Abstract
Membrane transporter proteins switch between conformational states to move substrates across membranes. The transition between these states can now be studied using single-molecule experiments.
Biologists who work with membrane transporters — proteins that ferry substrate molecules across membranes — have long been envious of their colleagues that study ion channels. For more than three decades, researchers have been able to observe the dynamic opening and closing of single ion channels in real time using single-channel recording techniques1. The ability to watch ion channels ‘dance’ as they conduct a current allows the functional states of these proteins to be identified, and provides clues about their gating mechanisms. In contrast, most secondary membrane transporters can’t be studied in this way, with a few notable exceptions2,3. But in this issue, Zhao et al.4 report their use of a fluorescence technique to visualize several steps in the cycle of a transporter as it transfers a substrate across a membrane, an important breakthrough for the field.
Secondary membrane transporters are ubiquitous, dynamic molecular machines that use electrochemical gradients across membranes to drive the movement of substrates ‘uphill’ through those membranes — that is, against the prevailing concentration gradient of the substrate5. Various research efforts, including several X-ray crystal structures6,7, have greatly enhanced our understanding of the substrate specificity and translocation pathways in these proteins.
But such spectacular structures can only suggest the motions that are critical to membrane transport cycles. Two important pieces of information therefore remain missing8. The first is the dynamics of the transport cycle: how much time does the transporter stay in a particular state or in transition between states, and how does the protein’s conformation change during transition? Single-channel recording cannot answer these questions, because the transport cycles are too slow to be studied using that technique, and because membrane transporters are often electroneutral.
The second area of ignorance is an even greater embarrassment: we don’t really know how many conformational states exist in the transport cycles of a secondary membrane transporter. Ion channels and primary transporters can be trapped in particular states with toxins and ATP analogs, respectively, but we lack such specific molecular tools for dissecting the individual conformations of secondary transporters. As a result, many of the mechanistic details of these proteins remain unresolved.
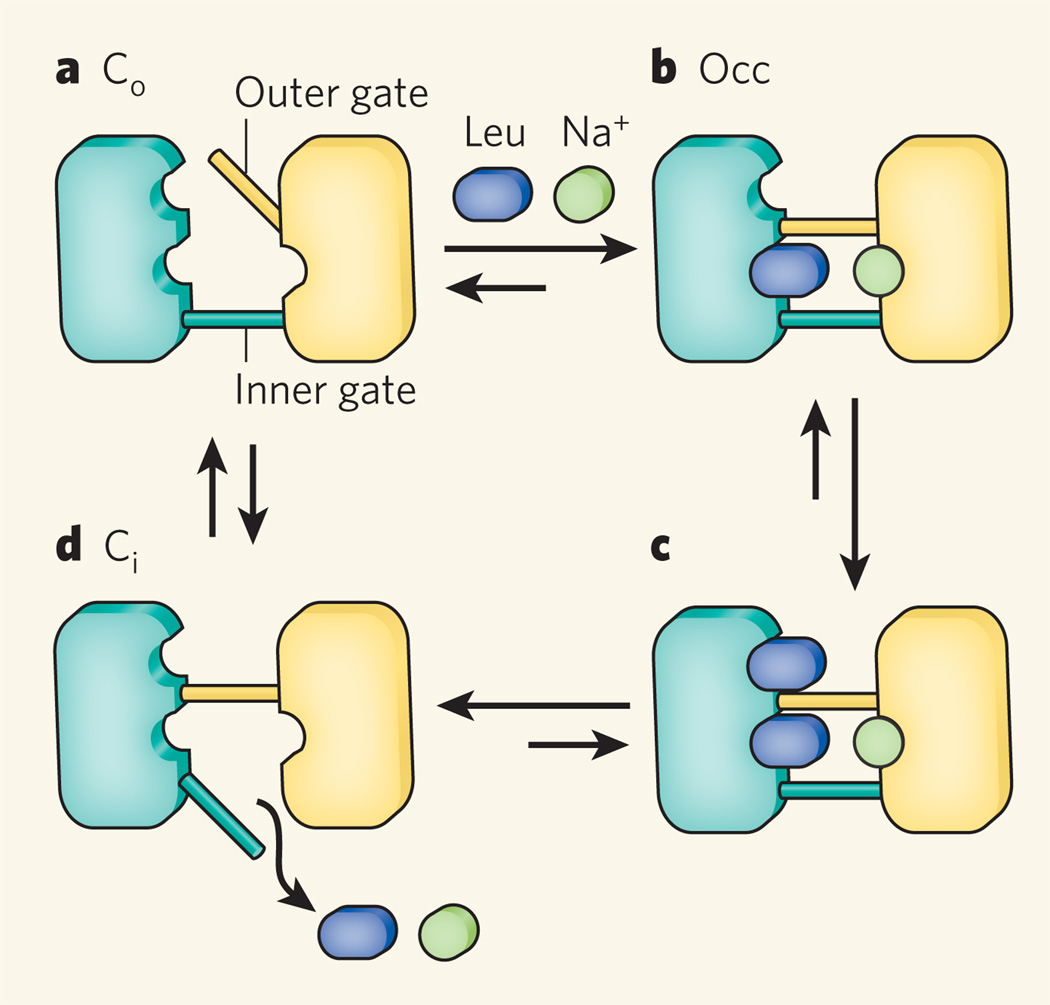
Enter Zhou et al.4, who have used a technique known as single-molecule fluorescence resonance energy transfer (smFRET) to study the LeuT transporter. Found in bacteria, LeuT belongs to the neurotransmitter/sodium symporter family of proteins, which couples the uphill uptake of substrates into cells to the downhill co-transport of sodium ions9. The LeuT transporter is a pore that contains two gates: an inner gate that opens to the cell lumen, and an outer gate that opens to the cell’s exterior. The transporter adopts so-called outward-facing (Co), occluded (Occ) and inward-facing (Ci) conformations (Fig. 1), probably among several others. Interconversion between these states facilitates translocation of the amino acid leucine across bacterial membranes. X-ray crystal structures of LeuT in the Co and Occ conformations have been determined9,10, but Zhao et al.4 now bring to light previously unknown features of the conformational cycle of the transporter.
Figure 1. Conformation states of a membrane transporter.
The LeuT protein adopts various conformational states as it transports the amino acid leucine across bacterial cell membranes. It consists of a pore containing two gates, the inner and outer gates. Four conformational states are shown here; the total number of states is unknown. a, The transport cycle begins with an outward-facing state, Co, in which the outer gate is open but the inner gate is closed. b, The binding of a leucine molecule and a sodium ion at sites between the gates causes the outer gate to close, generating the occluded state (Occ). c, A second leucine molecule can then bind to another site just outside the outer gate. d, Next, LeuT opens its inner gate and loses all its substrates to form the inward-facing state, Ci. Closure of the inner and gate and opening of the outer gate returns LeuT to the Co state. Zhao et al.4 have used a fluorescence-based, single-molecule technique to study the transition between Ci and Co.
Single-molecule FRET is uniquely suited to probing the functional dynamics of proteins, and have already bridged the gap between the statics and mechanics of protein function11,12. The technique involves measuring the distance between fluorescent probes strategically attached to different positions within a protein molecule. In this way, researchers can monitor the magnitude and timeframe of conformational changes for individual molecules within a population. The technique also allows the period spent in each observable conformation to be determined. These data are especially powerful when interpreted in the context of high-resolution structural information, such as crystal structures.
By attaching a fluorescent label to the gate on the intracellular side of LeuT, Zhao et al.4 used smFRET to characterize the transition of the transporter to the Ci conformation — something that has not been visualized crystallographically. In the absence of substrate, the authors observed two distinct fluorescent states for LeuT, presumably corresponding to the open and closed states of the inner gate. [OK] These data suggest that LeuT switches between the Ci and Co conformations, partially driven by Brownian motion.
Interestingly, when the authors added substrates to this dynamic trasnporter population, the inner gate closed, probably leading to formation of the Occ conformation in which a sodium ion and a leucine molecule are bound between the two closed gates (Fig. 1). In contrast, when Zhao et al. performed smFRET on mutants of LeuT in which the packing of the inner gate is disrupted, they observed that the transporter preferentially adopted the Ci conformation, and that substrate binding no longer closed the gate. By correlating their smFRET data with molecular dynamics simulations of the inner gate opening, the authors identified the rotation of a particular transmembrane helix in LeuT as being pivotal to the transporter’s conformational transition to the Ci state.
The authors also attached fluorescent labels to the extracellular surface of LeuT to probe the conformation of the outer gate. So, in the LeuT mutants biased towards the Ci state, the outer gate is closed. Conversely, if the outer gate was ‘pried’ open, then the inner gate closed. The closing of the inner gate is therefore coupled to the opening of the outer gate, suggesting long-distance coupling between the two gates in the transport across the membrane.
So what next? Zhao et al. performed their experiments in detergent solution, but the activities of membrane transporters in natural situations depend on their lipid-bilayer environment. Future single-molecule work on these transporters should therefore be performed in lipid membranes. Furthermore, the authors have reported only a limited part of the transport cycle of LeuT, characterizing the motion of a few helices, rather than the movement of the entire protein. The next step will be to characterize the entire transport cycle. In particular, it will be exciting to see how the binding of substrate to a second leucine binding site, recently identified above the extracellular outer gate13, triggers the opening of the inner gate to allow substrate to be released from the cytoplasm. A complete mechanistic description of a transport cycle also requires the identification of all the conformational states and the thermodynamic and kinetic relationships between them. Zhao and colleagues’ smFRET study4 lifts the curtain and shines a spotlight onto some of the choreography of LeuT — but now the audience is waiting to see the entire dance.
Contributor Information
Nathan K. Karpowich, Email: karpowic@saturn.med.nyu.edu.
Da-Neng Wang, Email: wang@saturn.med.nyu.edu.
References
- 1.Neher E, Sakmann B. Nature. 1976;260:799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- 2.Kang TM, Hilgemann DW. Nature. 2004;427:544–548. doi: 10.1038/nature02271. [DOI] [PubMed] [Google Scholar]
- 3.Majumdar DS, et al. Proc. Natl Acad. Sci USA. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, et al. Nature. 2010;465:188–193. doi: 10.1038/nature09057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maloney PC, Wilson TH. Soc. Gen. Physiol. Ser. 1993;48:147–160. [PubMed] [Google Scholar]
- 6.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 7.Abramson J, et al. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 8.Law CJ, Maloney PC, Wang DN. Annu. Rev. Microbiol. 2008;62:289–305. doi: 10.1146/annurev.micro.61.080706.093329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 10.Singh SK, Piscitelli CL, Yamashita A, Gouaux E. Science. 2008;322:1655–1660. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda R, Noji H, Kinosita K, Jr, Yoshida M. Cell. 1998;93:1117–1124. doi: 10.1016/s0092-8674(00)81456-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang X, et al. Science. 2000;288:2048–2051. doi: 10.1126/science.288.5473.2048. [DOI] [PubMed] [Google Scholar]
- 13.Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. Mol. Cell. 2008;30:667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]