Abstract
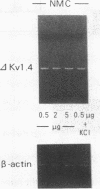
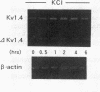
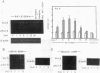
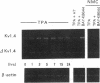
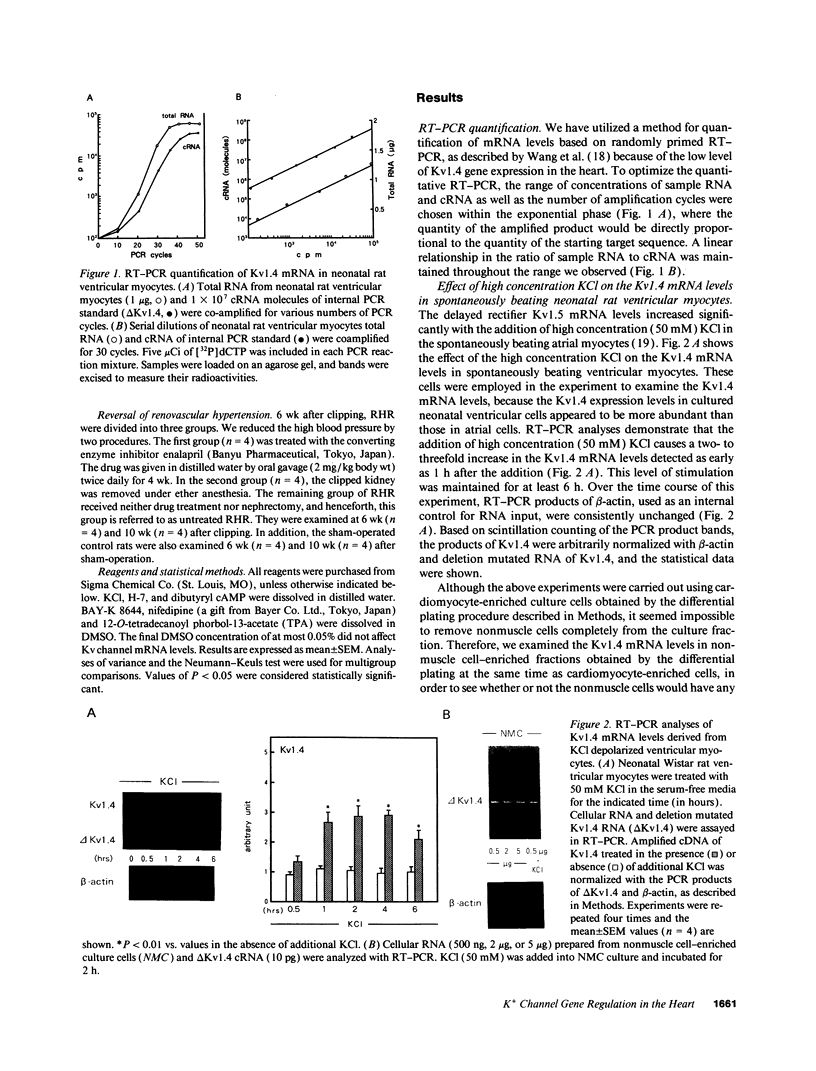
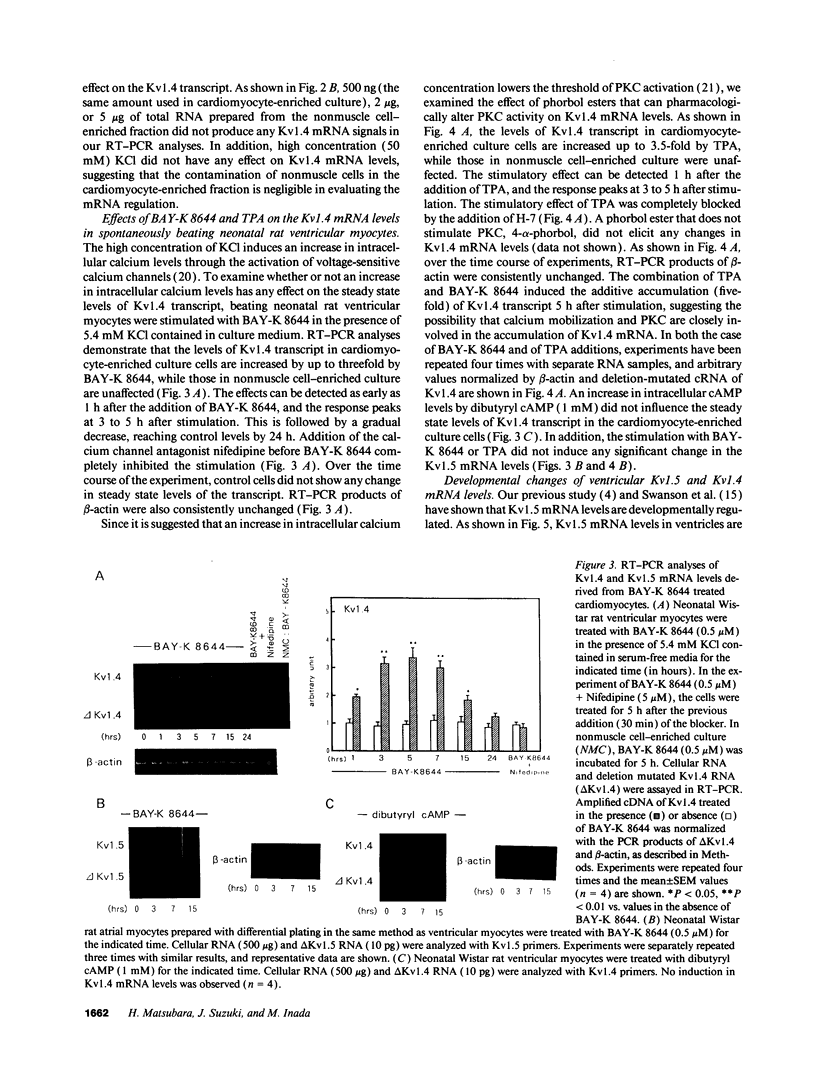
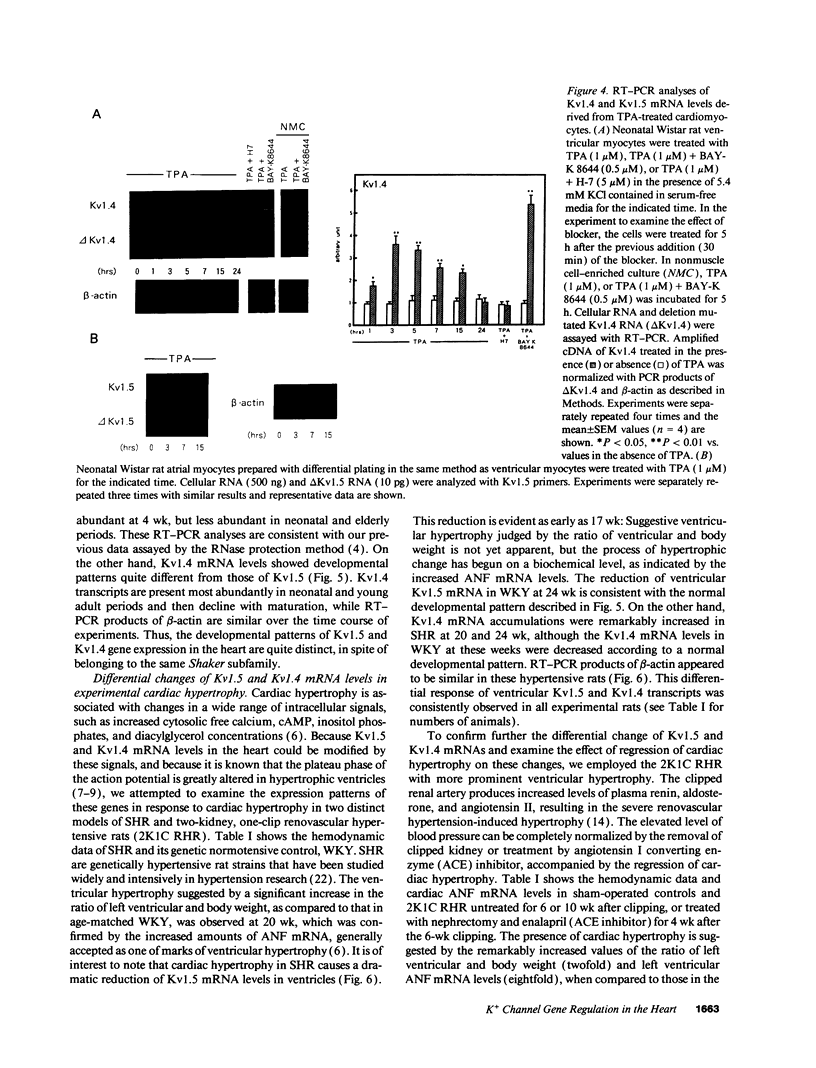
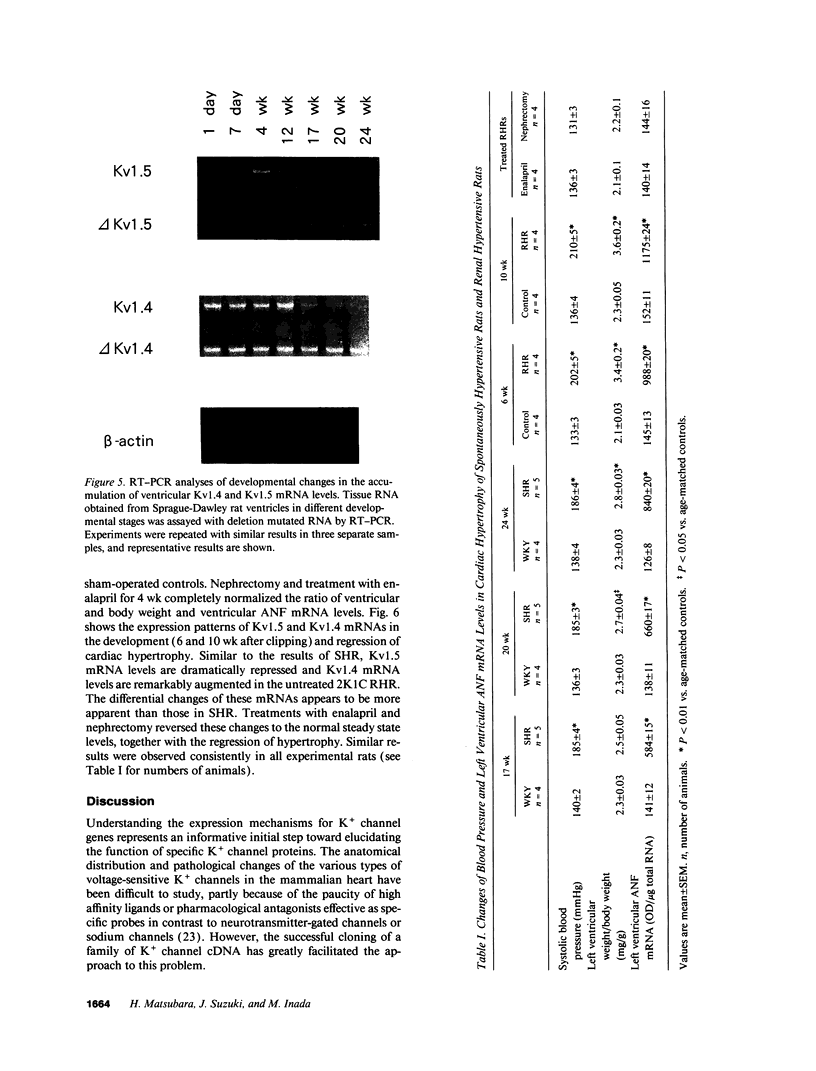
The multiple K+ channels are crucial for repolarization and configuration of the action potential in the neuronal and cardiac cells. In this study, we report the regulatory mechanisms of rapidly inactivating Shaker Kv1.4 channel transcript in the rat heart. Quantitative PCR analysis showed that stimulation with high concentration of KCl, BAY-K 8644, or 12-O-tetradecanoyl phorbol-13-acetate resulted in an immediate and substantial increase (two- to threefold) of Kv1.4 mRNA levels in spontaneously beating myocytes prepared from neonatal rat ventricles. The Kv1.4 mRNA in the ventricle remains at a steady state level after birth and gradually declines with maturation. These results suggest that the Kv1.4 mRNA level is not static and undergoes dynamic modulation by multiple factors that activate intracellular signals. In addition, the expression patterns of Kv1.4 as well as the delayed rectifier Shaker K+ channel Kv1.5 mRNAs were examined in hypertrophied ventricles in which a plateau phase of action potential is remarkably prolonged. The Kv1.5 mRNA level was dramatically repressed while the Kv1.4 mRNA level was remarkably increased. This differential regulation was completely reversed by the normalization of hypertrophy, suggesting that the pathological alterations of K+ channel gene regulation may be involved in the occurrence of ventricular arrhythmias in hypertrophic hearts.
Full text
PDF

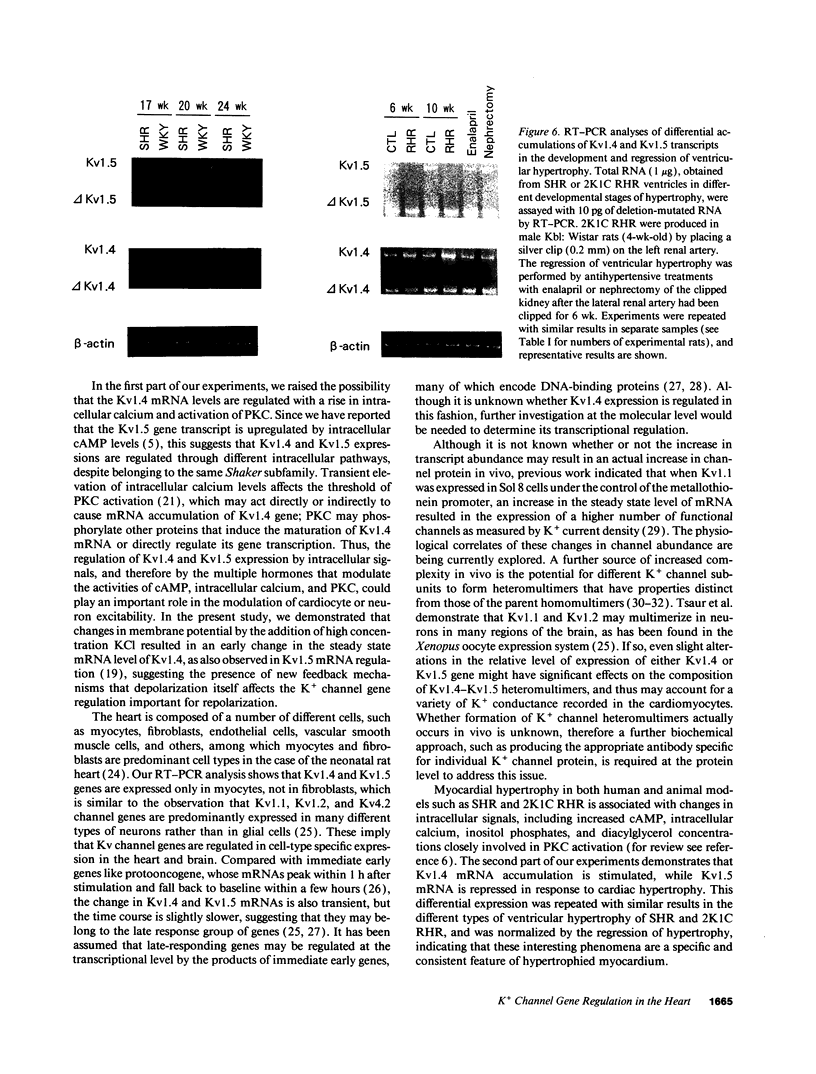

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson R. S. Characteristics of action potentials of hypertrophied myocardium from rats with renal hypertension. Circ Res. 1980 Sep;47(3):443–454. doi: 10.1161/01.res.47.3.443. [DOI] [PubMed] [Google Scholar]
- Christie M. J., Adelman J. P., Douglass J., North R. A. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989 Apr 14;244(4901):221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Memories of fos. Bioessays. 1987 Dec;7(6):255–258. doi: 10.1002/bies.950070606. [DOI] [PubMed] [Google Scholar]
- Dostal D. E., Rothblum K. N., Conrad K. M., Cooper G. R., Baker K. M. Detection of angiotensin I and II in cultured rat cardiac myocytes and fibroblasts. Am J Physiol. 1992 Oct;263(4 Pt 1):C851–C863. doi: 10.1152/ajpcell.1992.263.4.C851. [DOI] [PubMed] [Google Scholar]
- Döemeci A., Dhallan R. S., Cohen N. M., Lederer W. J., Rogers T. B. Phorbol ester increases calcium current and simulates the effects of angiotensin II on cultured neonatal rat heart myocytes. Circ Res. 1988 Feb;62(2):347–357. doi: 10.1161/01.res.62.2.347. [DOI] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990 Jun 7;345(6275):530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Voltage-sensitive ion channels. Cell. 1989 Jan 13;56(1):13–25. doi: 10.1016/0092-8674(89)90979-3. [DOI] [PubMed] [Google Scholar]
- Koren G., Liman E. R., Logothetis D. E., Nadal-Ginard B., Hess P. Gating mechanism of a cloned potassium channel expressed in frog oocytes and mammalian cells. Neuron. 1990 Jan;4(1):39–51. doi: 10.1016/0896-6273(90)90442-i. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Hirata Y., Yoshimi H., Takata S., Takagi Y., Umeda Y., Yamane Y., Inada M. Role of calcium and protein kinase C in ANP secretion by cultured rat cardiocytes. Am J Physiol. 1988 Sep;255(3 Pt 2):H405–H409. doi: 10.1152/ajpheart.1988.255.3.H405. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Liman E. R., Hess P., Koren G. Pretranslational mechanisms determine the type of potassium channels expressed in the rat skeletal and cardiac muscles. J Biol Chem. 1991 Jul 15;266(20):13324–13328. [PubMed] [Google Scholar]
- Matsubara H., Yamamoto J., Hirata Y., Mori Y., Oikawa S., Inada M. Changes of atrial natriuretic peptide and its messenger RNA with development and regression of cardiac hypertrophy in renovascular hypertensive rats. Circ Res. 1990 Jan;66(1):176–184. doi: 10.1161/01.res.66.1.176. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Baker K. M. Cardiac hypertrophy. Mechanical, neural, and endocrine dependence. Circulation. 1991 Jan;83(1):13–25. doi: 10.1161/01.cir.83.1.13. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Roberds S. L., Tamkun M. M. Cloning and tissue-specific expression of five voltage-gated potassium channel cDNAs expressed in rat heart. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1798–1802. doi: 10.1073/pnas.88.5.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. B., Gaa S. T., Allen I. S. Identification and characterization of functional angiotensin II receptors on cultured heart myocytes. J Pharmacol Exp Ther. 1986 Feb;236(2):438–444. [PubMed] [Google Scholar]
- Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Sewing S., Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990 Jun 7;345(6275):535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Jahn L., Takahashi T., Kulik T. J., Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem. 1992 May 25;267(15):10551–10560. [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R., Marshall J., Smith J. S., Williams J. B., Boyle M. B., Folander K., Luneau C. J., Antanavage J., Oliva C., Buhrow S. A. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990 Jun;4(6):929–939. doi: 10.1016/0896-6273(90)90146-7. [DOI] [PubMed] [Google Scholar]
- Ten Eick R. E., Baumgarten C. M., Singer D. H. Ventricular dysrhythmia: membrane basis or of currents, channels, gates, and cables. Prog Cardiovasc Dis. 1981 Sep-Oct;24(2):157–188. doi: 10.1016/0033-0620(81)90003-7. [DOI] [PubMed] [Google Scholar]
- Ten Eick R. E., Singer D. H. Electrophysiological properties of diseased human atrium. I. Low diastolic potential and altered cellular response to potassium. Circ Res. 1979 Apr;44(4):545–557. doi: 10.1161/01.res.44.4.545. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaur M. L., Sheng M., Lowenstein D. H., Jan Y. N., Jan L. Y. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992 Jun;8(6):1055–1067. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]