Abstract
Prostate cancer relies on signaling through the androgen receptor (AR) for maintenance and progression; and androgen-deprivation therapy remains a cornerstone of treatment for advanced prostate cancer. An effective clinical classification of prostate cancer should account for the extent of the disease as well as the mechanisms that are driving the growth of the tumor. The previous terms hormone-sensitive and hormone-refractory described response to treatment. It has become clear that these terms do not reflect the mechanism of disease relapse; however over the last decade there has been a better understanding of androgen-receptor mediated signaling effects and incomplete suppression of androgens in prostate cancer. The Prostate Cancer Clinical Trials Working Group 2 (PCWG2) now recommends addressing the spectrum of clinical states based on castration status as this ligand-centered terminology can more accurately describe the patients’ disease, and ultimately provides a useful framework for patient management and drug development. Optimized use of androgen-deprivation therapy, low molecular weight inhibitors of adrenal androgen biosynthesis, and new AR antagonists are promising new therapeutics that can further define the meaning of castrate state. As hormone resistance is redefined to include patients that are refractory to treatments that ablate adrenal and in situ tumoral androgens, a meaningful new clinical state in patients will be forged. We propose a model for incorporating these patients into the current PCWG2 conceptualization of the disease.
Keywords: androgen-deplete prostate cancer, androgen-deplete signaling pathways, androgen receptor signaling, castrate resistant prostate cancer, collateral androgen deplete, prostatic adenocarcinoma
Introduction
Prostate adenocarcinoma leads to the second most common cause of male cancer deaths [Jemal et al. 2009] and its tumorigenesis is primarily regulated by androgen binding and transcription signals of the androgen receptor (AR). Even as the majority of patients are cured with definitive primary treatments, those with high-risk locally advanced disease or metastatic disease are offered medical or surgical castration which can induce durable remissions for a median of 14–30 months [Singer et al. 2008]. Though nearly all respond to hormone maneuvers initially, most patients progress and recurrent tumors re-express active AR signaling as indicated by continuous target gene expression despite having castrate levels of androgens [Chen et al. 2004]. The return of AR signaling in the castrate patient (testosterone ≤50 ng/dL) can be attributed to mechanisms that are mediated by the AR — such as tumor in situ androgen production and amplification of AR protein — or those that bypass it such as coactivators and transactivators. In this setting, 10–30% of patients will respond to secondary hormonal maneuvers such as estrogens, antiandrogen therapy, or adrenal androgen targeted therapy [Small et al. 2006]. Docetaxel plus prednisone chemotherapy is considered for castrate-resistant prostate cancer (CRPC) patients who progress on hormonal therapy or have a rapid disease progression. There is no standard for patients who progress beyond this, and new therapies on the horizon are pushing the envelope of attenuating and ablating resurgent AR signaling in CRPC. Optimized use of androgen deprivation therapy (ADT), low molecular weight inhibitors of adrenal androgen biosynthesis, and new AR antagonists are promising new delivery strategies and therapeutics that can account for the more common causes of hormone resistance in CRPC. Achieving a depleted AR signal will likely forge a meaningful new clinical state in patients previously resistant to hormone therapy.
Castration-resistant disease is a fatal manifestation of prostate cancer and significant efforts are underway to better understand this phase of the disease and develop treatments. At present we understand that there is a significant degree of heterogeneity amongst CRPC patients with different profiles of progression from slow to aggressive. There are some known biologic associations with these heterogeneous populations. Examples are prostate specific membrane antigen (PSMA) status, loss of Phosphatase and tensin homolog (PTEN), gains of v-myc myelocytomatosis viral oncogene homolog (MYC), ErbB3 status, cadherin-11 status, and TMPRSS2-ERG fusion gene status. Cause and effect has not been adequately established for many of these; however, as we are beginning to understand this phase of disease, we are likely to see these candidate biologic profiles become a relevant subclassification of CRPC patients that will impact rational treatment decisions and prognosis. This more individualized approach is likely several years away and requires further elucidation of the relevant biologic pathways that most impact disease course and drug development and delivery strategies that can perturb them.
Currently, however, we are on the horizon of several new systemic therapies that are reinforcing biologic principles of ligand and AR signaling discovered in the past one to two decades. These therapies are showing enough promise with tolerability and efficacy that our current models will need to incorporate these patients into the current Prostate Cancer Clinical Trials Working Group 2 (PCWG2) conceptualization of the disease.
Castrate-resistant prostate cancer: clinical heterogeneity
Risk factors for prostate cancer include a male with advancing age and an intact hypothalamic-pituitary-gonadal axis. There is a relative risk increase of 1.4 for a brother of a man with prostate cancer, and having two to three first degree relatives with the disease can increase the risk by 5–11 times, respectively, over the general population. There are race disparities where African-American men are proportionately more severely affected. Between 2001 and 2005, the yearly prostate cancer incidence for African-American men was 249 (per 100,000) compared with 157 for Caucasians, and 138 for Hispanics [Jemal et al. 2009]. Furthermore, during the same 4-year period, the yearly mortality for African-Americans was 59 (per 100,000) exceeding twice that of Caucasian (25 per 100,000) and Hispanic (21 per 100,000) patients [Jemal et al. 2009]. It is unclear whether this increased mortality rate in African-American men is due to unique racial, biological, and genetic factors, rather than dietary influences, comorbid medical conditions, lifestyle differences, and/or access to healthcare.
Retrospective and prospective studies have shown, however, that race does not play a role in cause-specific survival once diagnosed with advanced CRPC [these studies are pre-PCWG2 and used cohorts of patients defined as having androgen independent prostate cancer (AIPC) which would now be considered CRPC patients], response to antiandrogens, the time to PSA nadir, PSA doubling time, or in overall prognosis [Wyatt et al. 2004; McLeod et al. 1999; Brawn et al. 1993]. Other studies have shown that race does not correlate with extent of disease in bone, number of metastases, or time to CRPC [Wyatt et al. 2004]. Nonetheless; African-American and Hispanic patients were younger at diagnosis with more advanced disease than Caucasian cohorts. There may be differences in biology that lead to difference in prevalence, and earlier onset of disease, but those differences do not appear to be driving the cancer at a disproportionate tempo once patients receive therapy.
Prostate cancer displays a range of disease tempo, from tumors of no clinical impact to those that are aggressive and lethal with multiple metastases. Between these two ends of the range are the locally advanced tumors with few metastases that insidiously infiltrate pelvic structures causing significant morbidity in the way of urinary urgency, incomplete voiding with risk for urinary obstruction, constipation and hematochezia, insomnia, fatigue, obstructive lymphedema, sacral plexus neuropathy, and pain. The clinical subsets of patients with prostate cancer become clear over time as a patient is treated or actively observed — unfortunately, current wisdom of identifying the low risk patient is only in hindsight.
Even as patients progress to advanced CRPC disease, there continues to be heterogeneity with a spectrum of disease progression. The range amongst patients with CRPC is from advanced pelvic disease with few metastases and relatively long survival of over 40 months [Assikis et al. 2004] to those that develop neuroendocrine features with a rapid tempo of disease. Accurate staging that represents the patients’ disease course is therefore important for prognostic and treatment decisions.
Castrate-resistant prostate cancer: terminology and current classification
Clinical staging describes the profile of the extent of the prostate tumor at one point in a time continuum of the patient; much like the Rai clinical staging system achieves in chronic lymphocytic leukemia [Rai et al. 1975]. As the Rai system has needed modification [Gale et al. 1987], prostate cancer clinical staging has needed to be updated or revised as new understanding of what drives castrate-resistant biology and as therapeutics are discovered that are tolerable and favorably impact prognosis. While pathologically staging based on morphologic appearance using the Gleason score is useful and provides the best indicator of patient outcome for localized disease [Maitland and Collins, 2008], it does not help in describing patients who progress, and becomes less clinically relevant when recommending treatment options.
Various terms have been used to describe patients who progress while on hormone therapy, including androgen-independent prostate cancer (AIPC), androgen refractory or hormone refractory prostate cancer (HRPC). However, the PCWG2 advised against using the term ‘hormone-refractory disease’ as it describes a response to treatment that we have learned is not absolute, as patients have had responses with therapies such as aminoglutethimide [Ahmann et al. 1987, Harnett et al. 1987], AR antagonists, ketoconazole [Figg et al. 2005], adrenalectomy or hypophysectomy [Raghavan, 1988], and 5α-reductase inhibitors [Shah et al. 2009; Eisenberger et al. 2004]. The current terminology for clinical classification is CRPC, which encompasses castrate or anorchid patients with a rising prostate specific antigen (PSA) alone; with or without metastases, radiographic progression or clinical progression [Scher et al. 2008].
Previous to the PCWG2, disease states and clinical classification were not as clearly defined and caused difficulty interpreting outcomes. Clinical experience with the antiandrogen flutamide in metastatic prostate cancer patients led to the observation of the flutamide withdrawal syndrome [Kelly et al. 1993]. The initial series described approximately a third of patients with a significant PSA decline (>50%) and clinical improvement upon discontinuation of flutamide therapy [Scher and Kelly, 1993]. It quickly became clear that antiandrogen withdrawal is a hormone maneuver in its own right. However, this important therapy and clinical state was not uniformly incorporated into clinical trial use for several more years and not doing so was likely responsible for false-positive results in many trials during that time [Scher et al. 1995]. There was confusion in interpreting trials as a hormone-sensitive patient in one trial may have been considered resistant in another [Scher et al. 1995].
A classification scheme was developed for prostate cancer based on hormone sensitivity in 1995 and used categories of hormone-naïve, androgen-independent and hormone-sensitive, and hormone-independent [Scher et al. 1995]. These clinical states correlated well with prognostic factors and treatment decisions. In practice, these clinical states had previously been loosely defined and were refined to achieve more uniform cohorts in trials that would yield relevance in the clinic [Scher et al. 1995].
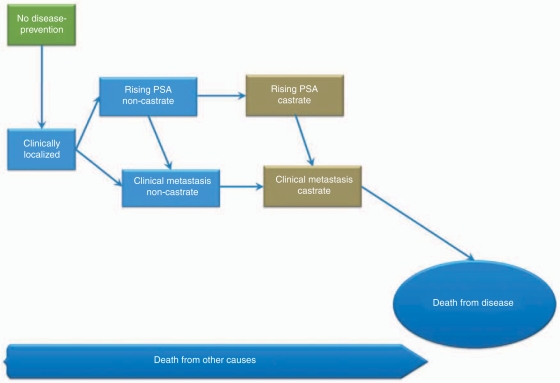
To have a common language amongst clinicians and investigators a vocabulary was established by the PCWG2 that is based on categorizing prostate cancer on a disease continuum [Scher et al. 2008]. The clinical states address presence of metastases and whether the patient has castrate levels of testosterone achieved either medically [luteinizing hormone releasing hormone (LHRH) agonist, and/or peripheral androgen receptor blockade] or by surgical orchiectomy. The states are localized disease, rising PSA and noncastrate, metastases and noncastrate, rising PSA and castrate, and metastases and castrate. This clinical staging (Figure 1) provides our most current framework to assess prognosis and define therapeutic objectives along the course of disease, rather than based on the tumor-node-metastasis (TNM) (TNM: Staging system of tumor size, regional lymph node involvement, and distant metastasis) stage of the tumor at diagnosis.
Figure 1.
Clinical states model of prostate cancer as conceptualized by the Prostate Cancer Working Group (PCWG2). Adapted from Scher et al. [2000, 2008] with permission.
Androgen-receptor signaling axis in prostate cancer
Testosterone is the major circulating androgen and 90–95% is synthesized in the Leydig cells of the testis, with 5–10% coming from the adrenals [Labrie, 2004]. Testosterone, as are all steroid hormones, is poorly soluble in water and upon release into circulation, associates with sex hormone-binding globulin (SHBG) which transports the hormone through the circulatory system to target tissues such as the prostate and skin. Circulating free testosterone enters prostate cells and is converted to dihydrotestosterone (DHT) by 5α-reductase within the prostate. DHT is the primary androgen in prostatic tissues as it binds the AR more stably.
Androgens are a requirement for growth and avoidance of apoptosis of prostate cancer cells, and castration triggers programmed cell death in both normal as well as malignant prostate cells [Denmeade et al. 1996]. Prostate cancer growth depends on androgens stimulating proliferation and inhibiting apoptosis. Castration causes a rate of cell death which is greater than the rate of cell proliferation. However, castration is rarely curative. It is not known if selection pressure leads to survival of a tumor clone that enables the CRPC state, or if alterations of the AR and/or AR signaling occur de novo [Maitland and Collins, 2008].
The AR gene is located in the X chromosome and the AR structure is similar to other steroid receptors with a modular structure of a well-conserved DNA binding domain and NH2-terminal transcriptional activation domain, in addition to a carboxyl terminal ligand binding domain [Culig et al. 2005]. The AR is identical in all androgen-responsive sites in the body and is required for normal prostate development, muscle hypertrophy, bone density, and cognition. Prior to ligand binding, the AR is sequestered in the cytoplasm with a complex of heat-shock proteins, molecular chaperones, and immunophilins which all help induce high-affinity conformation. Once bound to testosterone or DHT, the AR dissociates from the complex and forms a homodimer with exposure of the AR nuclear translocation where the bound AR engages specific chromatin regions, androgen response elements (AREs), to control target gene expression. The binding of AR with the AREs recruits coregulatory proteins which play an important role in initiation and fine tuning of transcription. PSA is the most familiar AR-regulated gene and is an important marker not only for screening but for disease progression including CRPC, indicating a functional AR signaling axis.
The AR was first noted to have an important role in resistant prostate cancer in 1991 [Van Der Kwast et al. 1991]. AR principally acts as ligand-inducible transcriptional enhancer factor. The AR signaling axis involves the physiologic ligands, testosterone and DHT, ligand regulation and transport through the bloodstream in association with SHBG, the AR, the regulation of unbound AR in the cytoplasm, and the regulation of dimerized and phosphorylated AR transport to the nucleus for engaging the ARE, and recruitment of coregulatory proteins for fine tuning transcriptional output. The axis is important in many diseases including prostate cancer, benign prostatic hyperplasia, alopecia, and hirsutism. A secondary role of the AR may mediate nongenomic signaling independent of the principal transcription factor role and does not require DNA binding, such as through the c-Src tyrosine kinase signaling cascade of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway [Migliaccio et al. 2000].
The concentration of AR in the cytosol is tightly controlled by a number of factors that includes the state of differentiation of the tissue. AR levels usually drop dramatically immediately after testosterone or DHT exposure as there is a net movement of the ligand-receptor complex to the nucleus. Eventually, the ARs are repopulated by new synthesis or recycling of receptor after dissociation from the ligand [Dehm and Tindall, 2007].
Resistance to hormonal therapy and mechanisms of AR signaling in CRPC
Achieving castrate or anorchid levels of testosterone (≤50 ng/dL) with ADT remains the most effective therapy for metastatic disease and has been long recognized as the first systemic anticancer maneuver for prostate cancer [Huggins and Hodges, 1941; Huggins et al. 1941]. Half of all men with prostate cancer are treated with ADT during the course of their disease and many for prolonged periods of time [Cooperberg et al. 2003; Meng et al. 2002]. The cancer usually responds well initially, resulting in prompt decrease in pain and PSA, with a decrease in tumor bulk. Relapse, however, is nearly inevitable and castrate-resistant disease is incurable with a time to death of approximately 2 years [Tannock et al. 2004]. The presence of SHBG, whose concentration in the circulation can change in response to a variety of physiologic conditions, can complicate efforts to accurately measure the active circulating testosterone. Nonetheless, measurement of serum testosterone is warranted in patients who are progressing on ADT with a goal of serum testosterone less than 50 ng/dL.
Though therapy is usually tolerated, side effects related to testosterone deficiency include muscle atrophy and weakness, erectile dysfunction, hair loss, insulin resistance and diabetes, increased fat mass and decreased lean body mass and anemia [Hang et al. 2005]. As estrogens are derived from aromatization of testosterone in men, estrogen deficiency commonly occurs in patients treated with ADT (either medically or surgically) and leads to side effects that include hot flashes, reduced bone mineral density and increased fracture risk, mood changes, memory loss, gynecomastia, and lipid changes [Freedland et al. 2009].
Despite these anticipated physiologic symptoms that impact nearly every organ system, a castrate serum value is not necessarily a reliable representation of the androgen level at the tumor site. In CRPC, prostate cancer cells develop a variety of aberrant pathways to survive in a castrate environment. Failure of castration therapy was presumed to be solely due to low levels of circulating nongonadal production of androgens; however tumor stem-cell, genetic, epigenetic, and tumor microenvironment in AR signaling are implicated.
The search for a prostate cancer stem cell (CSC) is difficult as it is complicated by the heterogeneity of tumors. A likely contender that retains self renewal and tumor generating capacity has the CD133+/α2β1 integrinhigh/CD44+ phenotype [Gu et al. 2007; Collins et al. 2005]. Heterogeneity and multifocality, in fact, may be the phenotypic depiction of stem cell tumorigenesis in the prostate as many stem cells become activated to acquire tumor-initiating properties. As the progeny cells either fail, expand and differentiate slowly, or expand and acquire a mutation to differentiate more rapidly; the various clones and failures contribute to the multifocality. The disease course, however, is determined by the more aggressive clone and overshadows the others. Interestingly, the prostate CSC population does not express AR, and therefore inherently resistant to hormone maneuvers. The CSC may be able to repopulate the tumor with AR dependent and AR independent progeny cells [Pienta and Bradley, 2006]. While this provides an explanation for minimal residual disease after hormone maneuvers, it is unclear why this important gain of function selection in AR is not seen at the cancer stem cell level [Maitland and Collins, 2008]. Perhaps steroid response is not conducive to activated CSCs maintaining an undifferentiated, or in some cases, a dedifferentiated (from a committed basal cell) state.
The axiom that prostate cancer is a hormone-dependent disease because it requires androgens for survival is becoming eroded by better understanding of the castrate state and now seems to be only partly true. The central dependence for tumor survival is on the AR signal, which may or may not involve an androgen ligand. Prostate cancer cells use five mechanisms mediated through the AR to promote tumor growth, three of which depend on ligand signaling. They are persistence of intratumoral androgens as a result of in situ steroidogenesis or adrenal source; AR mutations that allow promiscuous activation by otherwise nonsignaling ligands; wild-type AR gene amplification; alterations in AR coactivator to corepressor ratio that impact transcription; outlaw AR pathways that bypass the need for androgens by signaling through crosstalk with other ligand-bound receptors, cytokines, or transactivation of activated tyrosine kinase receptors in the cytosol [Mellado et al. 2009; Freeman et al. 2005; Scher and Sawyers, 2005; Eder et al. 2001; Feldman and Feldman, 2001]. One or many of these mechanisms may be playing a role, even within the same patient, and may be an explanation, in part, for heterogeneous responses.
Surreptitious tumor androgen exposure despite castrate serum levels
The first ligand-dependent mechanism of continued signaling through the AR can occur as prostate cancer tissues continue to be exposed to androgens. The source can be from extragonadal androgen conversion pathways. The adrenal gland imports cholesterol or synthesizes it de novo from acetate to make the 21-carbon pregnenolone which serves as the substrate for the multiple enzyme cascade of steroidogenesis that leads to 19-carbon androgens. 18-carbon estrogens are also produced, and through a peripheral tissue reversible interconversion pathway, can change estradiol to testosterone. An additional source is that CRPC tumors have the biochemical machinery for local intratumoral synthesis of androgens. For these reasons, tumor androgen levels may not be too far from baseline, despite what is measured in the serum [Chen et al. 2004; Mohler et al. 2004]. Recent data from human tissue samples show that castrate levels of serum androgens do not accurately or consistently represent the intratumoral androgen level which may be due to de novo synthesis of androgen within the tumor [Locke et al. 2008; Montgomery et al. 2008; Mostaghel et al. 2007; Page et al. 2006; Mohler et al. 2004]. Intratumoral levels are often close enough to baseline controls where prostate cancer cell proliferation, apoptosis, and androgen-regulated protein expression, including PSA, are unaffected [Page et al. 2006].
AR hypersensitivity: mutations
AR-activating mutations allow AR signaling by nongonadal androgens that would not normally cause AR activation. AR mutations have been identified in prostate cancer cell lines such as LNCaP and CWR22 as well as in patient tissue samples where adrenal androgens such as dihydroepiandrosterone (DHEA) or androstenedione causes a severalfold higher transcriptional response than wild-type AR [Koivisto et al. 1998; Tan et al. 1997; Taplin et al. 1995; Veldscholte et al. 1990]. According to one report, approximately 20% of metastatic tumors have an AR mutation that modulates steroid specificity [Marcelli et al. 2000]. Mutations can also increase sensitivity of the AR to nonandrogen steroids and molecules, including antiandrogens [Buchanan et al. 2004; Veldscholte et al. 1990].
AR hypersensitivity: amplification
More common is the third mechanism which is associated with increased levels of wild-type AR protein secondary to gene amplification which can impart a growth advantage without a specific mutation. This has been shown to be amplified in 20–30% of patients with CRPC and less than 5% of those with primary prostate cancer [Bubendorf et al. 1999; Koivisto et al. 1998, 1999; Visakorpi et al. 1995]. Elevated AR protein expression is necessary and sufficient to confer resistance to antiandrogen therapy in murine xenografts and sensitizes prostate cancer cells to respond to low levels of androgen ligand [Chen et al. 2004].
Alteration in coactivator to corepressor balance
Two additional AR-centric mechanisms of CRPC are considered that activate the AR with minimal to no ligand requirement. These are indirect mechanisms of continued AR signaling and involve coregulator molecules, and crosstalk with tyrosine kinases, cytokines, and other receptors. Though indirect, these mechanisms are not less significant; and on the contrary, perhaps have as much of a role in resistance to castration as the more intuitively proposed and parsimonious direct mechanisms. Interestingly, these mechanisms have the potential to be magnified by the other aberrant pathways, when present, such as AR amplification, AR mutation, and preserved intratumoral androgen levels.
Binding of AR homodimers at the AREs induces the attraction of coregulatory molecules such as the cointegrators c-AMP response element binding protein (CREB)-binding protein (in total ‘CBP’) and p300, coactivators of AR-associated (ARA) proteins and p160, and corepressors such as nuclear receptor corepressor (NCoR) and SMRT (silencing mediator for retinoic acid and thyroid hormone receptors). Transcription depends on the recruitment of RNA polymerase II to promoters of the AR target genes. The coregulator molecules make up the preinitiation complex (PIC) and direct specific interaction of RNA polymerase II and the promoter and can contribute to transcriptional synergy (coactivators), or in the case of competitive binding of these factors, transcriptional repression (corepressors) [Vo and Goodman, 2001] of the AR function. These molecules help regulate accessibility of gene promoters to transcription and DNA replication machinery through two active pathways or classes including ATP-dependent chromatin remodeling complexes that reorganize chromatin structure, and a class of enzymes that catalyze posttranslational modifications in histones, the best characterized are histone acetyltransferases (HAT) and histone deacetylases (HDAC).
AR-associated coregulators are important to the development of aberrant AR action. Not only is this true for prostate cancer, but is seen in other diseases such as the link of androgenic alopecia or hepatitis B virus to preponderance of male risk of hepatocellular cancer [Chiu et al. 2007; Lee et al. 2005]. In each of these, changing the coactivator to corepressor ratio can direct a change in AR function. Deregulated expression of AR coactivators tends to increase with tumor dedifferentiation and correlate with aggressive disease and poor prognosis [Need et al. 2009]. Specifically, the AR p160 coactivator family of steroid receptor coactivator-1, and others, can recruit HAT activity [Kang et al. 2004]. ARA proteins enhance AR-dependent transcriptional activation and are well studied in prostate cancer [Kikuchi et al. 2007; Mestayer et al. 2003; Li et al. 2002; Yeh et al. 1999]. For instance, ARA267α enhances AR-dependent transcription that is additive to HAT activity [Kang et al. 2004]. ARA54 and ARA70 can sensitize the AR to lower concentrations of testosterone or DHT, or even alternative ligands such as estradiol and hydroxyflutamide, or even allow ligand-independent enhancement of cell cycle progression via induction of cyclin D1, or via receptor tyrosine kinases such as HER2 [Kikuchi et al. 2007; Scher and Sawyers, 2005]. Corepressor proteins NCoR and SMRT can interact to form complexes with HDAC [Xu et al. 1999] and inhibit AR function in a ligand-dependent manner.
Outlaw pathways
The fifth mechanism is AR transcriptional activity that can be activated in the absence of androgen. Signaling to a genomic (via AREs) or nongenomic route is possible. Signaling of the AR to the AREs is possible through crosstalk with agonist occupied membrane receptors as described in many steroid hormone receptors including estrogen receptor, progesterone receptor, and AR [Scher and Sawyers, 2005; Pietras et al. 1995; Zhang et al. 1994; Power et al. 1991].
AR crosstalk and transactivation can occur with transduction pathways for proliferation, survival, or motility. This signaling mechanism does not signal to AREs and is considered a nongenomic pathway as opposed to the aforementioned. It may or may not involve ligand and can occur through transactivation of kinases, or crosstalk with cytokines or growth factor receptors.
Ability to transactivate through nongenomic mechanisms via cholesterol-rich lipid rafts is one way of linking AR signaling to the proliferative and cell survival transduction pathways [Baron et al. 2004; Migliaccio et al. 2000; Peterziel et al. 1999]. Transactivation with activated tyrosine kinase receptors has been shown between the AR-signaling path and activation of the MAPK and MAPK/extracellular signaling-regulated kinase kinase-1 (MEKK1), and the epidermal growth factor receptor (EGFR) pathway, including HER2/neu [Bonaccorsi et al. 2004; Abreu-Martin et al. 1999; Peterziel et al. 1999].
AR can also crosstalk with cytokines including nuclear factor kappa B (NF-κB), IL-6, IL-8, in addition to beta-catenin [Malinowska et al. 2009; Robinson et al. 2008; Seaton et al. 2008]. Crosstalk with other growth factor receptors such as EGFR, v-raf murine sarcoma viral oncogene homolog B1 (BRAF), and v-src sarcoma viral oncogene homolog (SRC) can induce activation of growth and survival pathways including MAPK, v-akt murine thymoma viral oncogene homolog (AKT), signal transducer and activator of transcription (STAT). This crosstalk enhancement of AR signaling has been shown to confer castration resistance in preclinical models [Wang et al. 2007].
Castrate-resistant prostate cancer: novel therapies for AR-signaling depletion
As prostate cancer continues to evade hormone manipulations and rely on AR signaling, drug development has focused efforts to target the pathway at many points for CRPC patients (Table 1). Therapies developed include novel and potent AR antagonists which block AR nuclear translocation and DNA binding, addressing prostate tumor in situ steroidogenesis with enzyme-specific adrenal androgen inhibitors, trials of 5α-reductase inhibition, addressing crosstalk and transactivation pathways with small molecule tyrosine kinase inhibition or mammalian target of rapamycin (mTOR) inhibition. Investigations for targeting coregulator molecules are underway, and arsenic trioxide has in vitro results that support a coregulator inhibition that attenuates the AR signal. Histone deacetylase (HDAC) inhibition may also impact coregulator ability of the PIC to access the chromatin structure.
Table 1.
Mechanisms of continued androgen receptor (AR) signaling axis despite castration and options for noncytotoxic chemotherapy therapeutic intervention.
| AR-signaling | Target | Therapeutic intervention |
|---|---|---|
| Ligand-dependent pathways | ||
| Testis androgen synthesis | H-P-G axis | LHRH or GnRH agonists |
| Degarelix (GnRH antagonist) | ||
| Orchiectomy | ||
| Adrenal androgen synthesis | Androgen feedback, DHEA, ERβ | DES [Kitahara et al. 1999; Pravettoni et al. 2007] |
| Non-specific CYP-17 | Ketoconazole | |
| 11β-hydroxylase | ||
| CYP 17A1 (17α hydroxylase & C17,20-lyase) | Abiraterone* | |
| Tumor androgen synthesis | 5α-R, primarily type II isozyme | Finasteride |
| 5α-R, primarily type I isozyme | Dutasteride | |
| AR mutation/alternate ligands | AR | Bicalutamide after flutamide |
| Nilutamide after bicalutamide | ||
| AAWD | ||
| BMS-641988* [Mellado et al. 2009] | ||
| AR | Antiandrogens | |
| Progestins | ||
| Glucocorticoids | ||
| AR gene amplification | AR, nuclear trans and ARE binding | MDV3100* [Scher et al. 2009] |
| RD162* [Tran et al. 2009] | ||
| Ligand-independent pathways | ||
| Coregulator | HDAC | SAHA |
| LBH589 | ||
| P160, TIF2 | Arsenic trioxide [Rosenblatt and Burnstein, 2009] | |
| Crosstalk/transactivation | Cell surface receptors | RTK inhibitors, antibodies |
| Src pathway | Dasatinib [Yu et al. 2008] | |
| PTEN loss with active mTOR pathway | Temsirolimus [Wu et al. 2005] | |
| PI3K activation by HER-2 signaling | Lapatinib [Whang et al. 2008] | |
| PKCβ/PI3K/AKT | Enzastaurin* |
5α-R, 5α-reductase; AAWD, anti-androgen withdrawal; AR, androgen receptor; ARE, androgen response elements; DES, diethylstilbestrol;, dehydroepiandrosterone; diethylstilbestrol; DHEA, dehydroepiandrosterone; CYP, cytochrome; cytochrome P450; ER, estrogen receptor; GnRH, gonadotropin releasing hormone; HDAC, histone deacetylase; H-P-G, hypothalamic-pituitary-gonadal; LHRH, luteinizing hormone release hormone; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinases; PTEN, phosphatase and tensin homolog; RTK, receptor tyrosine kinase; SAHA, suberoylanilide hydroxamic acid; Src, sarcoma; TIF2, transcriptional mediators/intermediary factor 2; trans, translocation.
not FDA approved, in clinical trials.
Of those that are not FDA approved, perhaps abiraterone acetate is the furthest along in clinical trials and showing tolerability and efficacy. Abiraterone acetate is a prodrug low molecular weight inhibitor of CYP17A1, also known in the adrenal steroid pathway as the dual role enzyme of 17α-hydroxylase, and 17, 20 desmolase. A different name is given to a different activity of the same enzyme as it adds a hydroxyl group to pregnenolone to form 17α-hydroxypregnenolone, and then acting as 17, 20 desmolase (also called a lyase) removes a side-chain carbon to form DHEA. The same enzymes are involved in the parallel progesterone pathway in the conversion from progesterone to 17α-hydroxyprogesterone to androstenedione. The phase I single center study of abiraterone acetate showed that 21 chemotherapy and ketoconazole naïve patients with metastatic CRPC tolerated the therapy well and had significant antitumor activity with over half of patients experiencing a greater than 50% PSA decline that lasted a minimum of 3 months [Attard et al. 2008a; De Bono et al. 2008]. This trial seamlessly expanded into a two stage, single-arm, phase II trial where abiraterone acetate was dosed once daily at 1000 mg for 28 day cycles until PSA progression at which point dexamethasone 0.5 mg daily was added. In the 42 phase II patients, 67% had a more than 50% decline in PSA, and declines of more than 90% were seen in eight patients [Attard et al. 2009a]. The median time to PSA progression (TTPP) was 225 days, at which point dexamethasone 0.5 mg daily was added and yielded an additional 151 days of median TTPP. The additional response gained with dexamethasone was independent of previous treatment with dexamethasone [Attard et al. 2009a].
While all these patients had progressive disease despite castration and further reduction in androgens were possible, this raises the question whether we can call the responding patients truly castrate resistant. Unfortunately biomarkers have not helped us to identify this subset of patients. This will be addressed in the phase III trial of abiraterone acetate which is a placebo-controlled trial in combination with prednisone. The primary endpoint is overall survival and also incorporates prospective evaluation of whether circulating tumor cell (CTC) counts after treatment can serve as a robust intermediate endpoint for overall survival [Attard et al. 2009a]. It has completed enrollment of over 1100 patients and results are maturing that will yield further information on the population that may have a clinical benefit. [National Institutes of Health et al. 2009]
MDV3100 is a second generation small-molecule antagonist of the AR that prevents nuclear translocation and DNA binding of AR without agonist activity [Tran et al. 2009] that recently reported interim results of a phase I/II trial of 140 patients that were either chemotherapy naïve or postchemotherapy with progression of CRPC. The dose-escalation study of 30–600 mg orally daily was well tolerated and nearly half of the patients had at least a 50% PSA decline at week 12; and 38% of evaluable patients receiving 240 mg/day dosing had a radiographic partial response (PR) [Scher et al. 2009]. An international phase III placebo-controlled trial is underway using the 240 mg/day dose [National Institutes of Health et al. 2009].
Systemic therapies in development that do not target the AR signal are also being investigated and include immunotherapy; bone targeted therapy with a monoclonal antibody against receptor activator of nuclear factor κ B ligand (RANK-L), oral endothelin inhibitors; Src kinase inhibitors such as dasatinib; altering tumor apoptotic sensitivity to chemotherapy using a clusterin inhibitor; monoclonal antibody to prostate specific membrane antigen, and an antisense oligonucleotide against B-cell leukemia/lymphoma-2 gene (BCL-2). Review of novel therapies is beyond the scope of this article and can be reviewed in recent publications [Fleming et al. 2009; Lassi and Dawson, 2009; Shah et al. 2009; Tran et al. 2009]
Molecular heterogeneity in CRPC: TMPRSS2:ETS and the lethal phenotype
It is clear the past decade has seen a significant increase in understanding the basis of prostate cancer progression and specifically the role of the AR and the factors it cooperates with while unbound in the cytosol or during activated signaling to the nucleus. The AR mechanisms of resistance are the survival traits prostate cancer has developed or acquired to flourish. Many of the ligand-independent crosstalk and transactivation mechanisms are important in enabling prostate cancer to share in the ‘hallmarks’ that define the common characteristics of cancer [Hanahan and Weinberg, 2000] and lend to the molecular lethal phenotype [Loberg et al. 2007]. These AR mechanisms enable genetic instability, limitless replicative potential, evasion of cell death, and can be rendered capable of growth factor independent growth.
The number of AR coregulators reported is over 150, indicating a daunting level of functional diversity among these proteins [Heemers and Tindall, 2007]. Also, crosstalk and transactivation lend AR the ability to interact with — and in many cases such as EGFR, regulate — other growth factor receptors and signals, tyrosine kinases, and cytokine pathways. There are also many molecular pathways that are important in the initiation and progression of prostate cancer. Germ-line mutations of several genes have been found in a fairly small subset of hereditary prostate cancers. Promoter hypermethylation leads to loss of expression of glutathione S-transferase gene (GSTP1) and is seen in many prostate (and other) cancers as well as prostatic intraepithelial neoplasia (PIN) [Lee et al. 1994]. Another frequent mutation seen in prostate cancers (many early, localized), is allelic loss of chromosome 8p. More recently discovered is the first epithelial malignancy fusion gene, and is found in over 50% of prostate tumors. This now links the pathogenic AR in CRPC to oncogenic transcription factors, ETS (erythroblastosis virus E26 oncogene like - avian).
The most common mechanism of ETS overexpression is the fusion of the ETS gene, ETS gene-related gene (ERG), with the highly AR-regulated transmembrane protease serine 2 (TMPRSS2) gene [Tomlins et al. 2005]. TMPRSS2 is expressed in normal and malignant prostate and dependent on androgen. ERG is a transcription factor involved in oncogenic translocations in Ewing’s sarcoma and myeloid leukemias [Saramaki et al. 2008]. Additionally, ERG interacts with histone methyltransferase and may impact silencing of target genes [Saramaki et al. 2008]. Both are close in proximity on chromosome 21, and interstitial deletion (Edel) of sequences 5′ to ERG has been found to be a common mechanism of TMPRSS2-ERG fusion in prostate cancer. Moreover, in CRPC, Edel was found to be the exclusive mechanism of rearrangement and with a particularly aggressive phenotype in a cohort of 30 men [Mehra et al. 2008].
TMPRSS2 also can become fused to other ETS transcription factors such as ETV1 and ETV4 and researchers have looked to correlate fusion events with histologic phenotype and prognosis. Researchers have identified a significant association between the fusion gene and prostate cancer specific death, as well as a link between ERG perturbations and a higher Gleason score [Demichelis et al. 2007]. The 5′ to ERG Edel fusion is found in approximately 60–70% of the cancers containing ERG rearrangements and has been found to correlate with a higher tumor stage and pelvic lymph node metastasis [Attard et al. 2008b; Perner et al. 2006]. Further clinicopathologic correlation has identified a subtype with poor survival using fluorescent in situ hybridization (FISH) analysis. These patients comprised nearly 7% of 445 previously untreated patients and were found to have a 2+ Edel (two or more FISH copies of 3′ to ERG without 5′ to ERG) which correlated with a poor cause-specific and overall survival in a multivariate analysis.
The estrogen receptor (ER) is also implicated as ERα stimulation results in TMPRSS2-ERG upregulation [Mertz et al. 2008; Taplin, 2007], even in an AR-negative cell line [Taplin, 2007]. This suggests that an ERα antagonist may have activity in prostate cancer through inhibition of TMPRSS2-ERG expression. In fact, antitumor activity of abiraterone acetate may be explained, in part, by suppressing estradiol and therefore attenuating upregulation of TMPRSS2-ERG expression via the ERα receptor [Ellem and Risbridger, 2007]. The abiraterone phase I study investigated this hypothesis as a correlative and found that of six patients with the TMPRSS2-ERG gene fusion, five responded to abiraterone acetate with a >50% PSA decline, a response that was 30% more than the overall group [Attard et al. 2008a]. It is not clear if the success of abiraterone acetate in this case was acting by decreasing estradiol availability which could otherwise act promiscuously with the AR directly, abolish extragonadal tissue androgen production through its intended CYP17A1 inhibition, or by inhibiting the reversible interconversion pathway of estradiol to testosterone.
Another treatment directed at the TMPRSS2-ERG gene fusion can take advantage of its dependence on HDAC for fusion gene activation. HDAC inhibitors have been shown to directly block genes involved in the TMPRSS2:ERG fusion and warrant further clinical studies to evaluate these drugs in this setting [Attard et al. 2009b; Welsbie et al. 2009; Bjorkman et al. 2008; Tomlins et al. 2008; Kelly et al. 2002].
Need for a new CRPC clinical state: androgen depleted
A Medline search of keywords ‘castration resistant’ or ‘castrate resistant’ yields its earliest relevant usage in 2000 and defined as prostate cancer that has progressed despite medical or surgical means of castration [Solit et al. 2003; Scher and Heller, 2000]. To define a patient’s clinical state as castrate resistant gives it a basis in physiology we can all understand; i.e. medical or surgical castration levels of circulating free testosterone. Reference to the clinical syndrome of ‘hormone resistance’ can be interpreted as a patient with a prostate tumor that is resistant to endogenous androgens, or, as more often used, a patient who no longer responds to hormone maneuvers. We now understand that it is more complicated than just what’s going on with the serum testosterone level.
A hormone-resistant tumor is now redefined as one that progresses in an environment of collateral androgen depletion (CoAD) where androgen levels are ablated beyond castrate levels. CoAD maneuvers target the ligand-dependent pathways of gonadal and extragonadal (physiologic), and in situ tumor (pathologic) androgenesis. A combination of ligand-dependent directed therapeutic interventions (see Table 1) can achieve the CoAD state.
Despite these interventions to ablate the ligand, AR signaling may persist via the ligand-independent crosstalk and transactivation pathways. Early data for treatment of the AR signal ligand-dependent pathways are demonstrating a substantial number of patients with potential long remissions. Unfortunately, however, there does appear to be a population of patients that progress despite castration plus CoAD treatments. Though the AR-signaling ligand-dependent pathways are maximally attenuated, there is likely a predominant AR-signaling ligand-independent pathway responsible as the principal cause for this group of resistant patients.
In the androgen-depleted state, AR signal ligand-independent pathways are likely a survival trait the prostate cancer developed through chance accumulation, or clonal expansion with or without selective therapeutic pressure. They are responsible for much of the latitude of cancer survival and ultimately, its lethal phenotype. Hormone-resistant tumors could perpetuate solely through the ligand-independent pathways. The proposed prostate CSCs do not express AR, and may impart in progeny tumor cells robust ‘workaround’ AR signal ligand-independent pathways that could serve to be the principal operator of progression in hormone-resistant tumors.
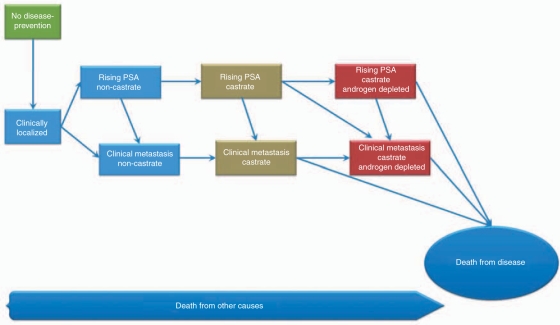
A modification to the current PCWG2 clinical states model is needed to classify this new population of castrate resistant patients with clinical metastasis. We propose a new category of ‘androgen depleted’ which is the clinical state of patients with tumor that have predominant AR-signaling ligand-independent pathways driving proliferation. A schematic to incorporate the new clinical state is shown in Figure 2. Androgen-depleted patients may or may not have metastases and the state is preceded by castrate patients that have either rising PSA or clinical metastases. Death from other causes can occur along this continuum, though death from disease represents a proportionately larger fraction of androgen-depleted castrate patients with a rising PSA and those with clinical metastases.
Figure 2.
Proposed clinical states model of prostate cancer to incorporate a new category of androgen-depleted clinical states which reflect tumors that are have predominant AR signaling via ligand-independent pathways.
Interest has turned to therapeutic intervention of the AR-signal ligand-independent pathways with familiar receptor targets such as EGFR, and HER2 and using antibodies and small-molecule receptor tyrosine kinase (RTK) inhibitors. Clinical trials are investigating the effect of dasatinib, gefitinib, cetuximab, mTOR inhibitors, the PKCβ inhibitor, enzastaurin, in combination with cytotoxic agents. Additionally, there are several AR-signal pathway inhibitors that are in preclinical and clinical testing, and of these, the second-generation antiandrogens like MDV 3100 with ability to degrade the AR and RD162 are likely the furthest along showing tolerability and efficacy [Tran et al. 2009]. As we develop therapeutics for this web of AR signaling, we anticipate a learning curve for clinical trialists to determine their optimal usage. Approaches being considered include sequential use in advanced disease, use in earlier disease, as well as adjuvant to primary therapy. In addition, due to AR-signal redundancy, clinical benefit may be gained from adding AR-signal active agents rather than switching after a failed therapy. Depleting the AR-signal redundancy would require an annihilation approach where CoAD treatment is combined with ligand-independent interventions. For example, an annihilation approach may include combination of an LHRH agonist, 5α-reductase inhibitor, a second-generation [Tran et al. 2009] antiandrogen, a CYP17A1 inhibitor, and an AR degrader, such as an HDACi.
Research has shown since the 1940s that the AR signal is crucial to attenuate in prostate cancer. The AR cascade interacts with many chaperones and other cell machinery and signals, and can become hypersensitive to ligand, use ligand that is produced in the tumor tissue or extragonadal pathways, use signals from other growth receptors, crosstalk with cytokines, transactivate tyrosine kinases, and can be preferentially activated by its coregulators. Now that we know an oncogene is a downstream target of AR in the majority of patients, namely the TMPRSS2-ETS fusion gene, depleting androgen and inhibiting the AR signal is paramount.
Conclusion
Prostate cancer has a heterogeneous clinical phenotype that can be correlated with key molecular events and signatures. Continuing to prospectively determine which molecular signatures correlate with disease specific outcome is important. The Gleason score is currently the best pathologic predictor of outcome for localized disease and novel biomarkers of AR-driven pathways are desperately needed as we continue our development of the new generation of androgen and AR therapeutics. However, the current classification schema for CRPC does not take into account these new AR therapeutics and we propose the clinical state ‘androgen depleted’ to reflect the AR-signal ligand-independent pathways for those patients who are refractory to castration plus CoAD. This clinical state is proposed to create a common vocabulary amongst clinical trialists and physicians so data sets can be interpreted accurately, and correlation with prognosis will be needed to qualify the new category.
Conflict of interest statement
None declared.
References
- Abreu-Martin M.T., Chari A., Palladino A.A., Craft N.A., Sawyers C.L. (1999) Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol Cell Biol 19: 5143–5154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmann F.R., Crawford E.D., Kreis W., Levasseur Y. (1987) Adrenal steroid levels in castrated men with prostatic carcinoma treated with aminoglutethimide plus hydrocortisone. Cancer Res 47: 4736–4739 [PubMed] [Google Scholar]
- Assikis V.J., Do K.-A., Wen S., Wang X., Cho-Vega J.H., Brisbay S., et al. (2004) Clinical and biomarker correlates of androgen-independent, locally aggressive prostate cancer with limited metastatic potential. Clin Cancer Res 10: 6770–6778 [DOI] [PubMed] [Google Scholar]
- Attard G., Clark J., Ambroisine L., Fisher G., Kovacs G., Flohr P., et al. (2008b) Duplication of the fusion of Tmprss2 to Erg sequences identifies fatal human prostate cancer. Oncogene 27: 253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G., Reid A.H.M., Yap T.A., Raynaud F., Dowsett M., Settatree S., et al. (2008a) Phase I clinical trial of a selective inhibitor of Cyp17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 26: 2564–4571 [DOI] [PubMed] [Google Scholar]
- Attard G., Jameson C., Moreira J., Flohr P., Parker C., Dearnaley D., et al. (2009b) Hormone-sensitive prostate cancer: a case of Ets gene fusion heterogeneity. J Clin Pathol 62: 373–376 [DOI] [PubMed] [Google Scholar]
- Attard G., Reid A.H.M., A'hern R., Parker C., Oommen N.B., Folkerd E., et al. (2009a) Selective inhibition of Cyp17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol 27: 3742–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S., Manin M., Beaudoin C., Leotoing L., Communal Y., Veyssiere G., et al. (2004) Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-oh kinase in androgen-sensitive epithelial cells. J Biol Chem 279: 14579–14586 [DOI] [PubMed] [Google Scholar]
- Bjorkman M., Iljin K., Halonen P., Sara H., Kaivanto E., Nees M., et al. (2008) Defining the molecular action of hdac inhibitors and synergism with androgen deprivation in Erg-positive prostate cancer. Int J Cancer 123: 2774–2781 [DOI] [PubMed] [Google Scholar]
- Bonaccorsi L., Carloni V., Muratori M., Formigli L., Zecchi S., Forti G., et al. (2004) Egf receptor (Egfr) signaling promoting invasion is disrupted in androgen-sensitive prostate cancer cells by an interaction between EGFR and androgen receptor (AR). Int J Cancer 112: 78–86 [DOI] [PubMed] [Google Scholar]
- Brawn P.N., Johnson E.H., Kuhl D.L., Riggs M.W., Speights V.O., Johnson C.F., et al. (1993) Stage at presentation and survival of white and black patients with prostate carcinoma. Cancer 71: 2569–2573 [DOI] [PubMed] [Google Scholar]
- Bubendorf L., Kononen J., Koivisto P., Schraml P., Moch H., Gasser T.C., et al. (1999) Survey of gene amplifications during prostate cancer progression by high throughput fluorescence in situ hybridization on tissue microarrays. Cancer Res 59: 803–806 [PubMed] [Google Scholar]
- Buchanan G., Yang M., Cheong A., Harris J.M., Irvine R.A., Lambert P.F., et al. (2004) Structural and functional consequences of glutamine tract variation in the androgen receptor. Hum Mol Genet 13: 1677–1692 [DOI] [PubMed] [Google Scholar]
- Chen C.D., Welsbie D.S., Tran C., Baek S.H., Chen R., Vessella R., et al. (2004) Molecular determinants of resistance to antiandrogen therapy. Nat Med 10: 33–39 [DOI] [PubMed] [Google Scholar]
- Chiu C.M., Yeh S.H., Chen P.J., Kuo T.J., Chang C.J., Chen P.J., et al. (2007) Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci U S A 104: 2571–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A.T., Berry P.A., Hyde C., Stower M.J., Maitland N.J. (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65: 10946–10951 [DOI] [PubMed] [Google Scholar]
- Cooperberg M.R., Grossfeld G.D., Lubeck D.P., Carroll P.R. (2003) National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst 95: 981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culig Z., Steiner H., Bartsch G., Hobisch A. (2005) Mechanisms of endocrine therapy-responsive and -unresponsive prostate tumours. Endocr Relat Cancer 12: 229–244 [DOI] [PubMed] [Google Scholar]
- De Bono J.S., Attard G., Reid A.H., Parker C., Dowsett M., Mollife R., et al. (2008) Anti-tumor activity of abiraterone acetate (AA), a Cyp17 inhibitor of androgen synthesis, in chemotherapy naive and docetaxel pre-treated castration resistant prostate cancer (Crpc). J Clin Oncol (Meeting Abstracts) 26: 5005–5005 [Google Scholar]
- Dehm S.M., Tindall D.J. (2007) Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol 21: 2855–2863 [DOI] [PubMed] [Google Scholar]
- Demichelis F., Fall K., Perner S., Andren O., Schmidt F., Setlur S.R., et al. (2007) Tmprss2: Erg gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene 26: 4596–4599 [DOI] [PubMed] [Google Scholar]
- Denmeade S.R., Lin X.S., Isaacs J.T. (1996) Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate 28: 251–265 [DOI] [PubMed] [Google Scholar]
- Eder I.E., Culig Z., Putz T., Nessler-Menardi C., Bartsch G., Klocker H. (2001) Molecular biology of the androgen receptor: from molecular understanding to the clinic. Eur Urol 40: 241–251 [DOI] [PubMed] [Google Scholar]
- Eisenberger M.A., Laufer M., Vogelzang N.J., Sartor O., Thornton D., Neubauer B.L., et al. (2004) Phase I and clinical pharmacology of a type I and II, 5-alpha-reductase inhibitor (Ly320236) in prostate cancer: elevation of estradiol as possible mechanism of action. Urology 63: 114–119 [DOI] [PubMed] [Google Scholar]
- Ellem S.J., Risbridger G.P. (2007) Treating prostate cancer: a rationale for targeting local oestrogens. Nat Rev Cancer 7: 621–627 [DOI] [PubMed] [Google Scholar]
- Feldman B.J., Feldman D. (2001) The development of androgen-independent prostate cancer. Nat Rev Cancer 1: 34–45 [DOI] [PubMed] [Google Scholar]
- Figg W.D., Liu Y., Arlen P., Gulley J., Steinberg S.M., Liewehr D.J., et al. (2005) A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. J Urol 173: 790–796 [DOI] [PubMed] [Google Scholar]
- Fleming M.T., Sonpavde G., Kondagunta G.V., Galsky M.D., Hutson T.E., Sternberg C.N. (2009) Systemic therapy and novel agents for metastatic castration resistant prostate cancer. Update Cancer Ther 3: 133–145 [Google Scholar]
- Freedland S.J., Eastham J., Shore N. (2009) Androgen deprivation therapy and estrogen deficiency induced adverse effects in the treatment of prostate cancer. Prostate Cancer Prostatic Dis 12: 333–338 [DOI] [PubMed] [Google Scholar]
- Freeman M.R., Cinar B., Lu M.L. (2005) Membrane rafts as potential sites of nongenomic hormonal signaling in prostate cancer. Trends Endocrinol Metab 16: 273–279 [DOI] [PubMed] [Google Scholar]
- Gale R.P., Rai K.R. (1987) New insights into a chronic lymphocytic leukemia. Leukemia 1: 677–679 [PubMed] [Google Scholar]
- Gu G., Yuan J., Wills M., Kasper S. (2007) Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res 67: 4807–4815 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Hang L., Katelyn M., Joel S.F., Matthew R.S. (2005) Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer 104: 1633–1637 [DOI] [PubMed] [Google Scholar]
- Harnett P.R., Raghavan D., Caterson I., Pearson B., Watt H., Teriana N., et al. (1987) Aminoglutethimide in advanced prostatic carcinoma. Br J Urol 59: 323–327 [DOI] [PubMed] [Google Scholar]
- Heemers H.V., Tindall D.J. (2007) Androgen receptor (Ar) coregulators: a diversity of functions converging on and regulating the Ar transcriptional complex. Endocr Rev 28: 778–808 [DOI] [PubMed] [Google Scholar]
- Huggins C., Hodges C.V. (1941) Studies on prostatic cancer – I the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1: 293–297 [DOI] [PubMed] [Google Scholar]
- Huggins C., Stevens R.E., Hodges C.V. (1941) Studies on prostate cancer II the effects of castration on advanced carcinoma of the prostate gland. Arch Surg 43: 209–223 [Google Scholar]
- Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M.J. (2009) Cancer statistics, 2009. CA Cancer J Clin 59: 225–249 [DOI] [PubMed] [Google Scholar]
- Kang Z., Janne O.A., Palvimo J.J. (2004) Coregulator recruitment and histone modifications in transcriptional regulation by the androgen receptor. Mol Endocrinol 18: 2633–2648 [DOI] [PubMed] [Google Scholar]
- Kelly W.K., Scher H.I. (1993) Prostate specific antigen decline after antiandrogen withdrawal – the flutamide withdrawal syndrome. J Urol 149: 607–609 [DOI] [PubMed] [Google Scholar]
- Kelly W.K., O'Connor O.A., Marks P.A. (2002) Histone deacetylase inhibitors: from target to clinical trials. Expert Opin Investig Drugs 11: 1695–1713 [DOI] [PubMed] [Google Scholar]
- Kikuchi H., Uchida C., Hattori T., Isobe T., Hiramatsu Y., Kitagawa K., et al. (2007) Ara54 Is involved in transcriptional regulation of the cyclin d1 gene in human cancer cells. Carcinogenesis 28: 1752–1758 [DOI] [PubMed] [Google Scholar]
- Kitahara S., Umeda H., Yano M., Koga F., Sumi S., Moriguchi H., et al. (1999) Effects of intravenous administration of high dose-diethylstilbestrol diphosphate on serum hormonal levels in patients with hormone-refractory prostate cancer. Endocr J 46: 659–664 [DOI] [PubMed] [Google Scholar]
- Koivisto P., Kolmer M., Visakorpi T., Kallioniemi O.P. (1998) Androgen receptor gene and hormonal therapy failure of prostate cancer. Am J Pathol 152: 1–9 [PMC free article] [PubMed] [Google Scholar]
- Koivisto P.A., Schleutker J., Helin H., Ehren-Van Eekelen C., Kallioniemi O.P., Trapman J. (1999) Androgen receptor gene alterations and chromosomal gains and losses in prostate carcinomas appearing during finasteride treatment for benign prostatic hyperplasia. Clin Cancer Res 5: 3578–3582 [PubMed] [Google Scholar]
- Labrie F. (2004) Adrenal androgens and intracrinology. Semin Reprod Med 22: 299–309 [DOI] [PubMed] [Google Scholar]
- Lassi K., Dawson N.A. (2009) Emerging therapies in castrate-resistant prostate cancer. Curr Opin Oncol 21: 260–265 [DOI] [PubMed] [Google Scholar]
- Lee W.H., Morton R.A., Epstein J.I., Brooks J.D., Campbell P.A., Bova G.S., et al. (1994) Cytidine methylation of regulatory sequences near the pi-class glutathione s-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci U S A 91: 11733–11737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P., Zhu C.C., Sadick N.S., Diwan A.H., Zhang P.S., Liu J.S., et al. (2005) Expression of androgen receptor coactivator Ara70/Ele1 in androgenic alopecia. J Cutan Pathol 32: 567–571 [DOI] [PubMed] [Google Scholar]
- Li P., Yu X., Ge K., Melamed J., Roeder R.G., Wang Z. (2002) Heterogeneous expression and functions of androgen receptor co-factors in primary prostate cancer. Am J Pathol 161: 1467–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loberg R.D., Bradley D.A., Tomlins S.A., Chinnaiyan A.M., Pienta K.J. (2007) The lethal phenotype of cancer: the molecular basis of death due to malignancy. CA Cancer J Clin 57: 225–241 [DOI] [PubMed] [Google Scholar]
- Locke J.A., Guns E.S., Lubik A.A., Adomat H.H., Hendy S.C., Wood C.A., et al. (2008) Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res 68: 6407–6415 [DOI] [PubMed] [Google Scholar]
- Maitland N.J., Collins A.T. (2008) Prostate cancer stem cells: a new target for therapy. J Clin Oncol 26: 2862–2870 [DOI] [PubMed] [Google Scholar]
- Malinowska K., Neuwirt H., Cavarretta I.T., Bektic J., Steiner H., Dietrich H., et al. (2009) Interleukin-6 stimulation of growth of prostate cancer in vitro and in vivo through activation of the androgen receptor. Endocr Relat Cancer 16: 155–169 [DOI] [PubMed] [Google Scholar]
- Marcelli M., Ittmann M., Mariani S., Sutherland R., Nigam R., Murthy L., et al. (2000) Androgen receptor mutations in prostate cancer. Cancer Res 60: 944–949 [PubMed] [Google Scholar]
- McLeod D.G., Schellhammer P.F., Vogelzang N.J., Soloway M.S., Sharifi R., Block N.L., et al. (1999) Exploratory analysis on the effect of race on clinical outcome in patients with advanced prostate cancer receiving bicalutamide or flutamide, each in combination with Lhrh analogues. Prostate 40: 218–224 [DOI] [PubMed] [Google Scholar]
- Mehra R., Tomlins S.A., Yu J., Cao X., Wang L., Menon A., et al. (2008) Characterization of Tmprss2-Ets gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res 68: 3584–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado B., Codony J., Ribal M.J., Visa L., Gascon P. (2009) Molecular biology of androgen-independent prostate cancer: the role of the androgen receptor pathway. Clin Trans Oncol 11: 5–10 [DOI] [PubMed] [Google Scholar]
- Meng M.V., Gary D.G., Natalia S., Shilpa S.M., Deborah P.L., Peter R.C. (2002) Contemporary patterns of androgen deprivation therapy use for newly diagnosed prostate cancer. Urology 60: 7–11 [DOI] [PubMed] [Google Scholar]
- Mertz K.D., Setlur S.R., Hoshida Y., Demichelis F., Lupien M., Perner S., et al. (2008) Tmprss2-Erg fusion prostate cancer is a molecularly distinct estrogen-sensitive subclass of aggressive prostate cancer, 97th Annual Meeting of the United-States-and-Canadian-Academy-of-Pathology, Nature Publishing Group: Denver, CO [Google Scholar]
- Mestayer C., Blanchere M., Jaubert F., Dufour B., Mowszowicz I. (2003) Expression of androgen receptor coactivators in normal and cancer prostate tissues and cultured cell lines. Prostate 56: 192–200 [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Castoria G., Di Domenico M., De Falco A., Bilancio A., Lombardi M., et al. (2000) Steroid-induced androgen receptor-oestradiol receptor beta-src complex triggers prostate cancer cell proliferation. EMBO Journal 19: 5406–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler J.L., Gregory C.W., Ford O.H., III, Kim D., Weaver C.M., Petrusz P., et al. (2004) The androgen axis in recurrent prostate cancer. Clin Cancer Res 10: 440–448 [DOI] [PubMed] [Google Scholar]
- Montgomery R.B., Mostaghel E.A., Vessella R., Hess D.L., Kalhorn T.F., Higano C.S., et al. (2008) Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 68: 4447–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostaghel E.A., Page S.T., Lin D.W., Fazli L., Coleman I.M., True L.D., et al. (2007) Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate Cancer. Cancer Res 67: 5403–5041 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health, U., Medicine, N.L.O. and Health and Human Services, U.D.O. (2009) Clinicaltrials.Gov, NIH, NLM, HHS – Clinical Trials Vol. 2009: Bethesda.
- Need E.F., Scher H.I., Peters A.A., Moore N.L., Cheong A., Ryan C.J., et al. (2009) A Novel androgen receptor amino terminal region reveals two classes of amino/carboxyl interaction-deficient variants with divergent capacity to activate responsive sites in chromatin. Endocrinology 150: 2674–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S.T., Lin D.W., Mostaghel E.A., Hess D.L., True L.D., Amory J.K., et al. (2006) Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab 91: 3850–3856 [DOI] [PubMed] [Google Scholar]
- Perner S., Demichelis F., Beroukhim R., Schmidt F.H., Mosquera J.M., Setlur S., et al. (2006) Tmprss2: Erg fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res 66: 8337–8341 [DOI] [PubMed] [Google Scholar]
- Peterziel H., Mink S., Schonert A., Becker M., Klocker H., Cato A.C.B. (1999) Rapid signalling by androgen receptor in prostate cancer cells. Oncogene 18: 6322–6329 [DOI] [PubMed] [Google Scholar]
- Pienta K.J., Bradley D. (2006) Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res 12: 1665–1671 [DOI] [PubMed] [Google Scholar]
- Pietras R.J., Arboleda J., Reese D.M., Wongvipat N., Pegram M.D., Ramos L., et al. (1995) Her-3 tyrosine kinase pathway targets estrogen-receptor and promotes hormone-independent growth in human breast-cancer cells. Oncogene 10: 2435–2446 [PubMed] [Google Scholar]
- Power R.F., Mani S.K., Codina J., Conneely O.M., O'Malley B.W. (1991) Dopaminergic and ligand-independent activation of steroid hormone receptors. Science 254: 1636–1639 [DOI] [PubMed] [Google Scholar]
- Pravettoni A., Mornati O., Martini P.G.V., Marino M., Colciago A., Celotti F., et al. (2007) Estrogen receptor beta (erbeta) and inhibition of prostate cancer cell proliferation: studies on the possible mechanism of action in Du145 cells. Mol Cell Endocrinol 263: 46–54 [DOI] [PubMed] [Google Scholar]
- Raghavan D. (1988) Non-hormone chemotherapy for prostate cancer: principles of treatment and application to the testing of new drugs. Semin Oncol 15: 371–389 [PubMed] [Google Scholar]
- Rai K.R., Sawitsky A., Cronkite E.P., Chanana A.D., Levy R.N., Pasternack B.S. (1975) Clinical staging of chronic lymphocytic leukemia. Blood 46: 219–234 [PubMed] [Google Scholar]
- Robinson D.R., Zylstra C.R., Williams B.O. (2008) Wnt signaling and prostate cancer. Curr Drug Targets 9: 571–580 [DOI] [PubMed] [Google Scholar]
- Rosenblatt A.E., Burnstein K.L. (2009) Inhibition of androgen receptor transcriptional activity as a novel mechanism of action of arsenic. Mol Endocrinol 23: 412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saramaki O.R., Harjula A.E., Martikainen P.M., Vessella R.L., Tammela T.L.J., Visakorpi T. (2008) Tmprss2:Erg fusion identifies a subgroup of prostate cancers with a favorable prognosis. Clin Cancer Res 14: 3395–3400 [DOI] [PubMed] [Google Scholar]
- Scher H.I., Heller G. (2000) Clinical states in prostate cancer: toward a dynamic model of disease progression. Urology 55: 323–327 [DOI] [PubMed] [Google Scholar]
- Scher H.I., Kelly W.K. (1993) Flutamide withdrawal syndrome – its impact on clinical-trials in hormone-refractory prostate-cancer. J Clin Oncol 11: 1566–1572 [DOI] [PubMed] [Google Scholar]
- Scher H.I., Sawyers C.L. (2005) Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 23: 8253–8261 [DOI] [PubMed] [Google Scholar]
- Scher H.I., Steineck G., Kevin Kelly W. (1995) Hormone-refractory (d3) prostate cancer: refining the concept. Urology 46: 142–148 [DOI] [PubMed] [Google Scholar]
- Scher H.I., Halabi S., Tannock I., Morris M., Sternberg C.N., Carducci M.A., et al. (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26: 1148–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher H.I., Beer T.M., Higano C.S., Taplin M., Efstathiou E., Anand A., et al. (2009) Antitumor activity of Mdv3100 in a phase I/II study of castration-resistant prostate cancer (CRPC). J Clin Oncol (Meeting Abstracts) 27: 5011–5011 [Google Scholar]
- Seaton A., Scullin P., Maxwell P.J., Wilson C., Pettigrew J., Gallagher R., et al. (2008) Interleukin-8 signaling promotes androgen-independent proliferation of prostate cancer cells via induction of androgen receptor expression and activation. Carcinogenesis 29: 1148–1156 [DOI] [PubMed] [Google Scholar]
- Shah S.K., Trump D.L., Sartor O., Tan W., Wilding G.E., Mohler J.L. (2009) Phase II study of dutasteride for recurrent prostate cancer during androgen deprivation therapy. J Urol 181: 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer E.A., Golijanin D.J., Miyamoto H., Messing E.M. (2008) Androgen deprivation therapy for prostate cancer. Expert Opin Pharmacother 9: 211–228 [DOI] [PubMed] [Google Scholar]
- Small E.J., Ryan C.J. (2006) The case for secondary hormonal therapies in the chemotherapy age. J Urol 176: S66–S71 [DOI] [PubMed] [Google Scholar]
- Solit D.B., Scher H.I., Rosen N. (2003) Hsp90 as a therapeutic target in prostate cancer. Semin Oncol 30: 709–716 [DOI] [PubMed] [Google Scholar]
- Tan J., Sharief Y., Hamil K.G., Gregory C.W., Zang D.Y., Sar M., et al. (1997) Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft Cwr22 and Lncap cells. Mol Endocrinol 11: 450–459 [DOI] [PubMed] [Google Scholar]
- Tannock I.F., De Wit R., Berry W.R., Horti J., Pluzanska A., Chi K.N., et al. (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351: 1502–1512 [DOI] [PubMed] [Google Scholar]
- Taplin M.E. (2007) Drug insight: role of the androgen receptor in the development and progression of prostate cancer. Nat Clinl Pract Oncol 4: 236–244 [DOI] [PubMed] [Google Scholar]
- Taplin M.E., Bubley G.J., Shuster T.D., Frantz M.E., Spooner A.E., Ogata G.K., et al. (1995) Mutation of the androgen-receptor gene in metastatic androgen-independent prostate-cancer. N Engl J Med 332: 1393–1398 [DOI] [PubMed] [Google Scholar]
- Tomlins S.A., Rhodes D.R., Perner S., Dhanasekaran S.M., Mehra R., Sun X.W., et al. (2005) Recurrent fusion of Tmprss2 and Ets transcription factor genes in prostate cancer. Science 310: 644–648 [DOI] [PubMed] [Google Scholar]
- Tomlins S.A., Laxman B., Varambally S., Cao X., Yu J., Helgeson B.E., et al. (2008) Role of the Tmprss2erg gene fusion in prostate cancer. Neoplasia 10: 177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C., Ouk S., Clegg N.J., Chen Y., Watson P.A., Arora V., et al. (2009) Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324: 787–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Kwast T., Schalken J., Ruizeveld De Winter J.A., Van Vroonhoven C.C.J., Mulder E., Boersma W., et al. (1991) Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer 48: 189–193 [DOI] [PubMed] [Google Scholar]
- Veldscholte J., Risstalpers C., Kuiper G., Jenster G., Berrevoets C., Claassen E., et al. (1990) A mutation in the ligand-binding domain of the androgen receptor of human Lncap cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commu 173: 534–540 [DOI] [PubMed] [Google Scholar]
- Visakorpi T., Hyytinen E., Koivisto P., Tanner M., Keinanen R., Palmberg C., et al. (1995) In-vivo amplification of the androgen receptor gene and progression of human prostate-cancer. Nat Genet 9: 401–406 [DOI] [PubMed] [Google Scholar]
- Vo N., Goodman R.H. (2001) Creb-binding protein and P300 in transcriptional regulation. J Biol Chem 276: 13505–13508 [DOI] [PubMed] [Google Scholar]
- Wang Y., Kreisberg J.I., Ghosh P.M. (2007) Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/akt pathway in prostate cancer. Curr Cancer Drug Targets 7: 591–604 [DOI] [PubMed] [Google Scholar]
- Welsbie D.S., Xu J., Chen Y., Borsu L., Scher H.I., Rosen N., et al. (2009) Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res 69: 958–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang Y.E., Moore C.N., Armstrong A.J., Rathmell W.K., Godley P.A., Crane J.M., et al. (2008) A phase II trial of lapatinib in hormone refractory prostate cancer. J Clin Oncol (Meeting Abstracts) 26: 16037–16037 [Google Scholar]
- Wu L.C., Birle D.C., Tannock I.F. (2005) Effects of the mammalian target of rapamycin inhibitor Cci-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res 65: 2825–2831 [DOI] [PubMed] [Google Scholar]
- Wyatt R.B., Sanchez-Ortiz R.F., Wood C.G., Ramirez E., Logothetis C., Pettaway C.A. (2004) Prognostic factors for survival among caucasian, African-American and hispanic men with androgen-independent prostate cancer. J Nat Med Assoc 96: 1587–1593 [PMC free article] [PubMed] [Google Scholar]
- Xu L., Glass C.K., Rosenfeld M.G. (1999) Coactivator and corepressor complexes in nuclear receptor function. Cur Opin Genet Devel 9: 140–147 [DOI] [PubMed] [Google Scholar]
- Yeh S., Kang H.-Y., Miyamoto H., Nishimura K., Chang H.-C., Ting H.-J., et al. (1999) Differential induction of androgen receptor transactivation by different androgen receptor coativators in human prostate cancer Du 145 cells. Endocrine 11: 195–202 [DOI] [PubMed] [Google Scholar]
- Yu E.Y., Wilding G., Posadas E., Gross M., Culine S., Massard C., et al. (2008) Dasatinib in patients with hormone-refractory progressive prostate cancer: a phase II study. J Clin Oncol (Meeting Abstracts) 26: 5156–5156 [Google Scholar]
- Zhang Y., Bai W., Allgood V.E., Weigel N.L. (1994) Multiple signaling pathways activate the chicken progesterone receptor. Mol Endocrinol 8: 577–584 [DOI] [PubMed] [Google Scholar]