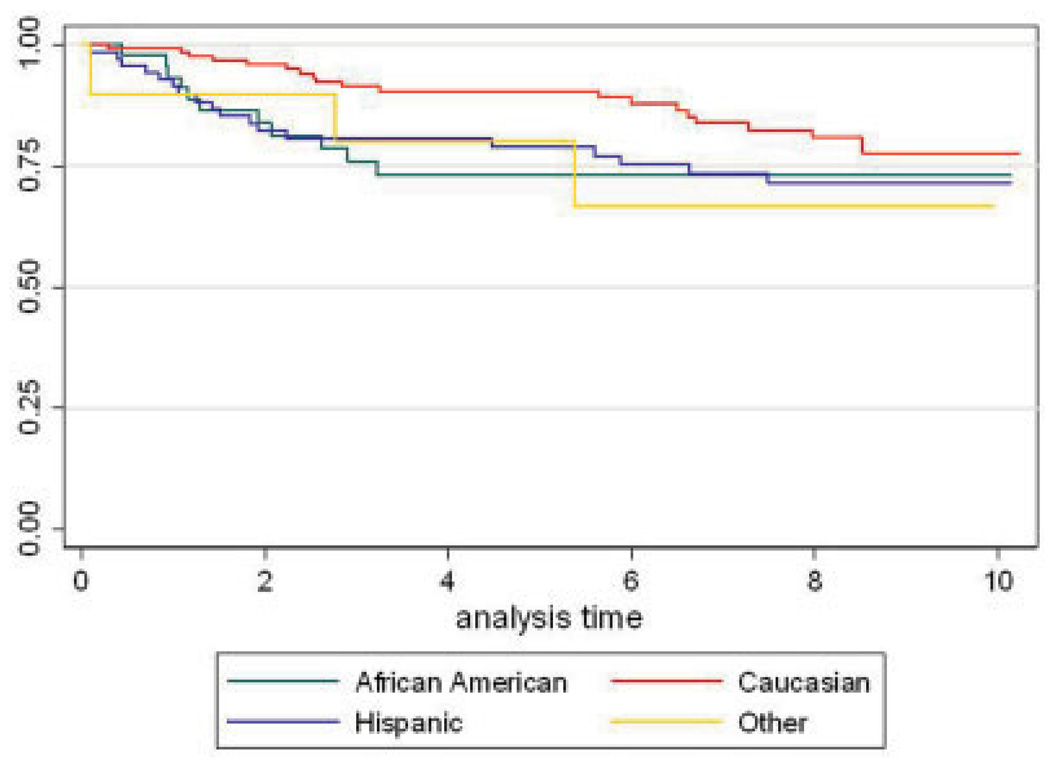
The set of demographic and clinical baseline variables considered in the model and the results from the two-at-a-time Cox models of these factors after adjustment for age at enrollment are shown in Table 2. Seven variables were age-adjusted predictors of mortality: a BMI <18.5 kg/m2, nonwhite ethnicity, clinically significant arrhythmia on EKG, FVC <50% of the predicted value, a DLco <60% of the predicted value, increased reticular markings on chest radiograph consistent with pulmonary fibrosis, and current steroid use. The age-adjusted association of African American and Hispanic ethnicity with mortality when compared with whites did not reach statistical significance, but when all nonwhite ethnicity groups were collapsed into one group, this group of patients had a significantly higher mortality risk (P = 0.028, hazard ratio [HR] 1.87, 95% confidence interval [95% CI] 1.06–3.29). The Kaplan-Meier survival curves of all patients within each ethnic group are shown in Figure 1.
Table 2.
The relationship between mortality and each variable adjusted for age*
| Prognostic variable | HR | 95% CI | P |
|---|---|---|---|
| Age at enrollment | 1.02 | 1.0–1.04 | 0.083 |
| Female sex | 0.99 | 0.47–2.11 | 0.987 |
| Disease duration† | 0.99 | 0.83–1.17 | 0.885 |
| Age at onset of RP | 1.00 | 0.95–1.05 | 0.948 |
| BMI, kg/m2 | 0.001‡ | ||
| 18.5–24.9 | 1.00 | ||
| 25–29.9 | 0.53 | 0.26–1.08 | 0.082 |
| >30 | 0.48 | 0.2–1.17 | 0.105 |
| <18.5 | 6.12 | 2.26–16.58 | < 0.001‡ |
| Ethnicity | 0.178 | ||
| White | 1.00 | ||
| African American | 1.88 | 0.89–3.93 | 0.096 |
| Hispanic | 1.83 | 0.96–3.48 | 0.066 |
| Other | 2.14 | 0.63–7.23 | 0.221 |
| Nonwhite versus white | 1.87 | 1.06–3.29 | 0.028‡ |
| Diffuse skin involvement | 1.45 | 0.82–2.55 | 0.193 |
| MRSS | 1.02 | 1.00–1.04 | 0.067 |
| Arrhythmia on EKG | 2.68 | 1.45–4.96 | 0.004‡ |
| Systolic BP ≥140 or diastolic BP ≥90 | 2.16 | 3.25–14.91 | 0.013‡ |
| FVC % predicted | 0.001‡ | ||
| >80 | 1.00 | ||
| 50–80 | 1.77 | 0.95–3.30 | 0.072 |
| <50 | 6.96 | 3.25–14.91 | < 0.001‡ |
| DLco % predicted | 0.006‡ | ||
| >60 | 1.00 | ||
| 40–60 | 2.16 | 1.06–4.43 | 0.035‡ |
| <40 | 4.64 | 1.85–11.63 | 0.001‡ |
| FVC/DLco ≥ 2 | 1.48 | 0.57–3.88 | 0.441 |
| Pulmonary fibrosis on chest radiograph | 3.56 | 2.03–6.22 | < 0.001‡ |
| Urine protein level | 1.76 | 0.74–4.18 | 0.23 |
| Creatinine level ≥ 1.5 | 2.09 | 0.82–5.31 | 0.12 |
| Hematocrit | 1.03 | 0.97–1.1 | 0.321 |
| Abnormal CPK | 1.78 | 0.75–4.19 | 0.188 |
| Joint contracture | 1.7 | 0.99–2.94 | 0.056 |
| Diarrhea | 1.45 | 0.81–2.62 | 0.227 |
| Dysphagia | 1.18 | 0.63–2.04 | 0.552 |
| Weight loss | 1.36 | 0.33–5.68 | 0.685 |
| Current steroid use | 2.26 | 1.28–3.96 | 0.005‡ |
| Current cyclophosphamide use | 1.83 | 0.44–7.53 | 0.446 |
| Autoantibodies | |||
| ANA | 1.31 | 0.32–5.39 | 0.697 |
| ACA | 0.42 | 0.15–1.16 | 0.06 |
| ATA | 1.23 | 0.61–2.44 | 0.585 |
| Anti-RNA polymerase III | 0.91 | 0.47–1.77 | 0.783 |
| Medsger Damage Index | |||
| General | 1.23 | 0.89–1.7 | 0.23 |
| Peripheral vascular | 1.47 | 1.16–1.87 | 0.002‡ |
| Skin | 1.35 | 1.02–1.8 | 0.047‡ |
| Joint/tendon | 1.18 | 0.98–1.43 | 0.097 |
| Muscle | 1.19 | 0.65–2.17 | 0.583 |
| Gastrointestinal tract | 1.51 | 1.01–2.26 | 0.054 |
| Lung | 1.76 | 1.34–2.31 | < 0.001‡ |
| Heart | 1.45 | 1.11–1.89 | 0.015‡ |
| Kidney | 1.48 | 1.01–2.17 | 0.087 |
| Total | 1.24 | 1.15–1.34 | < 0.001‡ |
| HLA–DRB1*0802§ | 1.46 | 0.48–4.46 | 0.502 |
| HLA–DQA1*0501§ | 1.75 | 0.98–3.17 | 0.064 |
HR = hazard ratio; 95% CI = 95% confidence interval; RP = Raynaud’s phenomenon; MRSS = Modified Rodnan skin thickness score; EKG = electrocardiogram; BP = blood pressure; CPK = creatine phosphoki-nase. See Table 1 for additional definitions.
Time interval between date of onset of first non-Raynaud’s symptom and enrollment.
Statistically significant.
Adjusted for ethnicity and age.
Figure 1.
Kaplan-Meier survival curves of the investigated ethnic groups.
Although all components of the Medsger Damage Index yielded age-adjusted HRs >1, the precision and strength of their association with mortality varied over a wide range. The components of the Medsger Damage Index relating to peripheral vascular, skin, pulmonary, and cardiac disease were significantly associated with mortality. The joint/tendon, GI tract, and kidney components of the Medsger score related to a slight, but nonsignificant, increase in mortality (P < 0.1). The likelihood for an association was low for the general and muscle components (P > 0.1). Ascertainment of the pulmonary and cardiac components of the Damage Index was incomplete because not all patients underwent an echocardiogram at baseline.
In the final multivariable Cox model that included non-genetic candidate risk factors simultaneously in the analysis, only 7 variables were independent predictors of mortality (Table 3). The following set of variables was significantly associated with higher mortality: age ≥65 years, FVC <50% of the predicted value, clinically significant arrhythmia on EKG, absence of ACAs, hypertension, chest radiograph suggestive of pulmonary fibrosis, and low BMI. Mortality for patients in the BMI subgroup 25–29.9 kg/m2 did not differ from mortality for patients in the ≥30 kg/m2 BMI subgroup; therefore, these subcategories were collapsed into a single reference group. Similarly, the mortality in the subgroup of patients with an FVC of 50–80% of the predicted value did not differ from the mortality in the subgroup with FVC ≥80% of the predicted value; therefore, these patients were collapsed into one reference group. For example, patients with a BMI <18.5 kg/m2 had a 12.94-times higher risk of mortality than patients with a BMI of ≥25 kg/m2. No first-order interaction terms were significant and they were therefore excluded from the final model.
Table 3.
Final model without genetic data*
| HR | 95% CI | P | |
|---|---|---|---|
| BMI, kg/m2 | < 0.001 | ||
| ≥25 | 1.00 | ||
| 18.5–24.9 | 2.39 | 1.21–4.72 | 0.012 |
| <18.5 | 12.94 | 4.32–38.80 | < 0.001 |
| Age ≥65 years | 4.37 | 1.98–9.67 | < 0.001 |
| FVC <50% of predicted | 4.83 | 2.06–11.31 | < 0.001 |
| sBP ≥140 or dBP ≥90 | 3.14 | 1.59–6.17 | < 0.001 |
| Arrhythmia on EKG | 2.18 | 1.05–4.50 | 0.035 |
| PF on chest radiograph | 2.46 | 1.20–5.02 | 0.014 |
| ACA | 0.17 | 0.04–0.76 | 0.02 |
HR = hazard ratio; 95% CI = 95% confidence interval; sBP = systolic blood pressure; dBP = diastolic blood pressure; EKG = electrocardiogram. See Table 1 for additional definitions.
We also examined the components of the Medsger Damage Index in the final model; only the heart component was significantly associated with increased mortality (HR 2.61, 95% CI 1.13–2.39; P = 0.009). When we included the Medsger heart component in the final multivariable model, it rendered the covariate arrhythmia on EKG insignificant. The addition of ethnicity to the final model did not show a significant association of this variable with mortality (P = 0.692 for African Americans, P = 0.349 for Hispanics, P = 0.564 for others; whites were the reference group) and did not result in any material changes in the HRs of the other covariates, indicating the lack of any serious confounding by ethnicity. Likewise, neither the disease duration at enrollment (HR 1.00, 95% CI 0.83–1.22; P = 0.969) nor the age at Raynaud’s symptom onset (HR 0.97, 95% CI 0.94–1.01; P = 0.13) contributed significantly to the final multivariable model. In addition, neither variable materially changed the HRs of the final set of significant covariates, indicating a lack of confounding by duration of disease (since the first non-Raynaud’s symptom) or the age at onset of Raynaud’s phenomenon.
In a separate purposeful model building procedure, we investigated the effect of HLA genotypes on mortality in our cohort. In Cox models that included only ethnicity and genotypes, the various HLA–DRB1, DQA1, and DQB1 alleles were not significantly associated with mortality. However, in a separate purposeful multivariable model building procedure that included the genotypes along with significant demographic and clinical features, the presence of an HLA–DRB1*0802 allele (HR 6.4, 95% CI 1.79–22.83; P = 0.004) and a DQA1*0501 allele (HR 2.78, 95% CI 1.27–6.09; P = 0.011) were significant independent predictors of mortality (Table 4). In our final Cox model, we collapsed across the heterozygous and homozygous patient subgroups for these alleles because the HRs for patients with 2 copies of DRB1*0802 or 2 copies of DQA1*0501 did not appear different from the HRs of patients with only 1 copy of the respective alleles, although homozygosity for either allele was relatively rare in the cohort. Ethnicity was included as an independent variable in all models assessing HLA genetic biomarkers in order to adjust appropriately for the known differences in allele frequencies across different ethnic groups in the general population. No first-order interaction terms were significant when added to the models, and thus they were excluded from the final model assessing genetic biomarkers (Table 4). HLA–DRB1*0802 is a mainly American Indian HLA allele. Although it was present in 13 Hispanic patients (18.3%) and 1 African American patient (2%), it was absent in white patients. HLA–DQA1*0501 was a more common HLA allele and was present across the ethnic groups; 65 white (53.3%), 18 African American (38.3%), and 27 Hispanic (38%) patients had ≥1 copy of this HLA gene.
Table 4.
Final model with genetic data*
| HR | 95% CI | P | |
|---|---|---|---|
| HLA–DRB1*0802 | 6.4 | 1.79–22.83 | 0.004 |
| HLA–DQA1*0501 | 2.78 | 1.27–6.09 | 0.011 |
| BMI, kg/m2 | |||
| ≥25 | 1.00 | ||
| 18.5–24.9 | 3.2 | 1.5–6.85 | 0.003 |
| <18.5 | 17.9 | 5.38–59.51 | < 0.001 |
| Age ≥65 years | 7.3 | 2.8–18.99 | < 0.001 |
| FVC <50% of predicted | 7.33 | 2.82–19.05 | < 0.001 |
| sBP ≥140 or dBP ≥90 | 4.05 | 1.88–8.71 | < 0.001 |
| Arrhythmia on EKG | 2.18 | 1.01–4.73 | 0.048 |
| PF on chest radiograph | 3.05 | 1.35–6.89 | 0.007 |
| ACA | 0.2 | 0.04–0.98 | 0.047 |
| Ethnicity | |||
| White | 1.00 | ||
| African American | 1.42 | 0.53–3.84 | 0.480 |
| Hispanic | 1.26 | 0.56–2.86 | 0.578 |
| Other | 1.1 | 0.2–5.88 | 0.913 |
HR = hazard ratio; 95% CI = 95% confidence interval; sBP = systolic blood pressure; dBP = diastolic blood pressure; EKG = electrocardiogram. See Table 1 for additional definitions.
The global tests of the proportional hazards assumptions based on Schoenfeld residuals could not be rejected for either of the final models with or without HLA genes (lowest P = 0.3168 for the model with HLA genes). Furthermore, none of the separate tests for each covariate within each of the 2 final models were significant at a 0.05 level, suggesting no violation of the proportional hazards assumption. The Cox-Snell residual plots (residuals were plotted against the cumulative mortality hazard, data not shown) had relatively small deviations from their reference lines, indicating good fit of the models. The final Cox model including the HLA genes showed the least deviation, and according to the Harrell’s C concordance statistic, patient outcomes were accurately predicted by this model 84% of the time.
DISCUSSION
We demonstrated that a limited number of clinical and genetic factors were able to predict mortality in a multiethnic prospective cohort of patients with early SSc. For the first time to our knowledge, predictors of mortality were investigated in a cohort that had a sizable Hispanic population. Two studies investigating the mortality of SSc in the US utilizing the National Center of Health Statistics database have demonstrated that the age-adjusted mortality was higher in the African American population. In these 2 studies, self-designated Hispanics were included within the white and African American ethnic groups and were not considered separately (29, 30). Nietert et al reported that African American and other nonwhite patients had a higher rate of in-hospital deaths even after adjustment for markers of socioeconomic status, disease severity and duration, and comorbidities (31). In our study, age-adjusted mortality did not differ significantly across the African American, Hispanic, other, and white groups. This finding might reflect insufficient power to detect ethnic differences. Only when all nonwhite patients were combined into a single group was their age-adjusted mortality significantly greater than that of the white patients. In the final multivariable models, ethnicity was not a significant independent predictor of mortality. This finding indicates that differences in clinical variables explain the increased age-adjusted mortality among nonwhite patients.
In our study, increased creatinine or proteinuria levels were not predictors of survival in the bivariate or final model, whereas previous studies have suggested that renal involvement (9, 10, 12, 15) and proteinuria (8, 11) are predictors of mortality in patients with SSc. This finding might reflect the shift in the primary cause of scleroderma-related mortality from underlying renal to pulmonary disease after the introduction of ACE inhibitors for prevention and treatment of scleroderma renal crisis (5). There are no published reports on the relationship between BMI and mortality in SSc. The strong association of low BMI with mortality in our study might be a surrogate for generalized deconditioning or GI involvement. Diarrhea, self-reported weight loss, and dysphagia were not predictors of mortality in our cohort, confirming findings from other studies that have investigated similar GI-related parameters (10). However, the ascertainment of these GI-related parameters was less objective and/or complete than that of BMI.
The date of the first non-Raynaud’s symptom was used as the disease onset date in our study, which is a commonly accepted practice, but even the mean time interval between Raynaud’s onset and enrollment was only 4.3 years (5.19 years in the limited SSc group), indicating that enrolled patients were captured in the early stages of their disease. In any case, neither disease duration nor the age at Raynaud’s onset contributed to the final models (as a significant predictor or an important confounder). The lack of association of disease type (i.e., limited or diffuse) with mortality in either the bivariate (age-adjusted) or the final multivariable models indicates that other markers of end organ involvement and serology are more important predictors of mortality.
Most of our study patients were recruited from tertiary care rheumatology clinics in South Texas, making it difficult to rule out a referral bias toward patients with more severe disease. Another limitation of our study was that an echocardiogram for the early detection of pulmonary hypertension and cardiac disease was not obtained from all patients as part of the study. In the assessment of mortality predictors, we used the FVC % predicted/DLco % predicted ratio as a surrogate for the presence of pulmonary hypertension. This parameter was not significantly associated with mortality (Table 2), and it had only a moderate reported sensitivity of 71% and specificity of 72% for the detection of pulmonary hypertension in another SSc cohort (32). We did not measure ESR in our study, therefore, we could not investigate the previously reported finding that high ESR is associated with increased mortality in SSc (8).
We determined the vital status and the cause of death for our participants mainly based on information obtained from the National Death Index. The National Death Index has a high sensitivity of 87.0 –97.9% for the ascertainment of death (33), but the information on cause of death can be inaccurate because it relies on information extracted from death certificates (34,35). To avoid bias due to misclassification of the cause of death, the overall mortality rather than the cause-specific mortality was chosen as our out come.
Our study demonstrated that the components of the Medsger score varied in their ability to predict survival; the lung component was associated with the highest age-adjusted HR and precision, whereas the muscle component had the lowest age-adjusted HR (Table 2). When the Medsger score was included in the final model, only the heart component was significantly associated with mortality and was able to replace arrhythmia on EKG as the other surrogate for cardiac involvement. These observations confirm the findings of other groups that the total Medsger Damage Index score weighting each individual organ sys tem score equally will not be an accurate predictor of damage in SSc (23). The predictive value of the heart and lung damage scores might have increased further if an echocardiogram had been available from all subjects at enrollment.
In our study, HLA–DRB1*0802 (primarily in Hispanics) and DQA1*0501 were significant predictors of mortality in the final model. Although these alleles did not appear to be significant predictors of mortality in the bivariate analyses (Table 2), they became highly significant when combined with the multiple clinical and demographic covariates in the final model (according to a separate purposeful model selection strategy). This finding supports the notion that discovering the role of genetic biomarkers in disease susceptibility, early detection, or prognosis requires that they be considered in the context of other clinical and demographic factors. In other words, we are able to accurately reflect the complex underlying biologic pathways and reveal the important and significant role of genetic factors only when we use the appropriate statistical models that allow for confounding and interaction among candidate prognostic (or risk) factors (36,37).
HLA–DRB1*0802 and DQA1*0501 are not known to be in linkage disequilibrium with each other in the general population (http://www.ihwg.org), supporting the notion that they are two independent prognostic risk factors in SSc. HLA–DRB1*0802 is an American Indian gene and was almost exclusively present in the Hispanic patients, which might partially explain their poorer survival in our study cohort. HLA–DRB5*0102 in linkage disequilibrium with DRB1*0802 has been associated with antitopoisomerase positivity in a Korean population with SSc (38). HLA–DQA1*0501 is not only a severity gene but also is a risk factor for disease susceptibility in a large cross-sectional population of patients with SSc (39). The HLA–DQA1*0501 allele is also reported to be associated with diffuse SSc in white men (40). Furthermore, the strongest risk factor for SSc in Oklahoma Choctaws, a Native American tribe with a high prevalence of SSc, was the presence of an HLA haplotype that included DQA1*0501. Choctaw patients with SSc have a severe form of the disease, exhibiting diffuse cutaneous involvement, pulmonary fibrosis, and antibodies to topoisomerase I (41). HLA–DRw6, currently designated as DRB1*13 and DRB1*14, is the only other HLA allele that has been previously reported to be associated with mortality in SSc (19). HLA–DRB1*0802 and DQA1*0501 are not linked to the HLA alleles DRB1*13 and DRB1*14.
Although previous studies have indicated that the association of MHC genes with susceptibility to SSc is primarily mediated by expression of SSc-related autoantibodies (17), the 2 significant HLA alleles in our final model were contributing to SSc mortality independent of the influence of the autoantibodies as examined in our multivariable modeling strategy. In other words, none of the autoanti-bodies we evaluated as candidate prognostic factors diminished the significance of the HLA alleles (DQA1*0501 or DRB1*0802) as predictors of mortality or altered the magnitude of the association of the HLA alleles with mortality in patients with SSc. It is also important to emphasize that the concern for multiple comparisons (which threatens the validity of studies seeking to identify one or more disease susceptibility genes from among many candidate genes tested) relates to the increase in Type I error as the number of separate univariate analyses (i.e., separate hypothesis tests) increases. In contrast, we sought to identify the single best set of independent SSc prognostic factors using multivariable modeling, in which candidate variables are examined in simultaneous combination and each variable’s influence on the outcome is thus adjusted for the influence of all the other covariates in the model. In such a multivariable framework, the adjustment of P values for the number of multiple comparisons (i.e., multiple hypothesis tests) is irrelevant because only a single test of the overall model is used to evaluate whether the variables (as a group) are associated with the outcome. The P values for our overall final models with and without the HLA genes were both less than 0.0001.
In conclusion, our data suggest that 7 easily obtainable demographic and clinical parameters (age ≥65 years, FVC <50% of the predicted value, clinically significant arrhythmia on EKG, absence of ACA, hypertension, chest radiograph suggestive of pulmonary fibrosis, and low BMI) were predictors of mortality in our multiethnic SSc cohort. In separate purposeful modeling strategies, we also demonstrated that HLA–DRB1*0802 and HLA–DQA1*0501 were significantly associated with mortality in addition to the above-mentioned factors, suggesting that these HLA alleles can be used as prognostic biomarkers in SSc.
ACKNOWLEDGMENTS
The authors thank Mrs. Deepthi Nair for her assistance in the database management and Mr. Julio Charles, Mr. William Babu, and Ms Yasamin Salehi for performing the laboratory studies.
Supported by an NIH Specialized Center of Research grant in scleroderma (P50-AR44888), by the NIH Centers for Research Translation (P50-AR054144), by University Clinic Research Center grants from the University of Texas Medical Branch and the University of Texas at San Antonio (M01-RR00073 and M01-RR01346), by an NIH Clinical and Translational Sciences award (1U54-RR23417-01), and by an American College of Rheumatology Clinical Investigator fellowship award.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Assassi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Assassi, Sutter, Reveille, Gourh, Estrada-Y-Martin, Arnett, Mayes.
Acquisition of data. Assassi, Sutter, McNearney, Reveille, Karnavas, Gourh, Estrada-Y-Martin, Fischbach, Arnett, Mayes.
Analysis and interpretation of data. Assassi, del Junco, Sutter, Reveille, Karnavas, Gourh, Estrada-Y-Martin, Arnett, Mayes.
REFERENCES
- 1.Bryan C, Howard Y, Brennan P, Black C, Silman A. Survival following the onset of scleroderma: results from a retrospective inception cohort study of the UK patient population. Br J Rheumatol. 1996;35:1122–1126. doi: 10.1093/rheumatology/35.11.1122. [DOI] [PubMed] [Google Scholar]
- 2.Bu-Shakra M, Lee P. Mortality in systemic sclerosis: a comparison with the general population. J Rheumatol. 1995;22:2100–2102. [PubMed] [Google Scholar]
- 3.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 4.Medsger TA, Jr, Masi AT, Rodnan GP, Benedek TG, Robinson H. Survival with systemic sclerosis (scleroderma): a life-table analysis of clinical and demographic factors in 309 patients. Ann Intern Med. 1971;75:369–376. doi: 10.7326/0003-4819-75-3-369. [DOI] [PubMed] [Google Scholar]
- 5.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altman RD, Medsger TA, Jr, Bloch DA, Michel BA. Predictors of survival in systemic sclerosis (scleroderma) Arthritis Rheum. 1991;34:403–413. doi: 10.1002/art.1780340405. [DOI] [PubMed] [Google Scholar]
- 7.Bennett R, Bluestone R, Holt PJ, Bywaters EG. Survival in scleroderma. Ann Rheum Dis. 1971;30:581–588. doi: 10.1136/ard.30.6.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryan C, Knight C, Black CM, Silman AJ. Prediction of five-year survival following presentation with scleroderma: development of a simple model using three disease factors at first visit. Arthritis Rheum. 1999;42:2660–2665. doi: 10.1002/1529-0131(199912)42:12<2660::AID-ANR23>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Ferri C, Valentini G, Cozzi F, Sebastiani M, Michelassi C, La MG, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002;81:139–153. doi: 10.1097/00005792-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Ioannidis JP, Vlachoyiannopoulos PG, Haidich AB, Medsger TA, Jr, Lucas M, Michet CJ, et al. Mortality in systemic sclerosis: an international meta-analysis of individual patient data. Am J Med. 2005;118:2–10. doi: 10.1016/j.amjmed.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Kaburaki J, Lee CC, Kuwana M, Tojo T, Ikeda Y, Takano M, et al. Initial predictors of survival in patients with systemic sclerosis (scleroderma) Keio J Med. 1992;41:141–145. doi: 10.2302/kjm.41.141. [DOI] [PubMed] [Google Scholar]
- 12.Nagy Z, Czirjak L. Predictors of survival in 171 patients with systemic sclerosis (scleroderma) Clin Rheumatol. 1997;16:454–460. doi: 10.1007/BF02238937. [DOI] [PubMed] [Google Scholar]
- 13.Scussel-Lonzetti L, Joyal F, Raynauld JP, Roussin A, Rich E, Goulet JR, et al. Predicting mortality in systemic sclerosis: analysis of a cohort of 309 French Canadian patients with emphasis on features at diagnosis as predictive factors for survival. Medicine (Baltimore) 2002;81:154–167. doi: 10.1097/00005792-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Simeon CP, Armadans L, Fonollosa V, Vilardell M, Candell J, Tolosa C, et al. Survival prognostic factors and markers of morbidity in Spanish patients with systemic sclerosis. Ann Rheum Dis. 1997;56:723–728. doi: 10.1136/ard.56.12.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simeon CP, Armadans L, Fonollosa V, Solans R, Selva A, Villar M, et al. Mortality and prognostic factors in Spanish patients with systemic sclerosis. Rheumatology (Oxford) 2003;42:71–75. doi: 10.1093/rheumatology/keg033. [DOI] [PubMed] [Google Scholar]
- 16.Steen VD, Medsger TA., Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437–2444. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC. HLA and autoimmunity in scleroderma (systemic sclerosis) Int Rev Immunol. 1995;12:107–128. doi: 10.3109/08830189509056707. [DOI] [PubMed] [Google Scholar]
- 18.Reveille JD, Fischbach M, McNearney T, Friedman AW, Aguilar MB, Lisse J, et al. Systemic sclerosis in 3 US ethnic groups: a comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Semin Arthritis Rheum. 2001;30:332–346. doi: 10.1053/sarh.2001.20268. [DOI] [PubMed] [Google Scholar]
- 19.Langevitz P, Buskila D, Gladman DD, Darlington GA, Farewell VT, Lee P. HLA alleles in systemic sclerosis: association with pulmonary hypertension and outcome. Br J Rheumatol. 1992;31:609–613. doi: 10.1093/rheumatology/31.9.609. [DOI] [PubMed] [Google Scholar]
- 20.McNearney TA, Reveille JD, Fischbach M, Friedman AW, Lisse JR, Goel N, et al. Pulmonary involvement in systemic sclerosis: associations with genetic, serologic, sociodemo-graphic, and behavioral factors. Arthritis Rheum. 2007;57:318–326. doi: 10.1002/art.22532. [DOI] [PubMed] [Google Scholar]
- 21.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 22.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–1285. [PubMed] [Google Scholar]
- 23.Medsger TA, Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26:2159–2167. [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 25.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 26.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 27.Hosmer DW, Lemeshow S. Applied survival analysis: regression modeling of time to event data. New York: John Wiley & Sons; 1999. pp. 159–180. [Google Scholar]
- 28.Leroy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 29.Krishnan E, Furst DE. Systemic sclerosis mortality in the United States: 1979–1998. Eur J Epidemiol. 2005;20:855–861. doi: 10.1007/s10654-005-2210-5. [DOI] [PubMed] [Google Scholar]
- 30.Mendoza F, Derk CT. Systemic sclerosis mortality in the United States: 1999–2002 implications for patient care. J Clin Rheumatol. 2007;13:187–192. doi: 10.1097/RHU.0b013e318124a89e. [DOI] [PubMed] [Google Scholar]
- 31.Nietert PJ, Silver RM, Mitchell HC, Shaftman SR, Tilley BC. Demographic and clinical factors associated with in-hospital death among patients with systemic sclerosis. J Rheumatol. 2005;32:1888–1892. [PubMed] [Google Scholar]
- 32.Hsu VM, Moreyra AE, Wilson AC, Shinnar M, Shindler DM, Wilson JE, et al. Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. J Rheumatol. 2008;35:458–465. [PubMed] [Google Scholar]
- 33.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 34.Pritt BS, Hardin NJ, Richmond JA, Shapiro SL. Death certification errors at an academic institution. Arch Pathol Lab Med. 2005;129:1476–1479. doi: 10.5858/2005-129-1476-DCEAAA. [DOI] [PubMed] [Google Scholar]
- 35.Ravakhah K. Death certificates are not reliable: revivification of the autopsy. South Med J. 2006;99:728–733. doi: 10.1097/01.smj.0000224337.77074.57. [DOI] [PubMed] [Google Scholar]
- 36.Khoury MJ. Commentary: epidemiology and the continuum from genetic research to genetic testing. Am J Epidemiol. 2002;156:297–299. doi: 10.1093/aje/kwf053. [DOI] [PubMed] [Google Scholar]
- 37.Yang Q, Khoury MJ. Evolving methods in genetic epidemiology. III. Gene-environment interaction in epidemiologic research. Epidemiol Rev. 1997;19:33–43. doi: 10.1093/oxfordjournals.epirev.a017944. [DOI] [PubMed] [Google Scholar]
- 38.Kang SH, Park MH, Song EY, Kang SJ, Lee EB, Song YW, et al. Association of HLA class II genes with systemic sclerosis in Koreans. J Rheumatol. 2001;28:1577–1583. [PubMed] [Google Scholar]
- 39.Arnett FC, Gourh P, Shete S, Ahn CW, Honey R, Agarwal SK, et al. Major histocompatibility complex (MHC) class II alleles, haplotypes, and epitopes which confer susceptibility or protection in the fibrosing autoimmune disease systemic sclerosis: analysis in 1,300 Caucasian, African American and Hispanic cases and 1,000 controls. Ann Rheum Dis. 2009 doi: 10.1136/ard.2009.111906. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambert NC, Distler O, Muller-Ladner U, Tylee TS, Furst DE, Nelson JL. HLA–DQA1*0501 is associated with diffuse systemic sclerosis in Caucasian men. Arthritis Rheum. 2000;43:2005–2010. doi: 10.1002/1529-0131(200009)43:9<2005::AID-ANR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Arnett FC, Howard RF, Tan F, Moulds JM, Bias WB, Durban E, et al. Increased prevalence of systemic sclerosis in a Native American tribe in Oklahoma: association with an Amerindian HLA haplotype. Arthritis Rheum. 1996;39:1362–1370. doi: 10.1002/art.1780390814. [DOI] [PubMed] [Google Scholar]