Abstract
Objectives
Pharmacologic therapy for intermittent claudication in patients with peripheral artery disease (PAD) is limited. We aimed to determine the durability of cilostazol treatment response over time, treatment effects in various subpopulations, and long-term safety.
Methods
This analysis pooled original data from nine randomized, controlled trials evaluating cilostazol in intermittent claudication, including 1258 subjects treated with cilostazol 100 mg bid. Analysis of covariance was used to compare differences in walking distance, and a pooled random-effects weighted mean difference in maximal walking distance (MWD) was determined. Temporal effects were analyzed by compiling data at 4 week intervals in studies of 24 weeks duration.
Results
Cilostazol was associated with a 50.7% improvement from baseline in MWD compared with placebo (24.3%) with an absolute improvement of 42.1 meters greater than the improvement with placebo (p<0.001) over a mean follow-up period of 20.4 weeks. Continued increases were demonstrated over the 24 week treatment period. These benefits were seen in all subgroups, after stratifying by age, gender, smoking status, duration of PAD, diabetes, hypertension, prior myocardial infarction, or prior beta-blocker use. Cilostazol did not increase the risk of all-cause mortality (RR 0.95 [0.68–1.35]).
Conclusions
Treatment with cilostazol achieves benefits in walking distance that are sustained at 24 weeks and observed irrespective of baseline clinical characteristics. Cilostazol demonstrated no increased risk of all-cause mortality.
Keywords: Peripheral Arterial Disease, Intermittent Claudication
INTRODUCTION
Management of intermittent claudication in patients with peripheral artery disease (PAD) has been limited by a relative dearth of effective pharmacotherapy. Only two medications, cilostazol and pentoxifylline, are FDA-approved for treating symptoms of intermittent claudication in the United States. Cilostazol, a phosphodiesterase 3 inhibitor, was approved in the United States in 1999 for use in patients with PAD and intermittent claudication. The precise mechanism of action by which cilostazol improves exercise performance is not completely understood, but increases in intracellular cyclic adenosine monophosphate (cAMP) levels may produce several potentially beneficial effects, including vasodilation and reversible inhibition of platelet aggregation.1–4 The efficacy and safety of cilostazol for treating intermittent claudication have been demonstrated in several multi-center, randomized, double-blind, placebo-controlled trials.5–10 Recent meta-analyses, including all but one recent unpublished study, have shown that cilostazol significantly improves maximal walking distance (MWD) or peak walking time, quality of life measures, and lipid profiles.11–13
Although an overall treatment effect has been demonstrated, individual patient responses can vary substantially, making it challenging for physicians to predict the likelihood of an individual response and the time course of response to treatment. Thus far, detailed subgroups analyses, which might assist physicians in identifying the patients most likely to benefit, have not been performed, and no prior meta-analysis have compared the relative time course of benefit and the durability of treatment response to cilostazol.
Accordingly, we pooled data from nine randomized, controlled trials of cilostazol in intermittent claudication. Using this pooled data, we examined 1) the overall improvement in MWD achieved by cilostazol in intermittent claudication and the frequency of response to treatment, 2) the effect of treatment in various subgroups, and 3) the temporal profile of the treatment response to cilostazol over 24 weeks.
Furthermore, long-term safety of cilostazol has been questioned because the drug is a phosphodiesterase 3 inhibitor, and other medications of the same class, notably milrinone, have been previously been associated with excess mortality in patients with heart failure.14 Therefore, we also evaluated long-term safety of cilostazol by pooling data on all-cause mortality from these nine cilostazol studies and including safety data from the recently published CASTLE study.15
METHODS
Study selection
This pooled analysis included individual patient-level data from nine randomized, double-blind, placebo-controlled trials evaluating the use of cilostazol in intermittent claudication. Data from six of these studies have been previously published independently,5–10 and the data from two additional randomized trials have been published as part of earlier meta-analyses.11, 13 Original data from the most recent study was made available to the authors for analysis by Otsuka America Pharmaceutical, Inc. All studies were designed specifically to assess the safety and efficacy of cilostazol in patients with intermittent claudication. The cilostazol safety analysis presented here also included data from these nine randomized controlled trials as well as the recently published CASTLE study,15 the aim of which was to assess long-term safety of cilostazol with up to 36 months of treatment. All data were made available without restriction from Otsuka and the authors had independence from the sponsor in terms of the analyses and publication.
Trial design
Patient selection
All trials enrolled patients aged ≥ 40 years with PAD and intermittent claudication with stable symptoms for the preceding three months. The diagnosis of PAD was defined as an abnormal resting ankle-brachial index (ABI) (≤0.90 in 8 trials and ≤0.80 in one trial). An additional decline in post-exercise ABI of ≥10 mmHg served as confirmation of a diagnosis of PAD. Symptomatic patients with normal resting ABIs but with a pressure drop of ≥20 mmHg with exercise were also eligible to include patients with suspected iliac disease. To ensure reproducibility, patients were only randomized if the MWD varied by no more than 20% on 2–3 consecutive treadmill tests.
Patients were excluded if they had limb-threatening ischemia; limb revascularization (surgical or percutaneous) within 3 months; unstable coronary artery disease; coronary revascularization within 6 months; thromboangiitis obliterans; deep vein thrombosis within 3 months; symptomatic arrhythmia; and conditions other than PAD that might limit exercise ability or preclude completion of the study. Subjects with congestive heart failure were specifically excluded only in the most recent study (98–213) which was conducted subsequent to US FDA approval in 1999 at which time congestive heart failure of any severity was identified as a contraindication to cilostazol use. Subjects using anticoagulant therapy, aspirin in doses greater than 81 mg/day, or uses of high dose ibuprofen (> 1200 mg/day) were ineligible to participate in these studies.
Medication administration
Patients were randomized in a double-blinded manner to receive cilostazol (50 mg, 100 mg, or 150 mg) twice daily (bid) or placebo. The primary dose of cilostazol used in all nine trials was 100 mg bid. Only two trials included a 50 mg bid dose and only one trial used 150 mg bid.
Treadmill exercise protocols
Two different exercise protocols were employed in the nine studies reviewed. Six trials used a constant load protocol during which patients walked at 2.0 mph (3.2 km/hr) at a constant 12.5% grade in 5 of these trials and 10% grade in the one study performed in the UK5, 6, 8 (and studies 94–301, 98–213, 95–201). Three studies used a progressive workload protocol where patients walked at 2.0 mph (3.2 km/hr) initially at 0% grade with a 3.5% increase in grade every 3 minutes.7, 9, 10 Treadmill exercise data from these studies were initially recorded as peak walking time and converted to walking distance given the constant treadmill speed. No adjustment was performed for the progression in work load with the graded protocol in the conversion from time to distance. Treadmill testing was performed at four-week intervals.
Quality of life measures
The Medical Outcomes Study 36-item Short-Form Health Survey (SF-36), an instrument that assesses eight domains of general physical and mental health, was used to evaluate overall health-related QOL16 The Walking Impairment Questionnaire (WIQ), a disease-specific instrument developed specifically for patients with peripheral arterial disease with intermittent claudication, was used as an additional measure of performance status.17, 18
Statistical Methods
In all trials, the primary outcome measure was change in MWD compared to baseline, with change in pain-free walking distance (PFWD) compared to baseline as a secondary outcome measure. For this analysis, we pooled the original individual patient data and used an intention-to-treat analysis. The pooled efficacy analysis included randomized patients who received study drug and had at least one post-baseline treadmill test. The primary dose of cilostazol used in all trials dose was 100 mg bid and as such, only subjects receiving the 100 mg doses were included in the efficacy analyses. Given the relatively small number of patients in the other dose groups (50 mg and 150 mg bid), subjects receiving these doses were not included in the efficacy analysis. The long-term safety analysis incorporated all patients who received any dose of cilostazol in order to ensure safety across all doses that have been utilized. Interval missing values were calculated with a last observation carried forward method. Because treadmill data were not normally distributed, change in treadmill walking distance for individual treatment groups was analyzed as the logarithm of the ratio of post-treatment distance divided by baseline distance (log [final distance/baseline distance]). Expressing data in this fashion accounts for the differences in treadmill protocols and reduces the impact of extreme values. Statistical comparison between groups was achieved using the analysis of covariance (ANCOVA) with study center and treatment group as primary effects and the baseline distance (log-transformed) as a covariate. Comparison of the effects of treatments on walking distance was achieved using estimated treatment effect, calculated as a ratio of geometric means and including 95% confidence intervals (CI). As this value is expressed in a log scale, we present a clinically interpretable average improvement in walking distance by performing a meta-analysis using a random effects weighted mean difference in MWD in meters comparing each treatment group to placebo. Random effects model was chosen after significant heterogeneity across studies was identified using the Q statistics for testing for heterogeneity (p<0.05) .We further compared frequency of response to treatment in each treatment group using various threshold definitions of treatment response. In order to characterize the determinants of response to treatment, subgroup analyses were performed stratifying subjects by age (<65 vs. ≥65 years), gender, duration of PAD, smoking status (current smoker or non-smoker), diabetes, CHF, hypertension, prior myocardial infarction, or beta-blocker use. Because treadmill testing was performed at 4 week intervals in all studies, we were able to evaluate the impact of treatment over time. Using only studies of 24 weeks duration, we compared the change from baseline in each treatment group to placebo at 4 week intervals. Analysis of all-cause mortality was performed by pooling data from the nine randomized controlled trials with data from the CASTLE study,15 and data are presented as a pooled risk ratio with 95% CI. For the safety analysis, data from subjects receiving all three doses of cilostazol were included. Analyses were conducted using SAS and Comprehensive Meta-Analysis Version 2.0. A p-value of <0.05 was considered statistically significant.
RESULTS
Study population
The nine placebo-controlled trials included 1258 subjects who received at least one dose of cilostazol 100 mg bid and 1233 who received placebo. As not all patients returned for follow-up testing at four week intervals, the efficacy analysis included only randomized patients who received a dose of study medication and who had at least one on-treatment treadmill test. Therefore the study population used for the efficacy analysis included 1116 subjects receiving cilostazol 100 mg bid and 1135 subjects receiving placebo. The safety analysis included all patients who took at least one dose of cilostazol whether or not they returned for post-treatment exercise treadmill testing. This included 1634 subjects receiving any dose of cilostazol from the nine randomized controlled trials with the majority of subjects (n=1258) receiving the 100 mg bid dose, as well as 717 additional subjects from the CASTLE study. There were no significant differences in the clinical characteristics among treatment groups (table 1). The mean age was not significantly different in the two groups (65.3 ± 9.2 in the cilostazol group vs. 65.9 ± 9.3 in the placebo group, p=ns). The prevalence of diabetes, hypertension, smoking, prior myocardial infarction, stroke and congestive heart failure were similar in the two groups.
Table 1.
Patient Characteristics
| Cilostazol 100 mg BID |
Placebo | |
|---|---|---|
| Total number, n | 1116 | 1135 |
| Gender (% male) | 76.3 | 76.4 |
| Age (years) | 65.3 ± 9.2 | 65.9 ± 9.3 |
| Race/ethnicity (%) | 89.2 | 86.2 |
| Body mass index (kg/m2) | 27.2 ± 4.5 | 27.1 ± 4.3 |
| PAD duration | ||
| ≥ 6 months to ≤ 5 years (%) | 59.4 | 62.5 |
| > 5 years (%) | 40.3 | 37.5 |
| Diabetes (%) | 26.4 | 27.3 |
| Hypertension (%) | 62.2 | 63.3 |
| Smoking status | ||
| Never (%) | 8.2 | 8.5 |
| Prior (%) | 53 | 52.5 |
| Current (%) | 38.7 | 38.9 |
| Prior myocardial infarction (%) | 20.6 | 21.2 |
| Prior stroke (%) | 3.9 | 4.9 |
| Prior congestive heart failure (%) | 3.3 | 3.5 |
| Systolic blood pressure (mmHg) | 144.8 ± 20 | 144.5 ± 20 |
| LDL cholesterol (mg/dL) | 136.5 ± 40 | 134.6 ± 40 |
| Ankle-brachial index | 0.64 ± 0.17 | 0.64 ± 0.16 |
| Maximal walking distance @ baseline (meters) | 172.2 | 180.1 |
Data are shown for patients included in the efficacy analysis. Continuous variables are shown as mean ± standard deviation. BID, twice daily. TID, three times a day. There were no statistically significant differences between the groups (all p-values >0.05).
Walking distance measurements
Treatment with cilostazol 100 mg bid resulted in a statistically significant improvement in MWD in 6 of 9 studies. In the remaining three trials, the point estimates for the estimated treatment effect marginally favored cilostazol 100 mg bid over placebo, but the results were not statistically significant (table 2). In the pooled analysis of all nine trials, cilostazol 100 mg bid resulted in a 50.7% mean change from baseline compared with a 24.3% mean change from baseline in the placebo group (p=0.0001), corresponding to an estimated treatment effect of 1.15 (95% CI 1.11–1.19) derived from the log-transformed data. There was no significant difference in treatment response based on the different treadmill protocols used (p interaction = 0.28). Improvements in pain-free walking distance (PFWD) were also seen, with subjects treated with cilostazol 100 mg bid experiencing a 67.8% mean increase from baseline in PFWD compared to a 42.6% mean increase from baseline in the placebo group (p=0.0001), corresponding to an estimated treatment effect of 1.15 (95% CI 1.10–1.20) on log-transformed data.
Table 2.
Estimated Treatment Effect* (95% CI) for Cilostazol 100 mg bid vs. Placebo on Maximal and Pain-free Walking Distance
| Trial | N | Maximal Walking Distance |
Pain-free Walking Distance |
|---|---|---|---|
| Beebe 19995 | 280 | 1.31 (1.17–1.47) | 1.31 (1.16–1.48) |
| Strandness 20026 | 249 | 1.21 (1.09–1.35) | 1.22 (1.08–1.38) |
| Study 94–301 | 245 | 1.06 (0.94–1.18) | 1.01 (0.90–1.14) |
| Dawson 20007 | 431 | 1.15 (1.06–1.25) | 1.23 (1.12–1.36) |
| Study 98–213 | 449 | 1.03 (0.95–1.12) | 1.02 (0.92–1.13) |
| Money 19989 | 219 | 1.29 (1.17–1.41) | 1.20 (1.06–1.36) |
| Dawson 19988 | 77 | 1.41 (1.14–1.74) | 1.35 (1.11–1.65) |
| Elam 199810 | 175 | 1.13 (1.01–1.26) | 1.08 (0.92–1.27) |
| Study 95–201 | 126 | 1.02 (0.88–1.18) | 1.05 (0.89–1.24) |
| Pooled Analysis | 2251 | 1.15 (1.11–1.19) | 1.15 (1.10–1.20) |
Estimated treatment effect is a ratio of the geometric means, calculated as the antilog of the difference in log(final distance/baseline distance) between cilostazol 100 mg bid and placebo.
N=subjects treated with cilostazol 100 mg bid (total n=1116) and placebo (total n=1135), CI=confidence interval.
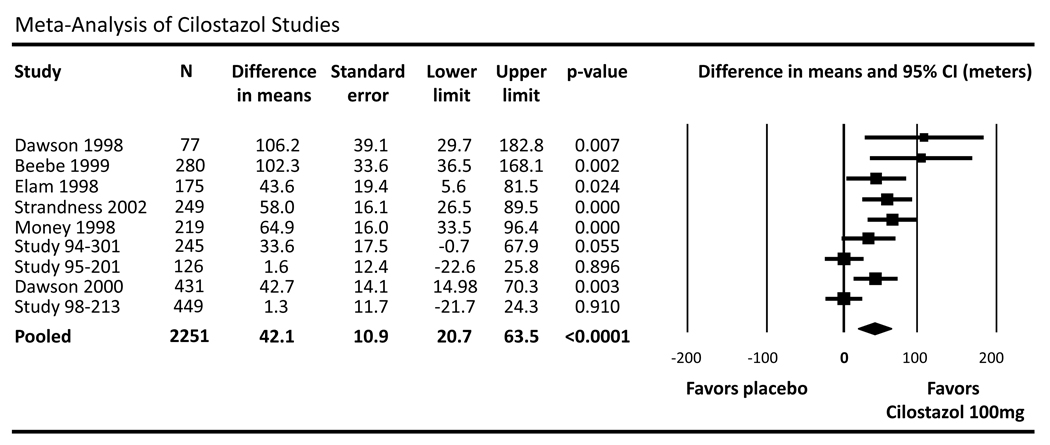
Using the weighted summary statistics from individual trials and a random effects model, these data demonstrate that subjects receiving cilostazol achieve an absolute 42.1 meter greater improvement in maximal walking distance than the placebo group (95% CI 20.7–63.5, p<0.001) over a mean follow-up period of 20.4 weeks (figure 1).
Figure 1.
Meta-analysis of randomized, controlled trials evaluating the effect of cilostazol versus placebo. Due to underlying heterogeneity, cilostazol analyses are performed using the random-effects weighted mean difference in maximal walking distance (MWD) with corresponding 95% confidence interval (CI).
Furthermore, a significantly greater percentage of subjects in the cilostazol group (compared to placebo) achieved a meaningful response to treatment. Defining treatment response by a >25% increase in maximal walking distance, 53% of subjects receiving cilostazol 100 mg bid were deemed responders, compared to only 40% in the placebo group (p<0.001). Consistent findings were observed when alternate definitions of response to treatment were used, including a >25% increase in pain-free walking distance (61% vs. 49%, p<0.001) and patient-reported outcomes (table 3). These findings are also supported by analyzing the percent of patients achieving various percent improvements in MWD (table 4). These data show that cilostazol was more likely than placebo to achieve higher percent changes in MWD and less likely to achieve lower percent increases (p<0.001).
Table 3.
Response to Treatment
| Response indicator | Cilostazol 100 mg bid | Placebo | p-value* |
|---|---|---|---|
| Maximal walking distance >25% increase | 53% | 40% | <0.0001 |
| Pain-free walking distance >25% increase | 61% | 49% | <0.0001 |
| SF-36 physical component >5 point increase | 50% | 42% | 0.0008 |
| SF-36 physical function >5 point increase | 56% | 46% | <0.0001 |
| WIQ walking distance >5 point increase | 61% | 52% | <0.0001 |
| WIQ walking speed >5 point increase | 44% | 36% | 0.0004 |
Statistical comparison achieved using χ2 test.
Table 4.
Percent of group achieving response to treatment
| Percent Improvement in MWD (%) | p-value* | ||||
|---|---|---|---|---|---|
| <0% | 0–50% | 50–100% | ≥100% | ||
| Treatment group | |||||
| Cilostazol 100 mg bid | 24.3% | 39% | 18.5% | 18.2% | <0.0001 |
| Placebo | 31.5% | 42.2% | 13.8% | 12.4% | |
Data are shown as the percent of individuals in each treatment group that achieve a given percent improvement in maximal walking distance (MWD); Analysis performed only using data from trials with 24 week treatment duration (5 trials)
p-value from Cochrane-Mantel-Haenzsel test stratified by study
Improvements in maximal walking distance correlated significantly with changes in patient-reported outcome measures. For cilostazol 100 mg bid, changes in MWD correlated with SF-36 physical function score (r=0.29, p<.0001) and with WIQ walking distance score (r=0.34, p<.001), walking speed score (r=0.23, p<.001), and pain score (r=0.20, p<.001).
Subgroup analyses
Given the significant improvements in MWD seen with cilostazol, we further sought to examine whether there were differences based on underlying patient characteristics. We found that cilostazol 100 mg bid has similar benefits on MWD irrespective of age (<65 vs. ≥65 years), gender, smoking status. Treatment effects were also similar irrespective of underlying medical conditions, such as diabetes, congestive heart failure, hypertension, PAD duration, or prior myocardial infarction, as well as active medications (current beta-blocker use) (table 5).
Table 5.
Subgroup Analyses of the Effect of Cilostazol 100 mg bid on Maximal Walking Distance
| N (cilostazol) |
N (placebo) |
Estimated Treatment Effect (95% CI) |
p-value | |
|---|---|---|---|---|
| Age | ||||
| < 65 years | 484 | 471 | 1.16 (1.09–1.23) | 0.0001 |
| ≥65 years | 632 | 664 | 1.14 (1.09–1.20) | 0.0001 |
| Gender | ||||
| Male | 852 | 867 | 1.17 (1.12–1.22) | 0.0001 |
| Female | 264 | 268 | 1.10 (1.02–1.19) | 0.014 |
| Duration of PAD | ||||
| ≤5 years | 666 | 710 | 1.15 (1.09–1.21) | 0.0001 |
| > 5 years | 449 | 425 | 1.13 (1.06–1.20) | 0.0001 |
| Smoking status | ||||
| Non-smoker | 558 | 563 | 1.18 (1.12–1.25) | 0.0001 |
| Smoker | 558 | 572 | 1.12 (1.07–1.18) | 0.0001 |
| Diabetes | ||||
| Yes | 295 | 310 | 1.10 (1.01–1.19) | 0.02 |
| No | 821 | 825 | 1.17 (1.12–1.22) | 0.0001 |
| CHF | ||||
| Yes | 37 | 40 | 1.42 (1.12–1.81) | 0.005 |
| No | 1079 | 1095 | 1.14 (1.1–1.19) | 0.0001 |
| Hypertension | ||||
| Yes | 694 | 718 | 1.12 (1.07–1.18) | 0.0001 |
| No | 422 | 417 | 1.18 (1.11–1.26) | 0.0001 |
| Prior myocardial infarction | ||||
| Yes | 230 | 241 | 1.18 (1.08–1.28) | 0.0002 |
| No | 886 | 894 | 1.13 (1.09–1.18) | 0.0001 |
| Beta-blocker use | ||||
| Yes | 200 | 183 | 1.15 (1.04–1.27) | 0.006 |
| No | 916 | 952 | 1.14 (1.1–1.19) | 0.0001 |
PAD = peripheral artery disease, CHF = congestive heart failure;
P-values were determined using ANCOVA. Estimated treatment effect is a ratio of geometric means.
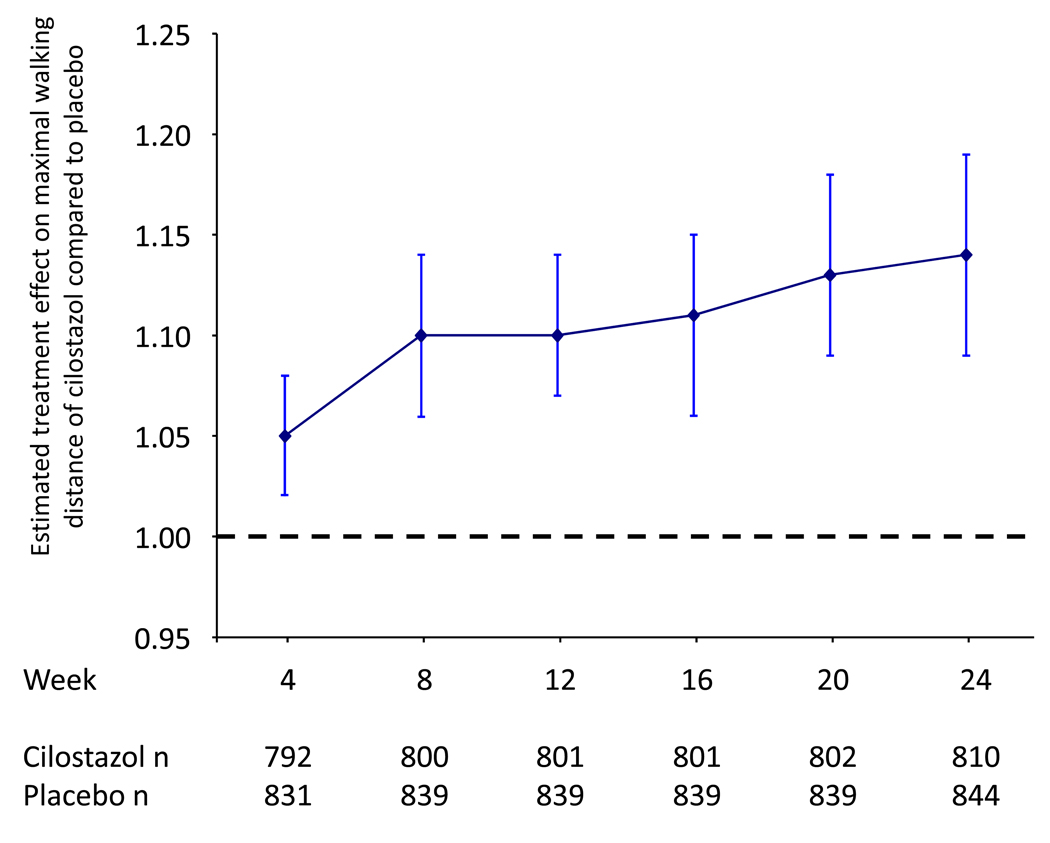
Treatment effects over time
As exercise testing was performed at 4-week intervals, we were able to assess the overall effects of cilostazol 100 mg bid over the 24-week treatment period compared to placebo. In order to analyze treatment effects over 24 weeks, this analysis only included data from the 5 studies in which subjects were treated for a full 24 weeks. Subjects randomized to cilostazol 100 mg bid achieved continuous increases in treatment effect over time (figure 2). At 4 weeks, there was a small but statistically significant treatment effect compared to placebo, with estimated treatment effect 1.05 (95% CI 1.02–1.08, p=0.0015). This treatment benefit remained significantly in favor of cilostazol and continued to increase over the 24 week treatment period, with estimated treatment effects of 1.10 (95% CI 1.07–1.14, p=0.0001) at week 12 and 1.14 (95% CI 1.09–1.19, p=0.0001) at week 24.
Figure 2.
Analysis of treatment effects over time for cilostazol versus placebo. Data are shown as the estimated treatment effect, a comparison of geometric means. Statistical comparison is achieved by ANCOVA.
All-cause mortality
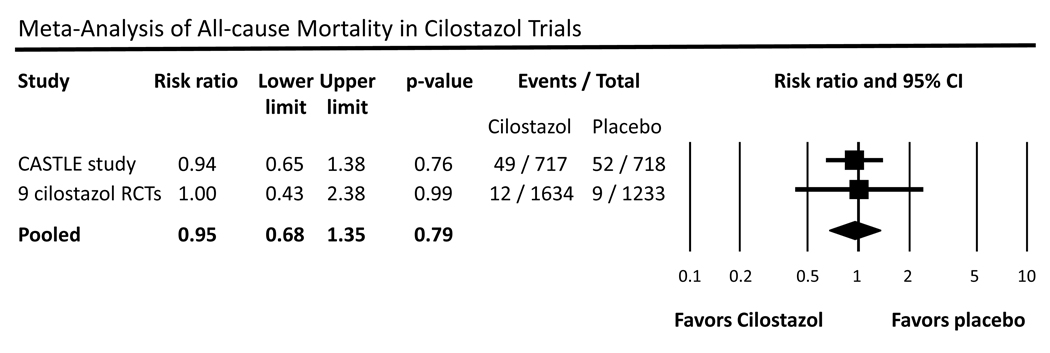
Given prior concerns about the potential increase in mortality with use of phosphodiesterase 3 inhibitors, we combined data from the nine prior randomized trials of cilostazol of 12 or 24 weeks duration with recent long-term safety data from the CASTLE study (36 months).15 In the pooled meta-analysis using a random effects model and combining all 9 prior cilostazol studies with the CASTLE study, we found that overall there was no increased risk of all-cause mortality in patients taking any dose of cilostazol compared to placebo (RR 0.95, 95% CI 0.86–1.35, p=0.79) (figure 3).
Figure 3.
Meta-analysis of all-cause mortality in cilostazol trials. Data are shown as a risk ratio with corresponding 95% confidence interval (CI) for the CASTLE study and the nine cilostazol randomized controlled trials (RCTs).
DISCUSSION
The results of this pooled analysis from nine randomized, multi-center, placebo-controlled trials demonstrate that at 24 week follow-up cilostazol produced significant improvements in maximal walking distance in patients with intermittent claudication, benefits that correlated with improvements in quality of life measures. Specifically, cilostazol 100 mg bid increased MWD by 42.1 meters compared to placebo, with the benefits of cilostazol achieved in all subgroups examined and correlating with improvements in quality of life measures. Furthermore, analysis of the temporal effects of cilostazol on walking ability showed that continued increases could be achieved with cilostazol 100 mg bid over 24 weeks of therapy. Improvements in pain-free walking distance were demonstrable as well.
An advantage of the present analysis was the ability to combine the patient-level data from nine trials with identical inclusion and exclusion criteria. Based on that, we are able to demonstrate in a large pooled sample that cilostazol achieves benefits in maximal walking time not only in the overall population, but also amongst multiple subgroups. However, the present study must be evaluated in the context of its limitations. First, given that the goals of treatment in intermittent claudication include improved exercise performance, assessment of treadmill walking ability is central to establishment of clinical efficacy. These nine cilostazol trials employed two different exercise tests, constant load and graded exercise protocols, and walking distances measured on the graded test tend to be greater than distances measured with the constant load protocol.19 However, the comparability of the two protocols has been previously established, particularly in patients able to walk greater than 100m.19 Moreover, to further account for potential effects of having different treadmill protocols, we used a value that incorporated both baseline walking distance and post-treatment walking distance in our analysis and we demonstrated using a test for interaction that there was no significant difference in treatment response based on the different treadmill exercise protocols. Second, as with all pooled analyses, it is important to recognize the potential limitations of combining data from different studies. Due to heterogeneity across these nine studies, we chose a random effects model to be most conservative the estimate of the treatment effect of cilostazol. Heterogeneity in these studies was felt to be due in large part to the widely varying point estimates for the treatment effect of cilostazol. Given that the studies had identical inclusion and exclusion criteria, similar outcome measures and similar periods of follow-up, we did not feel that the heterogeneity seen here was related to differences in patient characteristics. We may also have been limited in our ability to detect a greater response to cilostazol. The assessment of exercise capacity on a treadmill has been shown to underestimate actual patient walking distance,20 given that patients tend to walk at greater intensity levels on treadmill protocols than they would otherwise use for regular activities of daily living. Despite these limitations, these data demonstrate that cilostazol does produce a sustained improvement in walking distance over 24 weeks and among all subgroups analyzed.
The walking improvements observed with cilostazol in this meta-analysis were significant as assessed by objective parameters (treadmill exercise performance) and associated with subjective patient-based improvements in functional capacity. How these clinical benefits compare to other established therapies for claudication, such as a formal exercise program or a successful limb revascularization, remain to be determined. Comparative trials assessing these different treatment modalities need to be performed. These data also highlight the limitations in our treatment options for stable claudication and remind us of the critical need for continued research to seek novel therapies to improve symptoms and quality of life in patients with PAD.
Furthermore, the mechanisms of cilostazol’s benefit in intermittent claudication also remain unclear. By inhibiting phosphodiesterase 3 and increasing intracellular cAMP levels, cilostazol has been shown to cause vasodilation and improve peripheral blood flow.1–4 However, although flow limitation in PAD is a primary cause of intermittent claudication, PAD is not simply a hemodynamic disorder and severity of claudication pain cannot simply be explained by reduction in perfusion pressure and flow. Indeed, studies have shown a poor correlation between diminished calf blood flow and walking distance in patients with PAD.22 Additionally, restoration of flow via revascularization does not completely improve claudication symptoms,23 and the symptomatic improvements known to result from exercise training programs in patients with PAD do not occur solely as a result of increase blood flow.24 Compelling reasons therefore exist for the exploration of novel paradigms to explain the symptomatic limitation in patients with intermittent claudication.
In conclusion, the current analysis demonstrated that administration of cilostazol can achieve sustained improvements in walking distance and quality of life in patients with intermittent claudication. However, the improvements seen here should encourage us to continue to focus on the fundamental mechanisms of intermittent claudication as a guide to the development of novel therapies for this life-limiting condition. These efforts are essential in order to broaden the currently limited therapeutic options for patients with PAD and intermittent claudication.
Acknowledgments
Acknowledgements and disclosures
Dr. Pande is supported by a Research Career Development Award (K12 HL083786) from the National Heart, Lung, and Blood Institute (NHLBI). Drs. Zhang and Hittel are full-time employees of Otsuka Pharmaceuticals, Inc. Dr. Hiatt has received honoraria for speaking from Otsuka Japan. Dr. Creager is the Simon C. Fireman Scholar in Cardiovascular Medicine at Brigham and Women’s Hospital.
REFERENCES
- 1.Tanaka T, Ishikawa T, Hagiwara M, Onoda K, Itoh H, Hidaka H. Effects of cilostazol, a selective cAMP phosphodiesterase inhibitor on the contraction of vascular smooth muscle. Pharmacology. 1988;36(5):313–320. doi: 10.1159/000138400. [DOI] [PubMed] [Google Scholar]
- 2.Oida K, Ebata K, Kanehara H, Suzuki J, Miyamori I. Effect of cilostazol on impaired vasodilatory response of the brachial artery to ischemia in smokers. J Atheroscler Thromb. 2003;10(2):93–98. doi: 10.5551/jat.10.93. [DOI] [PubMed] [Google Scholar]
- 3.Igawa T, Tani T, Chijiwa T, Shiragiku T, Shimidzu S, Kawamura K, et al. Potentiation of anti-platelet aggregating activity of cilostazol with vascular endothelial cells. Thromb Res. 1990 Feb 15;57(4):617–623. doi: 10.1016/0049-3848(90)90079-r. [DOI] [PubMed] [Google Scholar]
- 4.Woo SK, Kang WK, Kwon KI. Pharmacokinetic and pharmacodynamic modeling of the antiplatelet and cardiovascular effects of cilostazol in healthy humans. Clin Pharmacol Ther. 2002 Apr;71(4):246–252. doi: 10.1067/mcp.2002.122474. [DOI] [PubMed] [Google Scholar]
- 5.Beebe HG, Dawson DL, Cutler BS, Herd JA, Strandness DE, Jr, Bortey EB, et al. A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial. Arch Intern Med. 1999 Sep 27;159(17):2041–2050. doi: 10.1001/archinte.159.17.2041. [DOI] [PubMed] [Google Scholar]
- 6.Strandness DE, Jr, Dalman RL, Panian S, Rendell MS, Comp PC, Zhang P, et al. Effect of cilostazol in patients with intermittent claudication: a randomized, double-blind, placebo-controlled study. Vasc Endovascular Surg. 2002 Mar–Apr;36(2):83–91. doi: 10.1177/153857440203600202. [DOI] [PubMed] [Google Scholar]
- 7.Dawson DL, Cutler BS, Hiatt WR, Hobson RW, 2nd, Martin JD, Bortey EB, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000 Nov;109(7):523–530. doi: 10.1016/s0002-9343(00)00569-6. [DOI] [PubMed] [Google Scholar]
- 8.Dawson DL, Cutler BS, Meissner MH, Strandness DE., Jr Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998 Aug 18;98(7):678–686. doi: 10.1161/01.cir.98.7.678. [DOI] [PubMed] [Google Scholar]
- 9.Money SR, Herd JA, Isaacsohn JL, Davidson M, Cutler B, Heckman J, et al. Effect of cilostazol on walking distances in patients with intermittent claudication caused by peripheral vascular disease. J Vasc Surg. 1998 Feb;27(2):267–274. doi: 10.1016/s0741-5214(98)70357-x. discussion 74–5. [DOI] [PubMed] [Google Scholar]
- 10.Elam MB, Heckman J, Crouse JR, Hunninghake DB, Herd JA, Davidson M, et al. Effect of the novel antiplatelet agent cilostazol on plasma lipoproteins in patients with intermittent claudication. Arterioscler Thromb Vasc Biol. 1998 Dec;18(12):1942–1947. doi: 10.1161/01.atv.18.12.1942. [DOI] [PubMed] [Google Scholar]
- 11.Thompson PD, Zimet R, Forbes WP, Zhang P. Meta-analysis of results from eight randomized, placebo-controlled trials on the effect of cilostazol on patients with intermittent claudication. Am J Cardiol. 2002 Dec 15;90(12):1314–1319. doi: 10.1016/s0002-9149(02)02869-2. [DOI] [PubMed] [Google Scholar]
- 12.Regensteiner JG, Ware JE, Jr, McCarthy WJ, Zhang P, Forbes WP, Heckman J, et al. Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials. J Am Geriatr Soc. 2002 Dec;50(12):1939–1946. doi: 10.1046/j.1532-5415.2002.50604.x. [DOI] [PubMed] [Google Scholar]
- 13.Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane database of systematic reviews (Online) 2008;(1):CD003748. doi: 10.1002/14651858.CD003748.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. The New England journal of medicine. 1991 Nov 21;325(21):1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 15.Hiatt WR, Money SR, Brass EP. Long-term safety of cilostazol in patients with peripheral artery disease: the CASTLE study (Cilostazol: A Study in Long-term Effects) J Vasc Surg. 2008 Feb;47(2):330–336. doi: 10.1016/j.jvs.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–483. [PubMed] [Google Scholar]
- 17.Regensteiner JGJFS, Panzer RJWRH. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–152. [Google Scholar]
- 18.McDermott MM, Liu K, Guralnik JM, Martin GJ, Criqui MH, Greenland P. Measurement of walking endurance and walking velocity with questionnaire: validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg. 1998 Dec;28(6):1072–1081. doi: 10.1016/s0741-5214(98)70034-5. [DOI] [PubMed] [Google Scholar]
- 19.Labs KH, Nehler MR, Roessner M, Jaeger KA, Hiatt WR. Reliability of treadmill testing in peripheral arterial disease: a comparison of a constant load with a graded load treadmill protocol. Vasc Med. 1999;4(4):239–246. doi: 10.1177/1358836X9900400406. [DOI] [PubMed] [Google Scholar]
- 20.Watson CJ, Phillips D, Hands L, Collin J. Claudication distance is poorly estimated and inappropriately measured. Br J Surg. 1997 Aug;84(8):1107–1109. [PubMed] [Google Scholar]
- 21.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. Jama. 1995 Sep 27;274(12):975–980. [PubMed] [Google Scholar]
- 22.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Prediction of claudication pain from clinical measurements obtained at rest. Med Sci Sports Exerc. 1992 Feb;24(2):163–170. [PubMed] [Google Scholar]
- 23.Regensteiner JG, Hargarten ME, Rutherford RB, Hiatt WR. Functional benefits of peripheral vascular bypass surgery for patients with intermittent claudication. Angiology. 1993 Jan;44(1):1–10. doi: 10.1177/000331979304400101. [DOI] [PubMed] [Google Scholar]
- 24.Johnson EC, Voyles WF, Atterbom HA, Pathak D, Sutton MF, Greene ER. Effects of exercise training on common femoral artery blood flow in patients with intermittent claudication. Circulation. 1989 Nov;80(5 Pt 2):III59–III72. [PubMed] [Google Scholar]