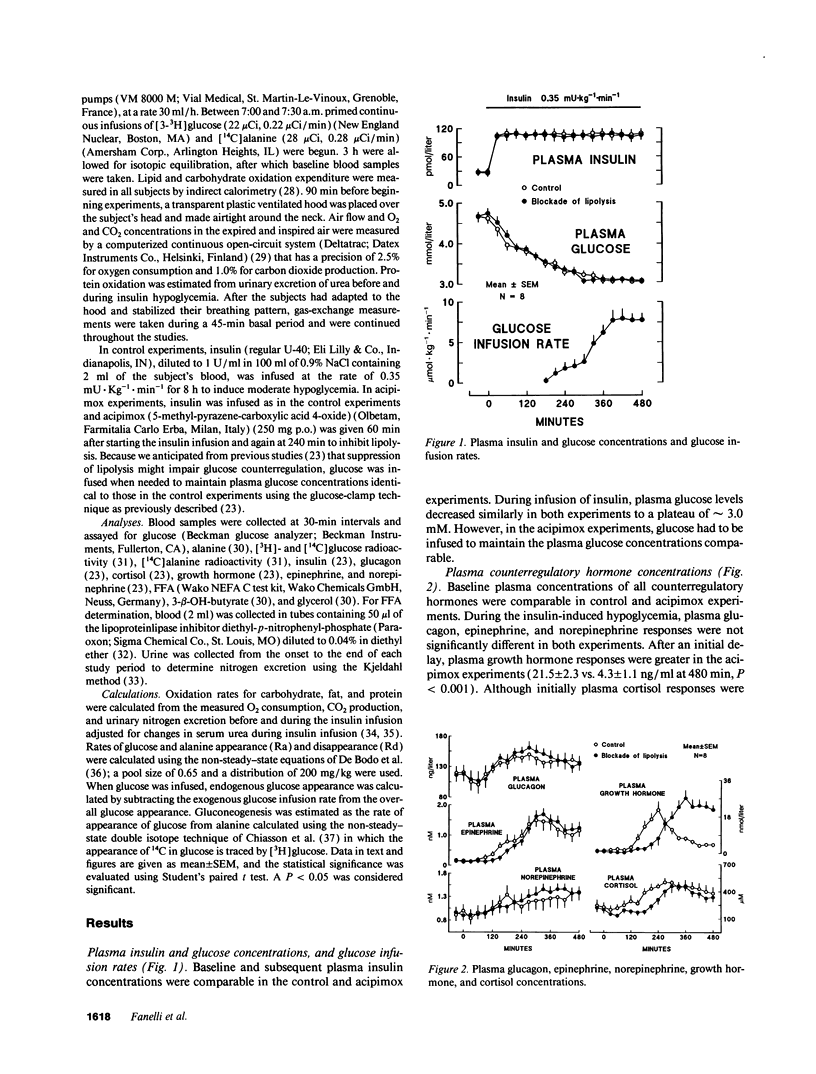
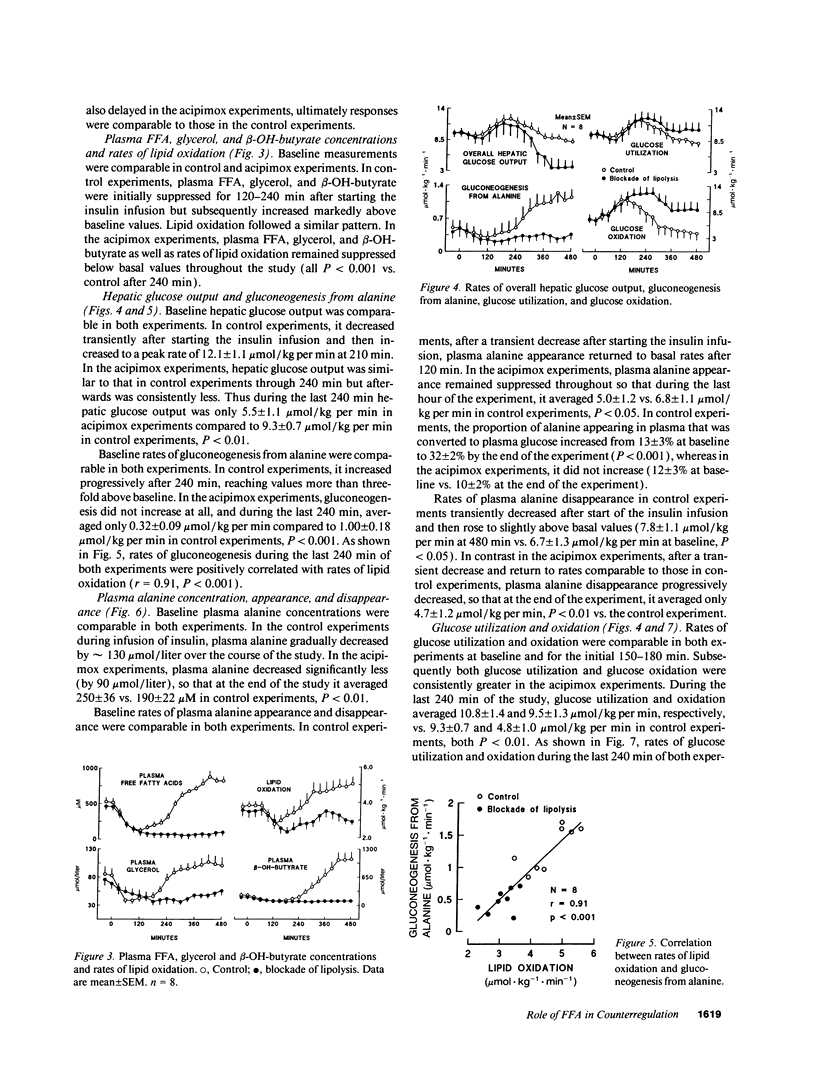
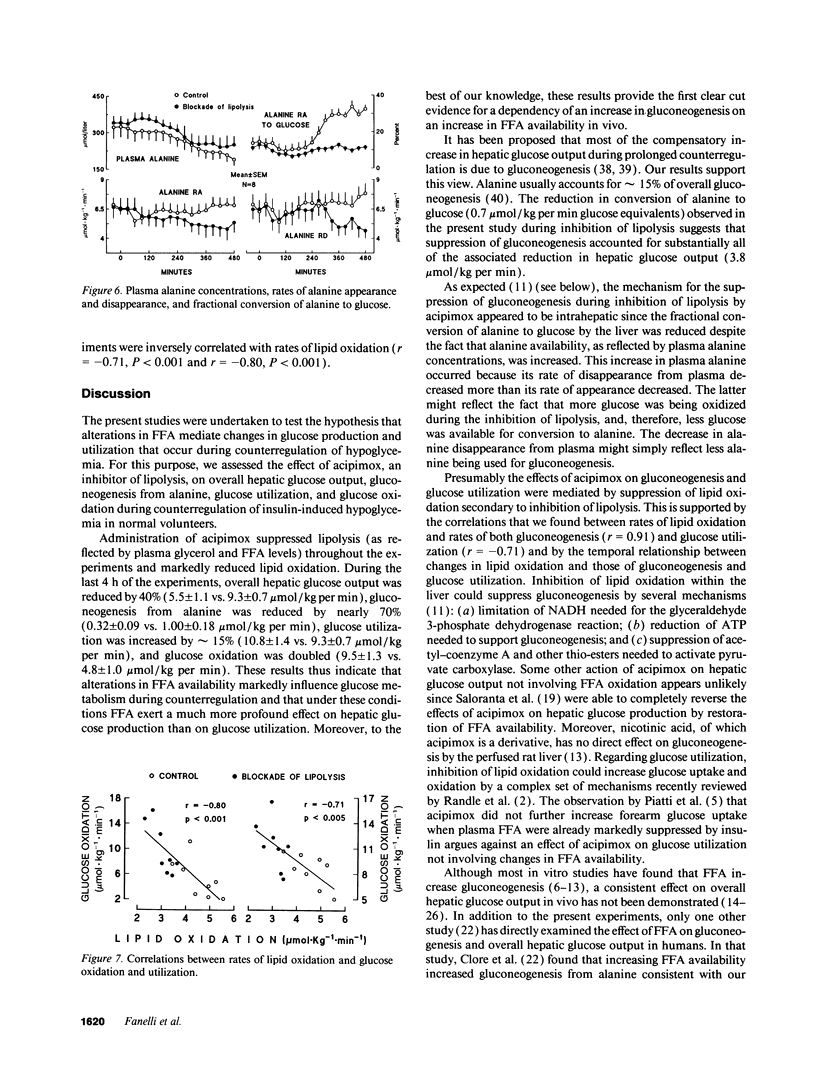
Abstract
In vitro studies indicate that FFA compete with glucose as an oxidative fuel in muscle and, in addition, stimulate gluconeogenesis in liver. During counterregulation of hypoglycemia, plasma FFA increase and this is associated with an increase in glucose production and a suppression of glucose utilization. To test the hypothesis that FFA mediate changes in glucose metabolism that occur during counterregulation, we examined the effects of acipimox, an inhibitor of lipolysis, on glucose production and utilization ([3-3H]glucose), and incorporation of [U-14C]-alanine into glucose during insulin-induced hypoglycemia. Eight normal volunteers were infused with insulin for 8 h to produce modest hypoglycemia (approximately 3 mM) on two occasions, first without acipimox (control) and then with acipimox administration (250 mg per os at 60 and 240 min). Despite identical plasma insulin concentrations, glucose had to be infused in the acipimox experiments (glucose-clamp technique) to maintain plasma glucose concentrations identical to those in control experiments. Acipimox completely prevented counterregulatory increases in lipolysis so that during the last 4 h plasma FFA were below baseline values and averaged 67 +/- 13 vs. 725 +/- 65 microM in control experiments, P < 0.001. Concomitantly, overall glucose production was reduced by 40% (5.5 +/- 11 vs. 9.3 +/- 0.7 mumol/kg per min, P < 0.001), and gluconeogenesis from alanine was reduced by nearly 70% (0.32 +/- 0.09 vs. 1.00 +/- 0.18 mumol/kg per min, P < 0.001), while glucose utilization increased by 15% (10.8 +/- 1.4 vs. 9.3 +/- 0.7 mumol/kg per min). We conclude that FFA play a critical role in mediating changes in glucose metabolism during counterregulation, and that under these conditions, FFA exert a much more profound effect on hepatic glucose production than on glucose utilization.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron A. D., Brechtel G., Edelman S. V. Effects of free fatty acids and ketone bodies on in vivo non-insulin-mediated glucose utilization and production in humans. Metabolism. 1989 Nov;38(11):1056–1061. doi: 10.1016/0026-0495(89)90040-1. [DOI] [PubMed] [Google Scholar]
- Bevilacqua S., Bonadonna R., Buzzigoli G., Boni C., Ciociaro D., Maccari F., Giorico M. A., Ferrannini E. Acute elevation of free fatty acid levels leads to hepatic insulin resistance in obese subjects. Metabolism. 1987 May;36(5):502–506. doi: 10.1016/0026-0495(87)90051-5. [DOI] [PubMed] [Google Scholar]
- Blumenthal S. A. Stimulation of gluconeogenesis by palmitic acid in rat hepatocytes: evidence that this effect can be dissociated from the provision of reducing equivalents. Metabolism. 1983 Oct;32(10):971–976. doi: 10.1016/0026-0495(83)90137-3. [DOI] [PubMed] [Google Scholar]
- Bougnères P. F., Castaño L., Rocchiccioli F., Gia H. P., Leluyer B., Ferré P. Medium-chain fatty acids increase glucose production in normal and low birth weight newborns. Am J Physiol. 1989 May;256(5 Pt 1):E692–E697. doi: 10.1152/ajpendo.1989.256.5.E692. [DOI] [PubMed] [Google Scholar]
- Caprio S., Gelfand R. A., Tamborlane W. V., Sherwin R. S. Oxidative fuel metabolism during mild hypoglycemia: critical role of free fatty acids. Am J Physiol. 1989 Mar;256(3 Pt 1):E413–E419. doi: 10.1152/ajpendo.1989.256.3.E413. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Lacy W. W., Jennings A. S., Cherrington A. D. Gluconeogenesis: methodological approaches in vivo. Fed Proc. 1977 Feb;36(2):229–235. [PubMed] [Google Scholar]
- Clore J. N., Glickman P. S., Helm S. T., Nestler J. E., Blackard W. G. Evidence for dual control mechanism regulating hepatic glucose output in nondiabetic men. Diabetes. 1991 Aug;40(8):1033–1040. doi: 10.2337/diab.40.8.1033. [DOI] [PubMed] [Google Scholar]
- Clore J. N., Glickman P. S., Nestler J. E., Blackard W. G. In vivo evidence for hepatic autoregulation during FFA-stimulated gluconeogenesis in normal humans. Am J Physiol. 1991 Oct;261(4 Pt 1):E425–E429. doi: 10.1152/ajpendo.1991.261.4.E425. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- Fanelli C. G., De Feo P., Porcellati F., Perriello G., Torlone E., Santeusanio F., Brunetti P., Bolli G. B. Adrenergic mechanisms contribute to the late phase of hypoglycemic glucose counterregulation in humans by stimulating lipolysis. J Clin Invest. 1992 Jun;89(6):2005–2013. doi: 10.1172/JCI115809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Friedmann B., Goodman E. H., Jr, Weinhouse S. Effects of insulin and fatty acids on gluconeogenesis in the rat. J Biol Chem. 1967 Aug 25;242(16):3620–3627. [PubMed] [Google Scholar]
- Frizzell R. T., Hendrick G. K., Biggers D. W., Lacy D. B., Donahue D. P., Green D. R., Carr R. K., Williams P. E., Stevenson R. W., Cherrington A. D. Role of gluconeogenesis in sustaining glucose production during hypoglycemia caused by continuous insulin infusion in conscious dogs. Diabetes. 1988 Jun;37(6):749–759. doi: 10.2337/diab.37.6.749. [DOI] [PubMed] [Google Scholar]
- Fröhlich J., Wieland O. Glucagon and the permissive action of fatty acids in hepatic gluconeogenesis. Eur J Biochem. 1971 Apr 30;19(4):557–562. doi: 10.1111/j.1432-1033.1971.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Fulcher G. R., Walker M., Catalano C., Agius L., Alberti K. G. Metabolic effects of suppression of nonesterified fatty acid levels with acipimox in obese NIDDM subjects. Diabetes. 1992 Nov;41(11):1400–1408. doi: 10.2337/diab.41.11.1400. [DOI] [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., Shank M., Petrides A. S., DeFronzo R. A. Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. J Clin Invest. 1991 Jan;87(1):83–89. doi: 10.1172/JCI115005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera M. G., Kamm D., Ruderman N., Cahill Non-hormonal factors in the control of gluconeogenesis. Adv Enzyme Regul. 1966;4:225–235. doi: 10.1016/0065-2571(66)90017-3. [DOI] [PubMed] [Google Scholar]
- Jahoor F., Peters E. J., Wolfe R. R. The relationship between gluconeogenic substrate supply and glucose production in humans. Am J Physiol. 1990 Feb;258(2 Pt 1):E288–E296. doi: 10.1152/ajpendo.1990.258.2.E288. [DOI] [PubMed] [Google Scholar]
- Jenssen T., Nurjhan N., Consoli A., Gerich J. E. Failure of substrate-induced gluconeogenesis to increase overall glucose appearance in normal humans. Demonstration of hepatic autoregulation without a change in plasma glucose concentration. J Clin Invest. 1990 Aug;86(2):489–497. doi: 10.1172/JCI114735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P., Hollenbeck C., Sheu W., Chen Y. D., Reaven G. M. Acute changes in plasma non-esterified fatty acid concentration do not change hepatic glucose production in people with type 2 diabetes. Diabet Med. 1990 Dec;7(10):871–875. doi: 10.1111/j.1464-5491.1990.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Kreisberg R. A., Siegal A. M., Owen W. C. Alanine and gluconeogenesis in man: effect of ethanol. J Clin Endocrinol Metab. 1972 May;34(5):876–883. doi: 10.1210/jcem-34-5-876. [DOI] [PubMed] [Google Scholar]
- Lecavalier L., Bolli G., Cryer P., Gerich J. Contributions of gluconeogenesis and glycogenolysis during glucose counterregulation in normal humans. Am J Physiol. 1989 Jun;256(6 Pt 1):E844–E851. doi: 10.1152/ajpendo.1989.256.6.E844. [DOI] [PubMed] [Google Scholar]
- Meriläinen P. T. Metabolic monitor. Int J Clin Monit Comput. 1987;4(3):167–177. doi: 10.1007/BF02915904. [DOI] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Piatti P. M., Monti L. D., Pacchioni M., Pontiroli A. E., Pozza G. Forearm insulin- and non-insulin-mediated glucose uptake and muscle metabolism in man: role of free fatty acids and blood glucose levels. Metabolism. 1991 Sep;40(9):926–933. doi: 10.1016/0026-0495(91)90068-8. [DOI] [PubMed] [Google Scholar]
- Puhakainen I., Koivisto V. A., Yki-Järvinen H. No reduction in total hepatic glucose output by inhibition of gluconeogenesis with ethanol in NIDDM patients. Diabetes. 1991 Oct;40(10):1319–1327. doi: 10.2337/diab.40.10.1319. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Kerbey A. L., Espinal J. Mechanisms decreasing glucose oxidation in diabetes and starvation: role of lipid fuels and hormones. Diabetes Metab Rev. 1988 Nov;4(7):623–638. doi: 10.1002/dmr.5610040702. [DOI] [PubMed] [Google Scholar]
- Saloranta C., Franssila-Kallunki A., Ekstrand A., Taskinen M. R., Groop L. Modulation of hepatic glucose production by non-esterified fatty acids in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991 Jun;34(6):409–415. doi: 10.1007/BF00403179. [DOI] [PubMed] [Google Scholar]
- Struck E., Ashmore J., Wieland O. Effects of glucagon and long chain fatty acids on glucose production by isolated perfused rat liver. Adv Enzyme Regul. 1966;4:219–224. doi: 10.1016/0065-2571(66)90016-1. [DOI] [PubMed] [Google Scholar]
- Tappy L., Owen O. E., Boden G. Effect of hyperinsulinemia on urea pool size and substrate oxidation rates. Diabetes. 1988 Sep;37(9):1212–1216. doi: 10.2337/diab.37.9.1212. [DOI] [PubMed] [Google Scholar]
- Thiébaud D., DeFronzo R. A., Jacot E., Golay A., Acheson K., Maeder E., Jéquier E., Felber J. P. Effect of long chain triglyceride infusion on glucose metabolism in man. Metabolism. 1982 Nov;31(11):1128–1136. doi: 10.1016/0026-0495(82)90163-9. [DOI] [PubMed] [Google Scholar]
- Tutwiler G. F., Joseph J., Wallace N. Effect of methyl palmoxirate, an oral hypoglycemic agent, on epinephrine-induced hyperglycemia in the rat. Biochem Pharmacol. 1985 Jun 15;34(12):2217–2220. doi: 10.1016/0006-2952(85)90424-1. [DOI] [PubMed] [Google Scholar]
- Vaag A., Skött P., Damsbo P., Gall M. A., Richter E. A., Beck-Nielsen H. Effect of the antilipolytic nicotinic acid analogue acipimox on whole-body and skeletal muscle glucose metabolism in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1991 Oct;88(4):1282–1290. doi: 10.1172/JCI115432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M., Fulcher G. R., Sum C. F., Orskov H., Alberti K. G. Effect of glycemia and nonesterified fatty acids on forearm glucose uptake in normal humans. Am J Physiol. 1991 Sep;261(3 Pt 1):E304–E311. doi: 10.1152/ajpendo.1991.261.3.E304. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Kreisberg R. A., Felts P. W. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A. 1966 Jul;56(1):247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B. M., Havel J. R., Marliss E. B., Kane J. P., Seymour J., Ahuja S. P. Effects of a 3-day fast and of ethanol on splanchnic metabolism of FFA, amino acids, and carbohydrates in healthy young men. J Clin Invest. 1976 Feb;57(2):329–340. doi: 10.1172/JCI108284. [DOI] [PMC free article] [PubMed] [Google Scholar]



