Abstract
Purpose
Acute gastrointestinal syndrome (AGS) due to ionizing radiation (IR) causes death within 7 days. Currently, no satisfactory agent exists for mitigation of AGS. A peptide derived from the receptor binding domain of fibroblast growth factor 2 was synthesized (FGF-P) and its mitigation effect on AGS was examined.
Methods and Materials
A subtotal-body irradiation (sub-TBI) model was created to induce gastrointestinal (GI) death while avoiding bone marrow death. After 10.5-16 Gy sub-TBI, mice received an intramuscular injection of FGF-P (10 mg/kg/day) or saline (0.2 ml/day) for 5 days; survival (frequency and duration) was measured. Crypt cells and their proliferation were assessed by H&E and BrdU staining. Additionally, GI hemoccult score, stool formation, and plasma levels of endotoxin, insulin, amylase, IL-6, KC, MCP-1, and TNF-α were evaluated.
Results
FGF-P rescued a significant fraction of four strains of mice (33-50%) exposed to a lethal dose of sub-TBI. FGF-P improved crypt survival and repopulation and partially preserved or restored GI function. Furthermore, while sub-TBI increased plasma endotoxin levels and several pro-inflammation cytokines (IL-6, KC, MCP-1, and TNF-α), FGF-P reduced these adverse responses.
Conclusions
The data support pursuing FGF-P as a mitigator for AGS.
Keywords: FGF2 peptide, Mitigation, Subtotal-body irradiation, AGS, GI function
Introduction
Acute gastrointestinal syndrome (AGS) is the predominant cause of death within the first week following exposure to high-dose radiation. AGS is due mainly to rapid and devastating damage to the GI epithelium, leading to loss of GI function, nutrition malabsorption, electrolyte imbalance, and translocation of GI pathogens. Subsequent bone marrow (BM) failure is worsened by AGS, leading to increased and more rapid BM syndrome-related deaths (1, 2).
Currently, there is no satisfactory mitigation agent for rescuing patients from AGS-related death. Antioxidant radio-protectors such as Amifostine® are preventative rather than mitigative; their benefit is minimal unless present in the blood stream at the time of irradiation (3).
FGF-2, a potent mitogen, promotes stem cell renewal, progenitor cell differentiation, and epithelial proliferation (1-3). Its receptor is widely distributed and functions in almost all types of cells (4-6). Since radiation damages a broad range of cells, the stimulation of multiple cell types can help cells and organs support each other and enhance tissue regeneration in response to multi-organ damage (5-12). The protective and mitigative potential of FGF-2 for radiation toxicity has been proven by a number of studies (13), including ours (14, 15). We have previously shown that: 1) FGF-2 protects the small bowel and reduces death from AGS in mice (16-18); and 2) circulating levels of FGF inversely correlate with toxicity in human acute radiation injury, indicating the potential for supplemental FGF-2 to reverse some deleterious situations (15).
Notably, the use of intact FGF-2 protein has several disadvantages: it is naturally unstable due to its required molecular confirmation (susceptible to temperature, pH, etc.), expensive to produce, and has a limited shelf-life. Recently we have synthesized a proprietary FGF-2 analog, designated FGF-P, which features the FGF receptor binding domain. The advantages of FGF-P over native FGF-2 are: 1) stability under severe conditions, for example boiling; 2) enzyme-resistant as dry powder and stable shelf-life; 3) can be administered by victims via intramuscular injection; and 4) this small peptide can be synthesized in large quantities with high purity. In this study, we examined the mitigation effect of FGF-P on AGS.
Methods and Materials
FGF-P synthesis and preparation
FGF-P is a synthetic peptide based on a 15 amino acid region of FGF-2 known to interact with FGF receptors. FGF-P was synthesized by standard, solid phase methods (Genemed Synthesis, San Antonio, TX) at a level of 97% purity, as determined by reverse-phase HPLC. The binding of peptide to FGF receptors was monitored by surface plasmon resonance using human FGFR1-IIIb (R&D Systems, Minneapolis, MN) immobilized to a Biacore CM5 sensor chip (GE Healthcare, Piscataway, NJ), demonstrating a KD of 322 × 10-9M; native, recombinant FGF-2 has a KD of 952 × 10-9M (data not shown). FGF-P is hydrophilic and was dissolved in normal 0.9% saline (1 mg/ml) for use.
Pre-clinical sub-TBI radiation model
To ensure that lethality measured during the first week after high-dose irradiation is predominantly due to AGS and only minimally perturbed by BM syndrome, a sub-TBI model was created. Four strains of mice were utilized: BALB/c, C57BL/6, C3H/NeN, and NIH Swiss. Mice were restrained in a custom jig with the right hind-legs extended outside the radiation field. The remainder of the body was irradiated at 1.84 Gy/min using a 137Cs source (J. L. Shepherd Irradiator), with a homogeneity better than ± 5%. Six to eight mice (male, 6-8 weeks old, NCI) per group received a single dose of 10.5 -16 Gy, depending on strain. In each case, the doses were selected to include both LD50/7 and ≈1.15 × LD50/7 for a given strain. Ten minutes to four hours after sub-TBI, the first of 5 daily FGF-P treatments was administered via i.m. injection (10 mg/kg in 0.2 ml saline). The controls received 0.2 ml saline. In some experiments, Amifostine (Ethyol, MedImmune Oncology Inc, Gaithersburg, MD) was given intravenously (i.v.) 30 min prior to irradiation (positive control) or 10 min after irradiation (time-matched control). The animal protocol was approved by the University's Institutional Animal Care and Use Committee.
Crypt counts and BrdU assay
Irradiated mice treated with/without FGF-P daily for 3 days were i.m. injected with 120 mg/kg BrdU, and sacrificed 12 hours later. The duodenal (Du), jejunal (Je), and ileal (Il) segments (3-4 pieces/segment) were harvested, fixed in formalin, processed in paraffin blocks, cut into transverse sections of full segment circumference (5 μm thick), and stained with H&E for crypt counts. Proliferating crypts were defined as containing 10 or more adjacent chromophilic non-Paneth cells and a lumen. The circumference of a transverse cross-section of intestine was used as a unit. The number of proliferating crypts was counted in each circumference. At least 10 circumferences were scored per mouse; 4–5 mice were used to generate each data point. For BrdU staining, the paraffin slides from each group were deparaffined, rehydrated, treated with 10% H2O2 to eliminate the activity of endogenous peroxidase, and stained with Biotinylated anti-BrdU (4 μg/ml in 10% calf serum-PBS) followed by Streptavidin conjugated horseradish peroxidase and AEC substrate.
Assays for GI bleeding, stool formation, and body weight
For GI bleeding, mouse stools were collected on day 2 post-IR and stool blood content was examined with a Hemoccult Sensa kit (Cat# 64151, BD Inc, Fullerton, CA). The score of each sample was determined by two researchers according to manufacturer's instructions. To assess stool formation, mice were sacrificed on day 3.5 post-IR; the entire colon starting from the anus was harvested. Loose, yellow content in the lumen was defined as poor stool formation or diarrhea, while solid, dark, granulated content was defined as formed stool. The body weight of each surviving mouse was measured daily.
Measurement of plasma endotoxin level
On day 3.5, mice were sacrificed, and plasma was collected to measure the level of endotoxin. The kit (Horseshoe Crab Reagent Manufactory, Xiamen, China) utilized tachypleus amebocyte lysate that determines a quantitative endotoxin level (<0.01 EU/ml). Endotoxin level was calculated using the standard from the same plate, according to manufacturer's instructions.
Measurement of plasma level of insulin and glucose
Insulin and glucose in plasma collected at day 3.5 were measured with a Rat/Mouse Insulin ELISA Kit (Cat. # EZRMI-13K, Linco Inc, St. Charles, Missouri) and the Glucose (GO) Assay Kit (Cat# GAGO-20, Sigma, St. Louis, MO), respectively, according to manufacturer's instructions.
Measurement of plasma pro-inflammatory molecules
To determine the levels of multiple cytokines in mouse plasma, an assay using bead array technology (0.025-0.05 ml per assay for several cytokines) was utilized. The paired anti-mouse cytokine antibodies (capture antibody and biotinylated antibody) and recombined mouse cytokines were purchased from R&D systems (Minneapolis, MN). The various types of xMAP Multi-Analyte COOH beads and the Luminex 200 instrument were purchased from Luminex (Austin, TX). Each capture antibody was covalently conjugated to free carboxyl groups on beads. The standard was calibrated with a mouse bead array kit (Linco Inc). The linear correlation coefficient of the fluorescence readings was >0.98 for each cytokine. Mouse plasma from different treatment groups was assessed for several pro-inflammatory molecules known to be involved in radiation injury (MCP-1, IL6, KC, and TNFα).
Statistical Analysis
All data are expressed as mean ± SD. Statistical significance of differences between treatment and control groups was determined by unpaired two-tailed Student's t tests. Kaplan-Meier analysis was used for assessment of survival data. Differences were considered statistically significant for P < 0.05.
Results
Sub-TBI model reduces impact of BM on AGS
Different strains of mice have different BM sensitivities and GI irradiation tolerances. In our hands, the BM LD50/30 for BALB/c, NIH Swiss, C3H/NeN, and C57BL/6 mice were approximately 6.0 ± 0.2, 7.3 ± 0.2, 7.4 ± 0.2, and 9.0 ± 0.3 Gy, respectively, after TBI. When mice were irradiated with a dose of LD50/30 × 1.15, 100% died within 20 days (LD100/20). At this dose, however, at 3.5 days post-TBI, there was only minor GI pathological damage (only a small decrease in villous length and crypt number) compared with normal mice. In a bioterrorism event, typical victims would receive an inhomogeneous dose that might preserve roughly 5% or more of their marrow. The sub-TBI model described protects about 5% of BM and is sufficient to separate the effects of BM collapse from those of GI toxicity and spare 100% of mice from death at the LD100/20 dose. Using the sub-TBI model in our specific pathogen-free facility, the LD50/7s for BALB/c, C57BL/6, C3H/NeN, and NIH Swiss mice were 10.5 ± 0.2, 13.5 ± 0.3 Gy, 12.2 ± 0.2, and 13.5 ± 0.2, respectively.
FGF-P rescues mice from otherwise lethal AGS
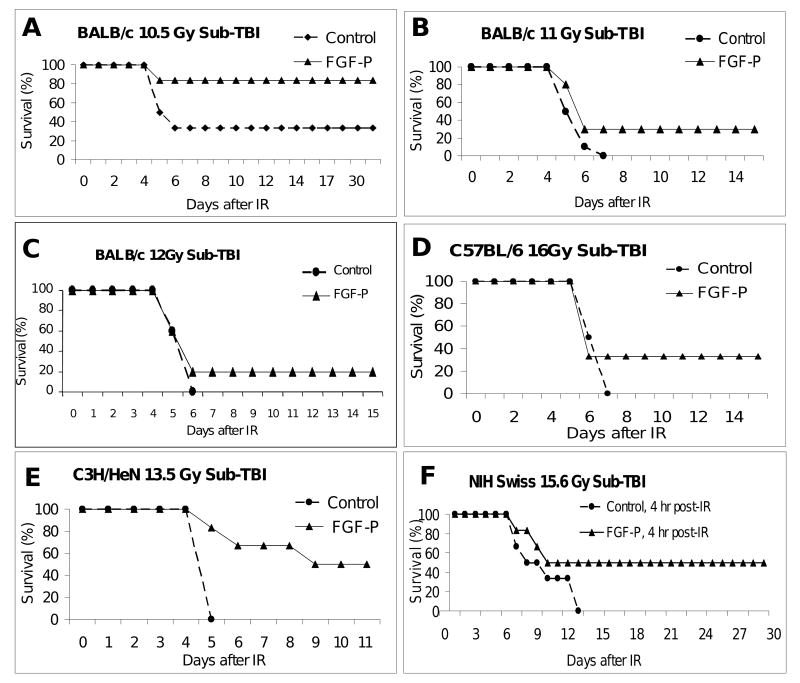
After defining the LD50/7s in the sub-TBI model, we examined the effect of FGF-P on mitigation of AGS following a lethal dose. BALB/c mice were exposed to sub-TBI at escalating doses and within 20 minutes administered 0.2 ml of either saline or FGF-P i.m., with subsequent doses daily for 4 days (5 doses total). At every dose, for all strains tested, survival was improved by FGF-P (Fig 1). The estimated DMF (dose modification factor) at 50% survival was 1.08, 1.15, 1.10, and 1.18 for BALB/c, NIH Swiss, C3H/NeN, and C57BL/6, respectively. Kaplan Meier analysis indicated that all differences between vehicle-alone and FGF-P-treated groups were statistically significant (P<0.05).
Figure 1. FGF-P reduces death from AGS.
Mice were restrained in a customized jig with right hind-legs extending outside the radiation field while they received sub-TBI (10.5-16 Gy). Ten minutes to four hours post-irradiation, the first of 5 daily i.m. injections of FGF-P was administered (10 mg/kg in 0.2 ml saline). Survival is shown as a function of time after irradiation. A-C) BALB/c mice received 10.5-12 Gy sub-TBI and i.m. injection of FGF-P 10 min post-IR. The survival of rate of the FGF-P-treated group was higher than that of controls (P<0.05). D-F) C57BL/6, C3H/NeN, and NIH Swiss mice received 13.5-16 Gy sub-TBI without/with FGF-P four hours post-IR; the survival rate was 33% to 50% (P<0.05).
FGF-P enhances proliferation of crypts of the small intestine
To determine whether FGF-P stimulates crypt regeneration, BALB/c and C57BL/6 mice were exposed to sub-TBI at 10.5-16 Gy. On day 3, they were injected i.m. with 120 mg/kg BrdU. Compared with normal, untreated bowel, numbers of proliferating crypts in all segments of bowel of BALB/c mice exposed to 10.5 Gy treated with vehicle alone decreased dramatically (control crypt counts were 110-130 vs. 10-15 after 10.5 Gy, vs. 3-4 at 12 Gy and ≈ 0 at 16 Gy: Fig 2). When mice were treated with FGF-P, numbers of proliferating crypts were higher. Similarly, there was an associated decreased BrdU staining of residual crypts after irradiation in saline-treated mice, whereas BrdU incorporation was increased in FGF-P-treated mice (10 ± 5 vs. 34 ± 19, P<0.05).
Figure 2. FGF-P increases the numbers of proliferating crypts.
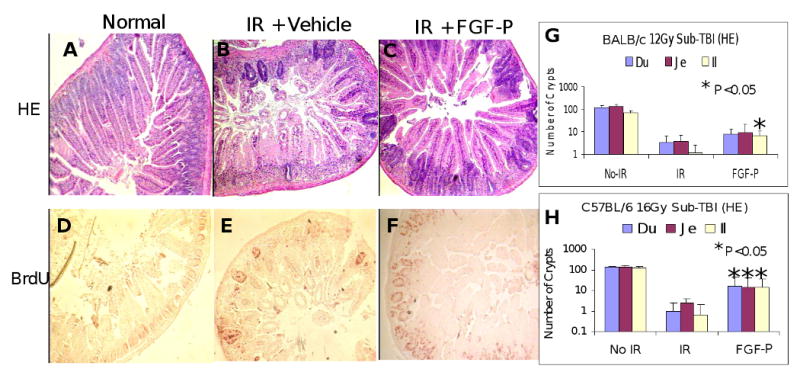
Mice that had been irradiated (sub-TBI 10.5 Gy, A-F; or 12 Gy, G; or 16 Gy, H) and treated without/with FGF-P (10 mg/kg) daily for 3 days were i.m. injected with 120 mg/kg BrdU and sacrificed 12 hours later. Duodenal, jejunal, and ileal segments (3-4 pieces/segment) in paraffin sections were processed for H&E and BrdU staining. A-C) H&E staining: FGF-P increased the number of chromophilic crypts compared with vehicle controls. D-F) BrdU staining: BrdU incorporated proliferating crypts were greater in the FGF-P groups than in controls (10 ± 5 vs. 34 ± 19, P<0.05). G and H) FGF-P improved crypt count and BrdU staining for all strains and radiation doses tested (*P <0.05).
FGF-P reduces GI bleeding and preserves GI function
Radiation damages both epithelium and endothelium of the GI mucosa, leading to loss of integrity of GI mucosa and vessels, manifested as GI bleeding. To determine whether FGF-P reduced GI bleeding, mice were exposed to 12 Gy sub-TBI, and on day 3.5, stool was collected and subjected to hemoccult analysis. The extent of GI bleeding was documented using a five scale scoring system, according to manufacturer's instructions. Mitigation of radiation-induced GI bleeding with FGF-P (given after radiation) was similar to that with Amifostine administered prior to irradiation (Fig 3C). Similar results were obtained from C57BL/6 mice (data not shown).
Figure 3. FGF-P improves stool formation, stool hemoccult, and body mass measures.
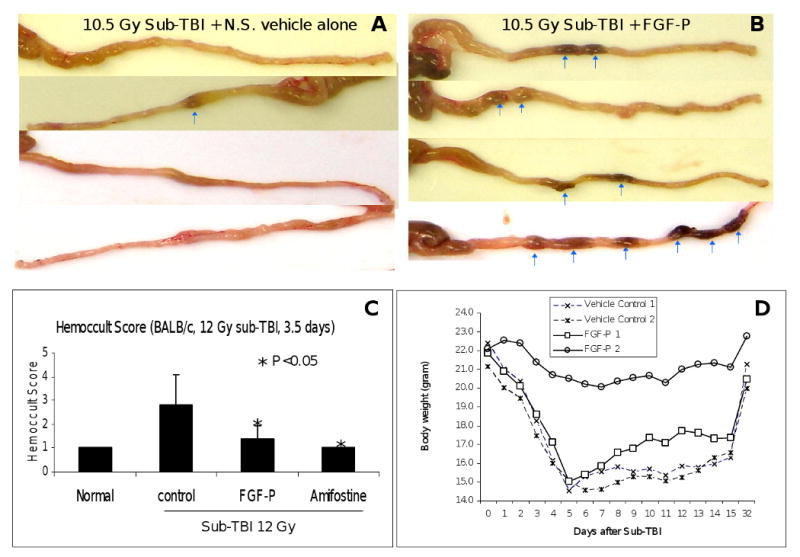
A and B) On day 3.5 post-IR, BALB/c mice were sacrificed, and the colon was harvested (10 cm). Loose, yellow content in the lumen is indicative of poor stool formation or diarrhea, while solid, dark, granulated content is well-formed stool (indicated by arrows). C) Stool was collected on day 3.5 post-IR and blood content was examined and scored. Mean ± SD of these scores is presented (n=5). IR-induced GI bleeding was reduced by FGF-P administered 10 min post-IR, and Amifostine administered 0.5 hr prior to IR. D) BW of representative surviving BALB/c mice treated with either vehicle alone or FGF-P after 10.5 Gy sub-TBI was measured daily for 2 weeks and on day 32. The daily measurement of two mice per group is presented.
To determine whether FGF-P preserved stool formation, mice were exposed to 10.5 Gy sub-TBI. At day 3.5, the large intestine (from anus to whole colon) was collected without disturbing its content. The formed stool was easily distinguished from liquid and semi-solid stool as it passed from ascending to descending colon. Representative images demonstrate that while irradiated mice given saline had loose, liquid, poorly formed stool throughout the colon (Fig 3A), FGF-P-treated mice had solid, dark, well-formed stools in the colon (Fig 3B).
Body weight (BW) is a prime gross measure of GI function. BW was measured for 15 days in BALB/c mice exposed to 10.5 Gy. During the initial 8 days following irradiation, BW loss was similar in both control and FGF-P-treated groups. However, the recovery of BW from day 9 to 15 was faster in FGF-P treated mice than vehicle-treated mice (Fig 3D).
FGF-P reduces toxemia
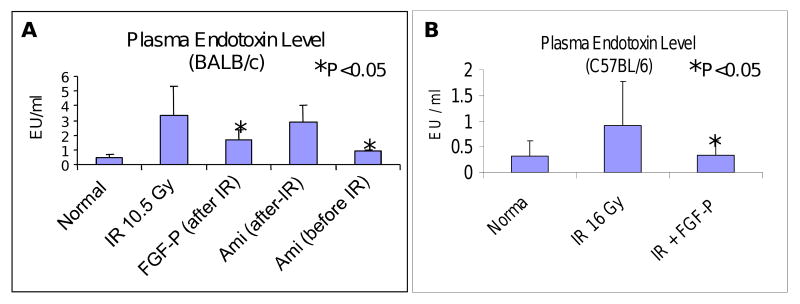
Radiation-induced GI mucosa barrier disruption and associated toxemia was detected with tachypleus amebocyte lysate reagents. On day 3.5 after 10.5 Gy sub-TBI, plasma endotoxin levels in BALB/c mice were elevated significantly in vehicle-treated groups, compared with normal controls (mean 0.45 vs. 3.32 EU/ml), while irradiated FGF-P-treated mice exhibited reduced endotoxin (mean 2.12 EU/ml) (Fig 4A). Amifostine (positive control) was delivered by i.v. 30 min before 10.5 Gy; this resulted in reduction of toxemia (mean 0.93 EU/ml), but it had no effect when given after irradiation (2.87 EU/ml). Similar results were obtained in C57BL/6 mice that were exposed to up to 16 Gy sub-TBI (Fig 4B).
Figure 4. FGF-P reduces toxemia.
On day 3.5, sub-TBI mice (A: BALB/c 10.5 Gy; B: C57BL/6 16 Gy) treated without/with FGF-P or Amifostine (indicated as Ami) were sacrificed and levels of endotoxin in plasma was measured. The level of endotoxin increased in control groups but was reduced in FGF-P- and Amifostine-treated groups (i.v. 0.5 hr pre-IR), but not with Amifostine 0.5 hr post-IR (*P<0.05).
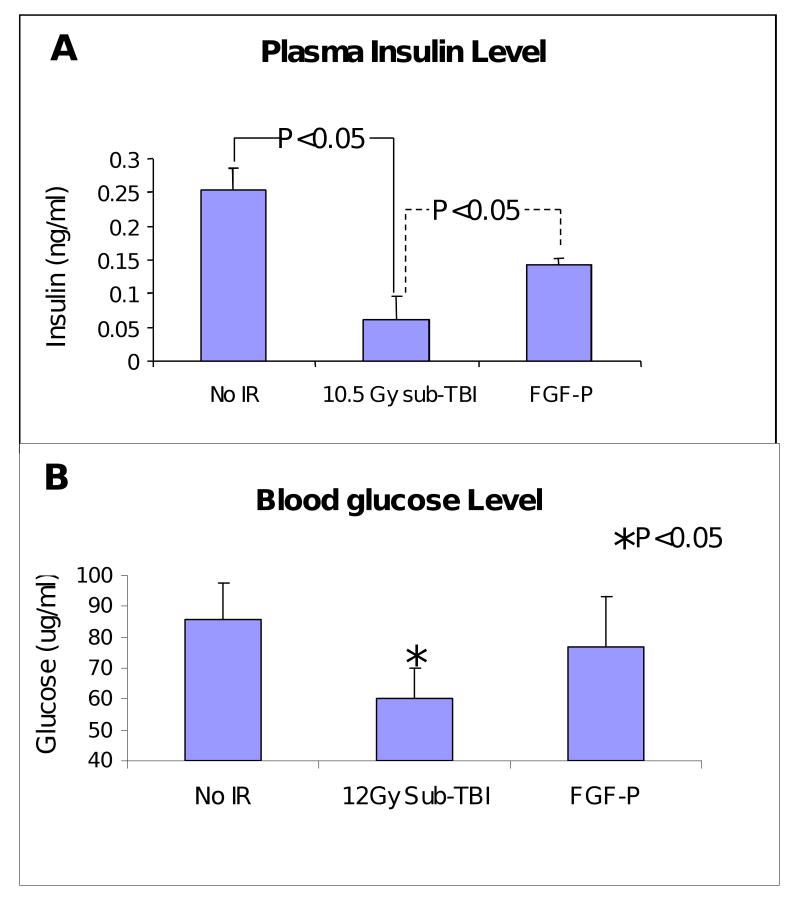
FGF-P partially preserves insulin and glucose level in plasma
While 10.5 Gy sub-TBI reduced plasma insulin levels at day 3.5, with corresponding dangerously low glucose levels, FGF-P treatment maintained glucose levels with little impact on insulin levels (Fig 5, P<0.05).
Figure 5. FGF-P increases insulin and glucose levels in plasma.
Insulin and glucose levels in plasma collected from BALB/c mice on day 3.5 after 10.5 Gy sub-TBI. Insulin and glucose were increased by FGF-P treatment (*P<0.05).
Effect of FGF-P on inflammatory molecules in plasma
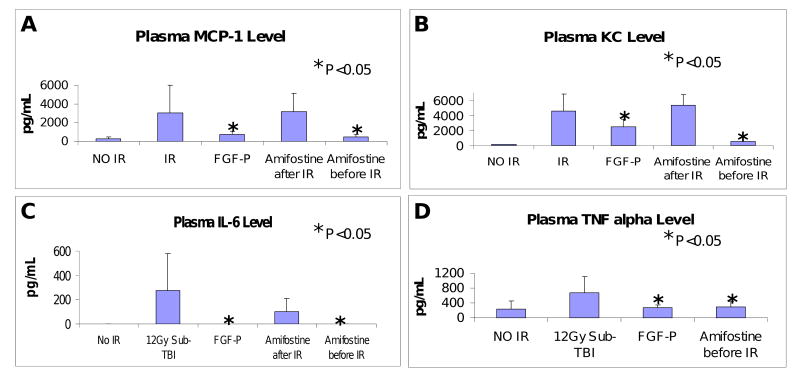
It is accepted that radiation is a multi-factorial pathological insult causing a surge of inflammatory molecules (IMs), including cytokines and chemokines. We focused on four key IMs: MCP-1, IL-6, KC, and TNFα. Specimens were collected on day 3.5 after 12 Gy sub-TBI; MCP-1, IL-6, KC, and TNFα were significantly increased by irradiation (Fig 6), consistent with expected radiation-induced systemic inflammation. FGF-P mitigated that effect, leading to nearly normal cytokine levels. Amifostine given i.v. 30 min before sub-TBI (positive control) also helped maintain normal cytokine levels, but, when given after irradiation, it demonstrated no benefit (19).
Figure 6. FGF-P reduces inflammatory molecules.
Levels of MCP-1, IL6, KC, and TNFα in plasma collected from BALB/c mice on day 3.5 after 10.5 Gy sub-TBI. These IR-induced pro-inflammatory molecules were reduced by FGF-P and Amifostine (0.5 hr pre-IR but not post-IR) (*P <0.05).
Discussion
Radiation-induced AGS causes death much faster than BM death (1, 20). Additionally, since most radiation events are expected to deliver inhomogeneous exposure, many victims will have some marrow shielding and therefore reduced risk of lethal BM syndrome. While the impact of AGS is likely to dominate many situations, to date, no satisfactorily effective mitigation agents have been demonstrated that can rescue or prolong life after near total body radiation-induced AGS.
An appropriate model system is critical for detecting gastrointestinal protection. Since BM LD50/30 is lower than GI LD50/7 (20, 21), a separation of the effects of these two syndromes must be achieved in a way that allows for severe GI disruption but prevents early BM lethality in order to evaluate the utility of AGS mitigators. This was achieved using our sub-TBI model, which has several advantages: 1) autologous marrow infusion from spared marrow of one limb avoids allogeneic transplantation and its complications; 2) the method used to protect marrow is reproducible with little variability; 3) the limited amount of marrow protected by this technique results in a combined injury that simulates expected clinical scenarios. The sub-TBI model allows us to screen GI mitigation agents efficiently and precisely.
This study demonstrated for the first time that a chemically synthesized FGF-2 analog, FGF-P, can be administered 10 min to 4 hours after irradiation as a mitigator of AGS in mice. A dose at 10 mg/kg (given i.m. daily for 5 days) was active, with a DMF similar to that previously described for human recombinant FGF-2 (DMF ≈ 1.1 to 1.15) (15-17). The response of human recombinant FGF-2, a 151aa protein, is modal, with a maximal benefit typically seen at about 0.3 mg/kg/mouse, when given in single or paired doses. Doses over 1 mg/kg i.m. resulted in loss of FGF-2 benefit in several mouse strains (15-17, 22). Compared with FGF-2, FGF-P was effective at a relatively high dose, possibly due to its smaller molecular weight (about 2210 Dalton) as compared with ≈46,000 Dalton for FGF-2, which might be cleared much faster. Due to its size and physical characteristics, a longer exposure time for receptor binding is also available to FGF-2 compared with FGF-P. In preliminary studies, response was maximal at 10 mg/kg, with nearly maximal response at 5 mg/kg (data not shown). In this study, the FGF-P administration schedule was selected for relevance to AGS. Obviously, different schedules and doses should be explored to better utilize FGF-P and improve its DMF.
FGF-P is promising for several reasons: 1) it can be chemically synthesized in large quantities with high purity; 2) it has no gross observable toxicity at the maximum dose we employed, 4 g/kg i.m., indicating the therapeutic window is likely to be > 400, a substantial safety factor; 3) the short amino acid sequence based on the FGF-2 receptor binding site lowers risk of autoimmune reaction; and 4) its production is inexpensive and can be stockpiled for long periods as a powder or solution.
An underlying mechanism through which FGF-P mediates its mitigation effect includes enhanced growth of crypt cells (increases number of crypts), and increased proliferation of crypt cells (BrdU staining). In addition, we also measured villi length of duodenum, jejunum, and ileum. 16 Gy sub-TBI reduced villi length compared with normal mice, and treatment with FGF-P better preserved villi length in the duodenum and jejunum. These observations with FGF-P mirror previous observations with native FGF-2 or its analog (17, 23-25). Progenitor crypt cells are responsible for replacing small bowel mucosa. The natural replacement rate of villi after minor radiation damage is very brisk (a few days) (26, 27) and can be enhanced by exogenous addition of FGF-2 (23, 25) or its analog (24). Growth factors also enhance NFκB phosphorylation and inhibit apoptosis. This process may preserve some progenitor cells, could help prolong survival and function of terminally differentiated cells in the villi, and thus, could reduce bacterial translocation and absorptive function.
Physical functions of the bowel were better preserved in FGF-P-treated mice. Reduced stool hemoccult score 3.5 days after IR suggests that GI tissue damage is reduced after FGF-P. Stool formation and weight loss were either less severe or of shorter duration in FGF-P-treated animals.
Notably, toxemia is a biomarker of GI integrity following irradiation, in addition to reflecting host immunological defenses. The extent of toxemia in FGF-P-treated mice was less severe than that of controls, indicating that FGF-P better maintained GI integrity and immunological defense.
The pancreas, itself damaged by irradiation, must also aid in physiological and GI recovery through endocrine and exocrine systems (28). Reduction of plasma insulin at 3.5 days after irradiation might therefore be due to: 1) reduced production of insulin from beta cells; or more likely, 2) down-regulation of insulin secretion due to low circulating glucose caused by reduced food intake or intestinal malabsorption. FGF-P given to irradiated animals both enhanced insulin levels and prevented severe hypoglycemia.
Finally, elevated circulating inflammation molecules, including IL-6, KC, MCP-1, and TNFα are commonly seen in animals and humans with severe side effects from irradiation and seem to predispose them to more severe fibrovascular consequences of irradiation. As in murine models, humans with intrinsic elevation of these cytokines have increased risk of early and late radiation complications. These inflammatory molecules were normalized in FGF-P-treated animals.
While the results of this study are promising, several questions need to be addressed regarding optimal dose and schedule, length of treatment, and effects of supportive care. We will address these questions in future studies.
Conclusion
FGF-P appears to produce the same mitigative benefits for GI as human recombinant basic FGF. In addition to previously shown effects of FGF-2, including reductions in apoptosis, increased proliferation, improved survival, and improved crypt survival, FGF-P also reduced hematochezia, hypoglycemia, and toxemia. Doses 400× higher than those used for therapy produced little or no lethal toxicity within 7 days. FGF-P therefore has many advantages over native FGFs. Further work is required to determine optimal dose and schedule, pharmacodynamics, target cells in the GI tract (e.g., endothelium, epithelium), impact on BM syndrome, and potential benefits to other radiation-sensitive organs such as skin.
Acknowledgments
This research was supported by 1RC1AI078519-01 and the Centers for Medical Countermeasures against Radiation program, U19-AI067733, National Institute of Allergy and Infectious Diseases (NIAID). The authors wish to thank Amy K. Huser for thoughtful research, writing, and editing assistance.
Footnotes
Conflict of Interest Notification: A conflict of interest exists; Drs. Lurong Zhang and Paul Okunieff developed FGF-P, and a U.S. Patent application has been filed by the University of Rochester.
References
- 1.Freshwater DA. Effects of nuclear weapons on the gastrointestinal system. J R Army Med Corps. 2004;150(3 Suppl 1):17–21. [PubMed] [Google Scholar]
- 2.Ishii T, Futami S, Nishida M, et al. Brief note and evaluation of acute-radiation syndrome and treatment of a Tokai-mura criticality accident patient. J Radiat Res (Tokyo) 2001;42(Suppl):167–182. doi: 10.1269/jrr.42.s167. [DOI] [PubMed] [Google Scholar]
- 3.Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27(1):127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 4.Cailliau K, Browaeys-Poly E, Vilain JP. Fibroblast growth factors 1 and 2 differently activate MAP kinase in Xenopus oocytes expressing fibroblast growth factor receptors 1 and 4. Biochem Biophys Acta. 2001;1538(2-3):228–233. doi: 10.1016/s0167-4889(01)00074-x. [DOI] [PubMed] [Google Scholar]
- 5.Diecke S, Quiroga-Negreira A, Redmer T, et al. FGF2 signaling in mouse embryonic fibroblasts is crucial for self-renewal of embryonic stem cells. Cells Tissues Organs. 2008;188(1-2):52–61. doi: 10.1159/000121282. [DOI] [PubMed] [Google Scholar]
- 6.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 7.Graham BM, Richardson R. Acute systemic fibroblast growth factor-2 enhances long-term memory in developing rats. Neurobiol Learn Mem. 2009;34(7):1875–1882. doi: 10.1038/npp.2009.14. [DOI] [PubMed] [Google Scholar]
- 8.House SL, Melhorn SJ, Newman G, et al. The protein kinase C pathway mediates cardioprotection induced by cardiac-specific overexpression of fibroblast growth factor-2. Am J Physiol Heart Circ Physiol. 2007;293(1):H354–365. doi: 10.1152/ajpheart.00804.2006. [DOI] [PubMed] [Google Scholar]
- 9.Krejci P, Masri B, Fontaine V, et al. Interaction of fibroblast growth factor and C-natriuretic peptide signaling in regulation of chondrocyte proliferation and extracellular matrix homeostasis. J Cell Sci. 2005;118(Pt 21):5089–5100. doi: 10.1242/jcs.02618. [DOI] [PubMed] [Google Scholar]
- 10.Li CF, Hughes-Fulford M. Fibroblast growth factor-2 is an immediate-early gene induced by mechanical stress in osteogenic cells. J Bone Miner Res. 2006;21(6):946–955. doi: 10.1359/jbmr.060309. [DOI] [PubMed] [Google Scholar]
- 11.Olson NE, Kozlowski J, Reidy MA. Proliferation of intimal smooth muscle cells. Attenuation of basic fibroblast growth factor 2-stimulated proliferation is associated with increased expression of cell cycle inhibitors. J Biol Chem. 2000;275(15):11270–11277. doi: 10.1074/jbc.275.15.11270. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi Y, Molyneaux K, Runyan C, et al. The roles of FGF signaling in germ cell migration in the mouse. Development. 2005;132(24):5399–5409. doi: 10.1242/dev.02080. [DOI] [PubMed] [Google Scholar]
- 13.MacVittie TJ. Therapy of radiation injury. Stem Cells. 1997;15 2:263–268. doi: 10.1002/stem.5530150735. [DOI] [PubMed] [Google Scholar]
- 14.Pateder DB, Eliseev RA, O'Keefe RJ, et al. The role of autocrine growth factors in radiation damage to the epiphyseal growth plate. Radiat Res. 2001;155(6):847–857. doi: 10.1667/0033-7587(2001)155[0847:troagf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Ding I, Huang K, Wang X, et al. Radioprotection of hematopoietic tissue by fibroblast growth factors in fractionated radiation experiments. Acta Oncol. 1997;36(3):337–340. doi: 10.3109/02841869709001273. [DOI] [PubMed] [Google Scholar]
- 16.Okunieff P, Wu T, Huang K, et al. Differential radioprotection of three mouse strains by basic or acidic fibroblast growth factor. Br J Cancer. 1996;27(Suppl):S105–108. [PMC free article] [PubMed] [Google Scholar]
- 17.Okunieff P, Mester M, Wang J, et al. In vivo radioprotective effects of angiogenic growth factors on the small bowel of C3H mice. Radiat Res. 1998;150(2):204–211. [PubMed] [Google Scholar]
- 18.Okunieff P, Cornelison T, Mester M, et al. Mechanism and modification of gastrointestinal soft tissue response to radiation: role of growth factors. Int J Radiat Oncol Biol Phys. 2005;62(1):273–278. doi: 10.1016/j.ijrobp.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Pamujula S, Kishore V, Rider B, et al. Radioprotection in mice following oral administration of WR-1065/PLGA nanoparticles. Int J Radiat Biol. 2008;84(11):900–908. doi: 10.1080/09553000802460198. [DOI] [PubMed] [Google Scholar]
- 20.Baranov AE, Selidovkin GD, Butturini A, et al. Hematopoietic recovery after 10-Gy acute total body radiation. Blood. 1994;83(2):596–569. [PubMed] [Google Scholar]
- 21.Gale RP, Butturini A. Medical response to nuclear and radiation accidents. Occup Med. 1991;6(4):581–596. [PubMed] [Google Scholar]
- 22.Wilkie AO. Bad bones, absent smell, selfish testes: the pleiotropic consequences of human FGF receptor mutations. Cytokine Growth Factor Rev. 2005;16(2):187–203. doi: 10.1016/j.cytogfr.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Houchen CW, George RJ, Sturmoski MA, Cohn SM. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am J Physiol. 1999;276(1 Pt 1):G249–58. doi: 10.1152/ajpgi.1999.276.1.G249. [DOI] [PubMed] [Google Scholar]
- 24.Motomura K, Hagiwara A, Komi-Kuramochi A, Hanyu Y, Honda E, Suzuki M, Kimura M, Oki J, Asada M, Sakaguchi N, Nakayama F, Akashi M, Imamura T. An FGF1:FGF2 chimeric growth factor exhibits universal FGF receptor specificity, enhanced stability and augmented activity useful for epithelial proliferation and radioprotection. Biochim Biophys Acta. 2008;1780(12):1432–1440. doi: 10.1016/j.bbagen.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Maj JG, Paris F, Haimovitz-Friedman A, Venkatraman E, Kolesnick R, Fuks Z. Microvascular function regulates intestinal crypt response to radiation. Cancer Res. 2003;63(15):4338–4341. [PubMed] [Google Scholar]
- 26.Li J, Hassan GS, Williams TM, et al. Loss of caveolin-1 causes the hyper-proliferation of intestinal crypt stem cells, with increased sensitivity to whole body gamma-radiation. Cell Cycle. 2005;4(12):1817–1825. doi: 10.4161/cc.4.12.2199. [DOI] [PubMed] [Google Scholar]
- 27.Semont A, Francois S, Mouiseddine M, et al. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol. 2006;585:19–30. doi: 10.1007/978-0-387-34133-0_2. [DOI] [PubMed] [Google Scholar]
- 28.Heijmans HJ, Mehta DM, Kleibeuker JH, et al. Intraoperative irradiation of the canine pancreas: short-term effects. Radiother Oncol. 1993;29(3):347–351. doi: 10.1016/0167-8140(93)90155-2. [DOI] [PubMed] [Google Scholar]