Abstract
Although it is generally accepted that patients with amnestic mild cognitive impairment (aMCI) and patients with Alzheimer's disease (AD) have significantly impaired recollection, recent evidence has been mixed as to whether these patients demonstrate impaired memorial familiarity. Recent work suggests that familiarity may remain intact for pictures, but not for words. Further, a recent ERP study suggests that enhanced conceptual processing of pictures may underlie this intact familiarity. However, to date there has been no direct comparison of perceptual and conceptual-based familiarity for pictures and words in patients with aMCI and AD. To investigate this issue, patients with aMCI, patients with AD, and healthy older adults underwent four study-test conditions of word-word, picture-picture, word-picture, and picture-word. When stimuli undergo form change, it has been suggested that only conceptual processing can help support recognition in the absence of recollection. Our results showed that patients successfully relied on perceptual and conceptual-based familiarity to improve recognition for the within format conditions over the across format conditions. Further, results suggested that patients with aMCI and AD are able to use enhanced conceptual processing of pictures compared to words to allow them to overcome the deleterious effects of form change in a similar manner as controls. These results help us begin to understand which aspects of memory are impaired and which remain relatively intact in patients with aMCI and AD. This understanding can then in turn help us to assess, conceptualize, and build behavioral interventions to help treat these patients.
Keywords: recognition memory, conceptual fluency, perceptual fluency, episodic memory
Introduction
Alzheimer’s disease (AD) is associated with gradually progressive deficits in several cognitive domains, with the initial and most notable impairment typically involving episodic memory. Mild cognitive impairment (MCI) is often thought of as a transitional state from normal aging to AD. Although not all cases of MCI progress to AD or another form of dementia, those with MCI progress to AD at much higher rates than age matched controls without cognitive impairment (Petersen et al., 1999; Petersen, 2004). In fact, the amnestic subtype of MCI (aMCI) has been associated with an estimated tenfold increase in yearly conversion rate to AD compared to age-matched controls with no cognitive impairment (Petersen, 2004). The importance of characterizing and understanding the precise nature of memory impairment in these patient populations has been increasingly emphasized due to its clinical relevance. Recent investigations into the exact nature of episodic memory impairment associated with aMCI and AD have typically examined recognition memory – the judgment as to whether a stimulus has been previously encountered. In order to better understand recognition memory, several theories have been proposed. The most widely recognized theory is the dual process model of episodic memory, which posits that recognition is supported by the distinct and independent processes of recollection and familiarity (Jacoby, 1991; Yonelinas, 2002). Recollection refers to retrieval of contextually specific episodic details about previously encountered stimuli, whereas familiarity refers to an acontexual sense that a stimulus has been previously encountered.
Although our understanding of recollection and familiarity in patients with aMCI and AD is still evolving, data suggest a significant impairment in recollection for both groups (Ally, Gold, & Budson (2009a); Ally, McKeever, Waring, & Budson, 2009; Budson, Daffner, Desikan, & Schacter, 2000; Christensen, Kopelman, Stanhope, Lorentz, & Owen, 1998; Dalla Barba, 1997; Gallo, Sullivan, Daffner, Schacter, & Budson, 2004; Mitchell, Sullivan, Schacter, & Budson, 2006; Knight, 1998; Koivisto, Porton, Seinela, & Rinne, 1998; Smith & Knight, 2002; Wolk, Signoff, & DeKosky, 2008; Westerberg et al., 2006). In contrast, results of studies examining familiarity are less clear. In general, studies of familiarity and gist memory suggest impairment in patients with AD (Budson et al., 2000; Budson, Todman, & Schacter, 2006; Dalla Barba, 1997; Smith & Knight, 2002; but see Rauchs et al., 2007; Wolk et al., 2005). Research also suggests that AD patients become over-reliant on familiarity (Gallo, Shahid, Olson, Solomon, Schacter, & Budson, 2006; Gold, Marchant, Koutstaal, Schacter, & Budson, 2007). It has been recently proposed that over-reliance on impaired familiarity may lead to the liberal response bias, or the tendency to respond “old” on a recognition memory test, demonstrated by patients with AD (Budson, Wolk, Chong, & Waring, 2006; Gold et al., 2007; Wolk, Gold, Signoff, & Budson, 2009).
Studies examining familiarity in patients with aMCI have been mixed. For example, Westerberg et al. (2006) administered two separate picture recognition memory tasks requiring subjects to make standard old/new recognition memory decisions or to make forced-choice recognition decisions in which the target was grouped with highly similar foils. Based on earlier evidence (Bastin and Van der Linden, 2003; Gardiner, Java, & Richardson-Klavehn, 1998), Westerberg and colleagues suggested that the standard single-item recognition memory test allowed participants to rely on both recollection and familiarity, whereas the forced-choice test forced participants to rely more on familiarity. The results of this study showed that patients with aMCI and mild AD performed significantly worse on the standard old/new test compared to the healthy older adults, but performance on the forced-choice test was indistinguishable for the aMCI group and the healthy older adults. Westerberg et al. (2006) concluded that while recollection is impaired in patients with aMCI, familiarity remains intact, and that it could be successfully used even in difficult tasks requiring subjects to identify a target from highly similar foils. More recent studies using the process-dissociation procedure and the Remember / Know paradigm support the findings of drastically impaired recollection with intact estimates of familiarity in aMCI (Anderson et al., 2008; Hudon, Belleville, & Gauthier, 2009).
In contrast, several other studies have suggested that familiarity is impaired in these patients. For example, Wolk et al. (2008) used three experimental paradigms (two modifications of the process-dissociation procedure and one procedure that utilized the task-dissociation methodology) to provide converging evidence that familiarity, like recollection, is impaired. Algarabel et al. (2009) used a Spanish version of the Parkin letter-fluency paradigm (Parkin et al., 2001) to estimate the use of familiarity in recognition decisions. Here, healthy older adults and older adults with nonamnestic cognitive impairment were able to use familiarity based on perceptual fluency of letters to improve recognition, but patients with aMCI could not (Algarabel et al., 2009). Moreover, using confidence-based receiver operating characteristic (ROC) curves and a depth of processing manipulation, Ally, Gold, & Budson (2009a) reported decreased estimates of familiarity in both shallow and deep encoding conditions for patients with aMCI and AD compared to the healthy older adults.
Most recently, Ally, McKeever et al. (2009) used event-related potentials (ERPs) to investigate recognition of pictures and words in patients with aMCI. Historically, the neural correlate of familiarity has been associated with an early bifrontal old/new effect where more familiar items elicit a more positive waveform between 300 and 500 ms post-stimulus, and the correlate of recollection has been associated with a parietal old/new effect between 500 and 900 ms post-stimulus where old items elicit a more positive waveform than new items (see Rugg & Curran, 2007 for review). Ally, McKeever et al. (2009) reported no evidence of the neural correlate of recollection for pictures or words. However, the neural correlate of familiarity was similar for pictures, but diminished for words, in patients with aMCI compared to healthy older adults, suggesting that perhaps familiarity remained intact for pictorial stimuli but not for words. Based on work from Voss and Paller (2006, 2007, 2008) suggesting that the early frontal ERP effect may reflect conceptual processing of the test item, Ally, McKeever et al. (2009) speculated that perhaps the enhanced early frontal ERP old/new effect for pictures reflected intact conceptual implicit memory processes, such as priming or fluency, that may engender the sense of memorial familiarity in these patients. Priming and processing fluency are known to be important factors contributing not only to item recognition based on familiarity (Jacoby & Whitehouse, 1989; Kelly & Jacoby, 2000; Rajaram & Geraci, 2000; Westerman, 2001; Whittlesea, 1993; Whittlesea & Williams, 2000, 2001a, 2001b; Jacoby and Dallas, 1981), but also to the subjective sense of memorial familiarity (Kelley & Rhodes, 2002; Jacoby & Whitehouse, 1989). The results of Ally, McKeever et al., (2009) warrant a further look at how these implicit processes, such as conceptual or perceptual fluency, play a role in memorial familiarity in patients with aMCI and AD, and was the impetus for the current investigation.
The role of processing fluency is particularly important in familiarity and successful recognition. Processing fluency, or the relative speed and ease with which a stimulus is processed, can be conceptual (meaning-based) or perceptual (form-based) in nature. Stimuli that are processed faster and more effortlessly tend to be judged as having been seen before. Previous research suggests that perceptual fluency remains intact and can contribute to increased recognition performance in patients with aMCI and mild AD (Ballesteros, Reales, & Mayas, 2007; Fleischman & Gabrieli, 1998; Willems, Germain, Salmon, & Van der Linden, 2009), likely through enhanced familiarity (Algarabel et al., 2009). Although it has been suggested that tasks facilitated by perceptual processing show far less compromise in patients with AD than those facilitated by conceptual processing (see Fleischman & Gabrielli, 1998 for review), studies have shown that under certain circumstances patients with aMCI and AD can successfully use conceptual fluency to support recognition judgments (Martins & Lloyd-Jones, 2006) through enhanced familiarity (Gold, Marchant, Koutstaal, Schacter, & Budson, 2007; Wolk, Gold, Signoff, & Budson, 2009; Wolk et al., 2005). However, it must be acknowledged that for Alzheimer’s patients, relying on conceptual fluency does not come without consequence. Gold et al., (2007) showed that when patients with AD rely on conceptual cues at test, response bias becomes very liberal, leading to a high rate of false recognition.
In the current study, we set out to understand the role of perceptual and conceptual fluency in the familiarity of pictures and words in patients with aMCI and AD. Based on previous ERP work, Ally, McKeever et al., (2009) hypothesized that the intact early frontal effect for pictures reflected the use of familiarity based on enhanced conceptual fluency of pictures during recognition. This is somewhat counterintuitive given evidence that semantic networks and conceptual fluency may be somewhat impaired, and perceptual fluency tends to remain intact in patients with AD (Fleischman & Gabrieli, 1998). To further examine whether conceptual-based familiarity of pictures remains intact in aMCI and AD, we elected to use the form change paradigm using pictures and words in the present investigation. Form change refers to a change in stimulus format between study and test conditions. For example, stimuli that are presented as words at study would be said to undergo a form change if they are presented as pictures at test. Changes in stimulus format between study and test result in a decrease in recognition accuracy, referred to as form change cost, likely stemming from the reduced or eliminated contribution of perceptual familiarity to recognition (Ally & Budson, 2007; Schloerscheidt & Rugg, 2004; Yonelinas, 2002). When stimuli are presented in the same modality at study and test (e.g., study-test stimuli of word-word or picture-picture), it is speculated that both conceptual and perceptual fluency can contribute to recognition (Stenberg, Radeborg, & Hedman, 1995). In contrast, when stimuli are presented in different formats at study and test (e.g., study-test stimuli of word-picture or picture-word conditions), it has been suggested that only conceptual processing can help support recognition (Thapar & Westerman, 2009; Willems et al., 2009). In the current study, healthy older adults, patients with aMCI, and patients with AD underwent four study-test phases of word-word, picture-picture, word-picture, and picture-word.
Overall, we hypothesized that if patients with aMCI and AD were able to utilize perceptual and conceptual fluency based familiarity to support successful recognition decisions, they would perform better on the within format conditions, which allows for greater reliance on familiarity, compared to the across format conditions, which reduce or eliminate the ability to rely on familiarity. More specifically, we predicted that this discrepancy of difference between within versus across format conditions would be greater for patients than for healthy older adults, as the latter may be able to rely on recollection to compensate for the reduced ability to rely on familiarity in the across format conditions. With respect to within-subjects comparisons, we hypothesized that if conceptual processing of pictures remains intact in patients with aMCI and AD, then patients would perform similar, if not better, on the across format condition when pictures were studied (picture-word) compared to the within format condition when words were studied (word-word). In contrast, this effect would not be present for words when comparing the across format condition when words were studied (word-picture) to the within format condition when pictures were studied (picture-picture). In other words, we predict that the memorial power of pictures will offset the deleterious effects of stimulus change from study to test in patients as well as healthy older adults. Finally, when the individual across format conditions are compared, which rely on conceptual processing, we would expect performance to be better for the picture-word condition compared to the word-picture condition for all groups.
Methods
Design Overview
The experimental design systematically varied words and color pictures of individual items at study and test to generate four separate study-test conditions (two within format and two across format): word-word (WW); picture-picture (PP); word-picture (WP); picture-word (PW). All conditions presented 50 stimuli at study and 100 stimuli at test (50% old, 50% new). All subjects completed the four study-test conditions in two 1.5-hour sessions over the course of two days, with breaks between each condition. ERPs were recorded at test for each condition. However, due to inadequate bin sizes for the WP and PW conditions for patients with aMCI and AD, only the behavioral data were analyzed. It should also be noted that the PP and WW data were analyzed for a subset of the aMCI and AD patients, and were presented in Ally, Gold, and Budson (2009a).
Participants
Sixteen healthy older adults, sixteen patients with a diagnosis of amnestic-type MCI (multiple or single domain; Petersen, 2004), and sixteen patients with a clinical diagnosis of mild AD were recruited for this study. Patients with MCI reported a subjective memory complaint, showed abnormal memory performance for their age as evidenced by performing more than 1.5 standard deviations below the healthy control group on either the recall or the recognition portion of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List Memory Test (Morris et al., 1989), and did not display functional impairment in activities of daily living according to caregiver report. Patients with probable mild AD met criteria described by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (McKhann et al., 1984), and were in the mild range of the disease based on MMSE scores ranging from 20 to 26 (Folstein, Folstein, & McHugh, 1975). Healthy older adults were screened in the same manner as patients with MCI and AD and were defined as demonstrating no cognitive impairment on neuropsychological testing and having no first-degree relatives with Alzheimer's disease or other neurodegenerative diseases. It should be noted that 15 of the 16 AD patients and 12 of the 16 aMCI patients were undergoing pharmacological treatment with cholinesterase inhibitors at the time of the study. The participants with mild AD and MCI were recruited from the Memory Disorders Unit at Brigham and Women’s Hospital and the Boston University Alzheimer’s Disease Center, both in Boston, Massachusetts. Healthy older adult controls were recruited from online and community postings in the Boston area, or were spouses and friends of the AD and MCI patients who participated in the study. The human subjects committees of the Bedford VA Hospital, Brigham and Women’s Hospital, and Boston University School of Medicine approved the study. Participants were paid $25/hour for their participation.
Participants were excluded if they were characterized by clinically significant depression, alcohol or drug use, cerebrovascular disease, traumatic brain damage, other neurological or psychological disease that could affect cognitive function, or if English was not their primary language. Subjects were also required to have corrected 20/30 or better color vision. ll subjects completed a brief neuropsychological battery to evaluate their cognitive functioning, administered in a separate 45-minute session. Subjects were first administered the Mini Mental State Examination (Folstein et al., 1975), for which a score of 27 or better was required for the healthy older adults. Subjects were then administered the CERAD word list memory test (Morris et al., 1989), Trail Making Test Part B (Adjutant General’s Office, 1944), Verbal Fluency (Monsch et al., 1992), and the 15-item Boston Naming Test (Mack, Freed, William, & Henderson, 1992). Demographic and neuropsychological test data can be seen in Table 1.
Table 1.
Demographic and standard neuropsychological test data by group. Notes: OC = healthy older adults; MMSE = Mini Mental State Examination (Folstein, Folstein, & McHugh, 1975); CERAD = CERAD Word List Memory Test (Morris et al., 1989); Trails-B = Trail Making Test Part B (Adjutant General’s Office, 1944); FAS & CAT = Verbal Fluency (Monsch et al., 1992); BNT-15 = 15-item Boston Naming Test (Mack, Freed, Williams, & Henderson, 1992).
| OC | MCI | AD | ||
|---|---|---|---|---|
| Age | 74.3 (4.3) | 72.5 (8.7) | 73.6 (9.7) | |
| Years education |
15.8 (3.7) | 16.9 (3.6) | 15.1 (3.9) | |
| MMSE | 29.4 (0.8) | 28.5 (1.6) | 23.9 (2.1) | |
| CERAD | ||||
| Immediate | 22.5 (3.2) | 14.1 (4.5) | 10.1 (3.8) | |
| Delayed | 7.7 (1.6) | 2.4 (1.9) | 0.7 (1.3) | |
| Recognition | 9.8 (0.4) | 7.9 (1.7) | 4.9 (3.7) | |
| Trails-B | 86.5 (27.4) | 127.4 (86) | 251 (98) | |
| FAS | 49.1 (8.9) | 40.2 (11.1) | 26.4 (17.8) | |
| CAT | 49.6 (10.7) | 35.8 (13.1) | 37.1 (12.1) | |
| BNT-15 | ||||
| No cue | 14.6 (0.9) | 13.6 (1.5) | 11.9 (3.1) | |
| Semantic cue | 0.1 (0.2) | 0.2 (0.5) | 0.4 (0.7) | |
| Phonetic Cue | 0.4 (0.8) | 0.8 (1.2) | 1.5 (1.5) | |
Stimuli and Procedure
The color pictures used in the current study were the same stimuli set used by Ally and Budson (2007). The original pool of experimental stimuli consisted of 480 high-resolution clip-art style color pictures of nameable objects (nouns) and 480 words corresponding to the names of the objects. From the total pool, 400 pictures were randomly selected and were piloted in a naming study involving 5 subjects. Pictures with poor naming reliability scores were replaced as needed. The final 400 items were counterbalanced across study-test lists for word length, concreteness, and imagibility ratings. In addition, condition order (WW, PP, WP, PW) and study status (old, new) were counterbalanced across subjects. Color pictures were presented in central vision on a white background, with an average height of 13 cm and an average width of 15 cm, and a visual angle subtended of 7°. Words were presented in central vision in black uppercase letters 4 cm in height, also on a white background, with a visual angle subtended of 5.6°. All stimuli were presented on a 21-inch flat screen computer monitor positioned 48 inches from the subject.
During the study portion of each condition, participants were asked to make like/dislike judgments of the stimuli and to remember the stimuli for a subsequent memory test. Study stimuli were presented for two seconds each and were followed by the question, “Do you like this item?” Healthy older adult participants were then prompted to button press to signify their like/dislike judgment, while MCI and AD subjects reported their judgments aloud to an experimenter. There was a one second pause between each study trial. Test stimuli were each presented for 1.5 seconds, followed by the question, “Is this item old or new?” Healthy older adults were prompted to button press to signify their old/new judgment, while MCI and AD participants reported their judgments aloud and had responses input by the experimenter.
It should be noted that one healthy older adult and one patient with AD were excluded from analyses because performance was significantly (2 SD) lower than the group means. The patient with AD that was excluded responded “old” for virtually every item. Over the 4 conditions, this patient responded “old” 94% of the time, even when the instructions were repeated multiple times. The healthy older adult performed 1.5 SD lower on the PP and WW conditions, and greater than 2 SD on the WP and PW conditions. Re-running the analyses with these individuals in the dataset did not change the overall pattern of results.
Results
To directly examine the effect of form change on recognition performance, our initial analysis used ANOVA to examine accuracy and response bias with the factors of Group (healthy older adults, aMCI, AD) and Condition (WW, PP, WP, PW). Follow-up ANOVA or t-tests were performed for significant main effects and interactions. In our analyses of accuracy, we used the statistic Pr [% hits - % false alarms] and in our analyses of response bias, we used the statistic Br [% false alarms / 1 – Pr] (Snodgrass & Corwin, 1988). The ANOVA using Pr revealed effects of Group [F(2, 43) = 55.62, p < .001] and Condition [F(3, 129) = 66.69, p < .001], and an interaction of Group and Condition [F(6, 129) = 2.54, p = .024]. The effect of Group was present because discrimination was higher for the healthy older adults compared to the aMCI [F(1, 29) = 25.99, p < .001] and AD [F(1, 28) = 156.68, p < .001] groups. In addition, the aMCI group performed better than the AD group [F(1, 29) = 24.79, p < .001]. The effect of Condition was present because performance was greatest in the PP condition compared to the WW condition [t(45) = 7.53, p < .001], the WP condition [t(45) = 12.24, p < .001] and the PW condition [t(45) = 8.76, p < .001]. Performance on the WW condition was greater than the WP condition [t(45) = 6.38, p < .001], but not compared to the PW condition [t(45) = 1.38, p = .174]. Finally, performance on the PW condition was greater than on the WP condition [t(45) = 5.26, p < .001]. Follow up analyses also revealed that the magnitude of this picture superiority effect was greater for the patient groups (collapsed across aMCI and AD patients) compared to the healthy older adults [t(44) = 2.37, p = .022]
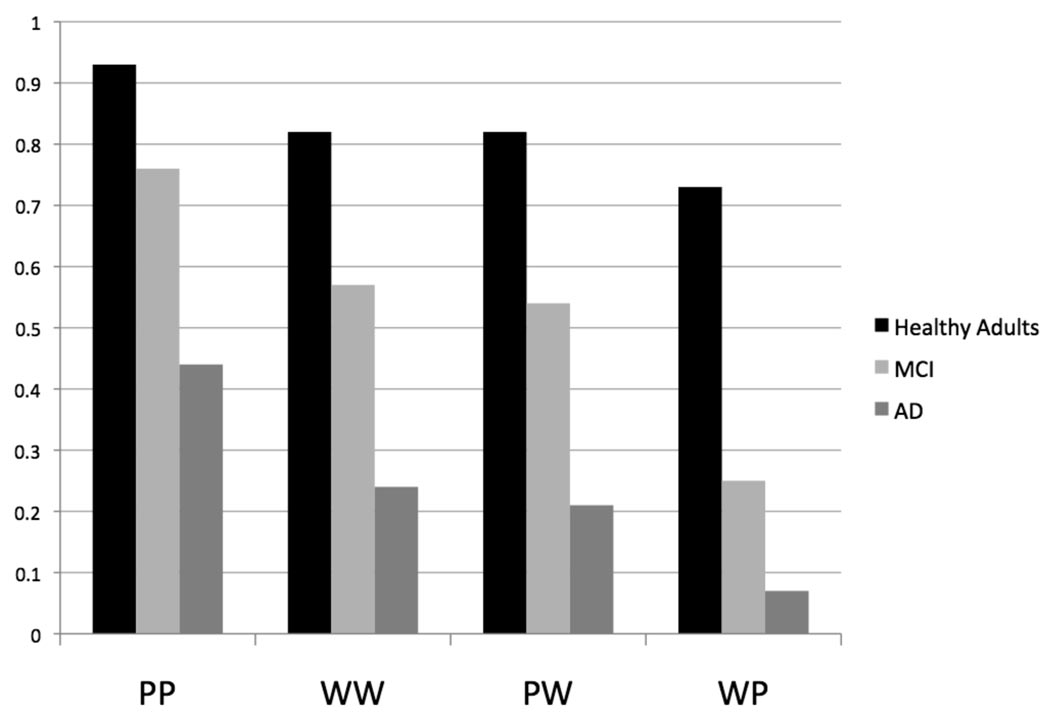
The interaction of Group and Condition was followed-up with ANOVAs between each group pair. The ANOVAs revealed individual interactions of Group and Condition for the healthy older adults compared to the MCI patients [F(3, 87) = 4.62, p = .005] and for the healthy older adults compared to the AD patients [F(3, 84) = 3.48, p = .019], but not for the MCI patients compared to AD patients [F(3, 87) <1]. The interaction between the healthy older adults and the patient groups and Condition was present because the difference in performance between the within and across format conditions was greater in the patient groups than in the healthy older adults. Discrimination was better by .10 for the within format conditions (.87) compared to the across format conditions (.77) for the healthy older adults. In contrast, discrimination was better by .22 for the within format conditions (.66) compared to the across format conditions (.44) in patients with MCI, resulting in a greater difference between the across and within format conditions compared to healthy older adults [t(29) = 3.03, p = .005]. Similarly, discrimination was better by .18 for the within format conditions (.32) compared to the across format conditions (.14) in patients with AD, resulting in a greater difference between the across and within format conditions compared to healthy older adults [t(28) = 2.20, p = .031]. No significant difference was observed between the within and across format conditions in the MCI group compared to the AD group [t(29) < 1]. Figure 1 shows accuracy (Pr) values for the within format and across format conditions for all three groups.
Figure 1.
Pr values for healthy older adults, patients with amnestic mild cognitive impairment (MCI), and patients with Alzheimer's disease (AD) on the four study-test conditions. PP = picture-picture, WW = word-word, PW = picture-word, WP = word-picture.
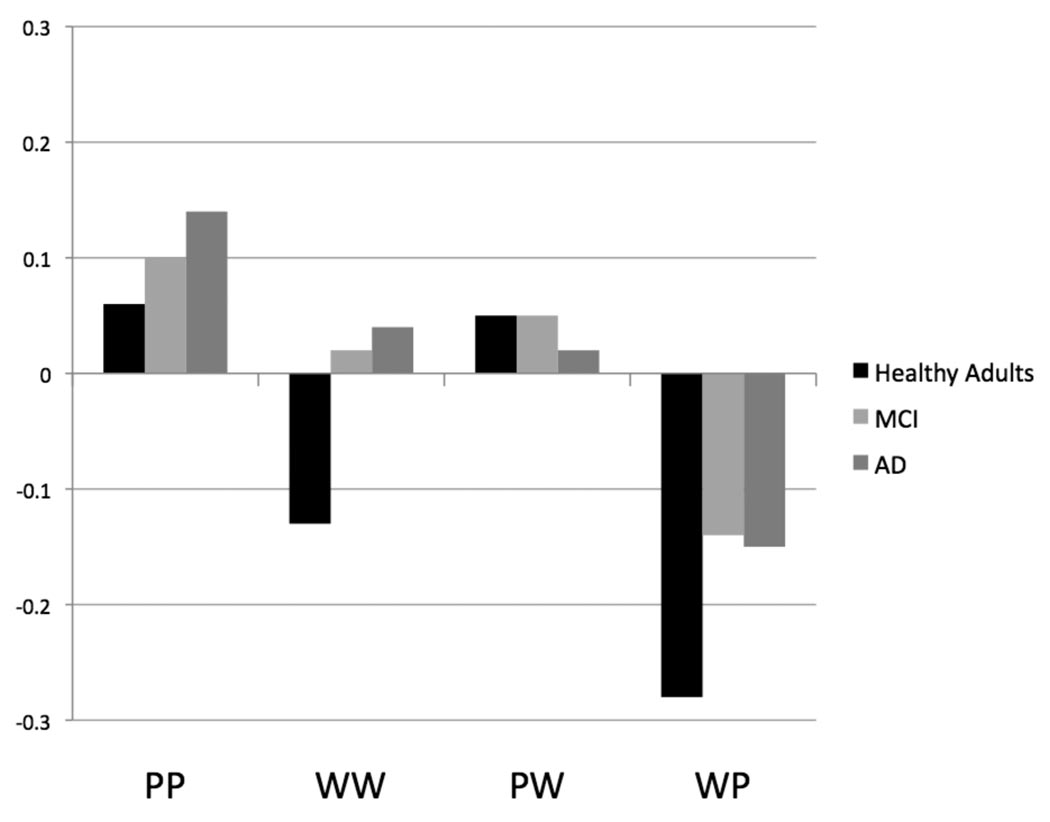
In a similar manner to discrimination, to understand the effect of form change on response bias (Br), we performed a repeated measures ANOVA with the factors of Group (healthy older adults, MCI, AD) and Condition (WW, PP, WP, PW). The ANOVA revealed only an effect of Condition [F(3, 129) = 22.69, p < .001]. There was no effect of Group [F(2, 43) = 1.15, p = .327] or interaction of Group and Condition [F(6, 129) = 1.18, p = .322]. The effect of Condition was present because response bias was more liberal for the PP condition compared to the WW [t(45) = 3.25, p = .002] and WP [t(45) = 8.52, p < .001] conditions. Response bias was also more liberal for the WW condition compared to the WP condition [t(45) = 4.34, p < .001], and for the PW condition compared to the WP condition [t(45) = 7.93, p < .001]. Overall, subjects demonstrated a more liberal response bias for the within format conditions compared to the across format conditions [t(45) = 3.70, p = .001]. Figure 2 shows response bias (Br, Snodgrass & Corwin, 1988) values for the within form and across form conditions for all three groups. Negative values indicate a conservative response bias, while positive values indicate a liberal response bias.
Figure 2.
Br values (negative values equate to a conservative response bias and positive value equate to a liberal response bias) for healthy older adults, patients with amnestic mild cognitive impairment (MCI), and patients with Alzheimer's disease (AD) on the four study-test conditions. PP = picture-picture, WW = word-word, PW = picture-word, WP = word-picture.
Discussion
The goal of the current study was to examine the role of perceptual and conceptual based familiarity for pictures and words in patients with aMCI and mild AD. Ally, McKeever et al. (2009) found that the ERP correlate of familiarity for pictures, but not words, was similar in patients with aMCI compared to healthy older adults. It was hypothesized that this intact early frontal effect for pictures reflected the ability of patients to use of familiarity based on enhanced conceptual fluency of pictures during recognition. However, this argument was somewhat speculative given evidence that semantic networks and conceptual fluency may be somewhat impaired, and that perceptual fluency tends to remain intact in these patients. In the present investigation, utilizing the form change paradigm allowed us to examine performance on within format conditions, in which patients would be able to rely on conceptually based and perceptually based familiarity, compared to across format conditions which are thought to rely only on conceptually based familiarity in the absence of recollection.
Consistent with our a priori hypothesis regarding perceptual and conceptual based familiarity, discrimination in patients with aMCI and AD suffered more from changes in stimulus format than healthy older controls, with the discrepancy of difference between within versus across format conditions being almost double for both patient groups compared to healthy older controls. It is very likely that in the absence of recollection, increased perceptual processing fluency allowed patients in the current study to perform significantly better on the within format conditions compared to the across format conditions through an enhanced sense of familiarity. As expected, analysis of the within format conditions revealed that all groups showed increased recognition for pictures compared to words. In fact, the magnitude of this picture superiority effect was greater for the patient groups compared to the healthy older adults, suggesting a preferential sparing of picture memory compared to verbal or word memory in patients. However, enthusiasm for this finding is somewhat dampened by the fact that healthy older adults were approaching ceiling on the PP condition.
For the across format conditions, consistent with previous work in young adults (Ally & Budson, 2007; Mintzer & Snodgrass, 1999; Schloerscheidt & Rugg, 2004), the cost of form change was significant for all groups when stimuli were studied as words and retrieved using pictures (WP) compared to when stimuli were studied and tested using pictures (PP). In contrast, all three groups showed very little cost of form change when stimuli were studied as pictures and retrieved using words compared to when stimuli were studied and tested using words. Stated another way, there was no difference in accuracy for the PW condition compared to the WW condition. It has been speculated that the memorial power of pictures can buffer the effects of stimulus form change through the use of recollection (Ally & Budson, 2007; Schloerscheidt & Rugg, 2004; Rajaram, 1993). This certainly may be the case in healthy individuals, who can use recollection of studied pictures. However, what about in patient groups who have severely impaired recollection?
For possible insight, we turn to the results of the form change conditions. In a similar manner as healthy young adults (Mintzer & Snodgrass, 1999; Schloerscheidt & Rugg, 2004; Stenberg et al., 1995), all groups in the current study better remembered stimuli presented as pictures at study with words as the test cue (PW) than stimuli presented as words at study with pictures as the test cue (WP). For the patient groups, who demonstrate significant impairment in recollection, conceptual processing should be the major contributing factor to recognition in these two conditions. For example, in the PW condition one would expect both conceptual and perceptual encoding at study, but only the ability to rely on conceptual information at test. Similarly in reverse, in the WP condition one would expect conceptual processing and possibly a small contribution of perceptual processing at study, with the ability to rely only on conceptual information at test. However, recognition accuracy for the patient groups was far greater for the PW condition compared to the WP condition, suggesting that there is something special about the encoding of pictures outside of perceptual distinctiveness. Although the patients with aMCI showed a greater difference in performance between the PW and WP conditions compared to the patients with AD, we suspect this was likely due to the fact that patients with AD were at floor for the WP condition.
We cannot discount the possibility that patients, particularly those with aMCI, were able to use recollection of studied pictures to improve performance on the PW condition compared to the WP condition, however there is likely an alternative explanation. Building on previous work in healthy memory, perhaps we have specialized conceptual processing of pictures that differs from our conceptual processing of words (e.g, see Weldon & Roediger, 1987), and this differential processing appears to remain intact in patients with aMCI and mild AD. Alternatively, perhaps our conceptual processing of words becomes differentially affected during the early course of Alzheimer's disease. Hamilton and Geraci (2006) proposed that it may not be perceptual distinctiveness, specifically, that provides the memorial advantage of pictures, but rather this perceptual distinctiveness provides the basis for information that is conceptually diagnostic of the item. This may be extremely beneficial to patients with aMCI and AD. Whereas these patients may have limited access to semantic information or relationships, or perhaps even completely forget the meaning of a word (Chertkow & Bub, 1990), pictures combat this by providing easy access to semantic and conceptual meaning, information, and relationships (Budson et al., 2002). This conceptual distinctiveness process for pictures may work to boost memory compared to words in patients with aMCI and AD. In other words, pictures may provide patients with aMCI and AD with numerous benefits that enhance processes that could be impaired in the disease and affect their memory for words (e.g., disordered semantic networks, impaired mental image generation, etc.).
Some support for this hypothesis comes form the results of the response bias data. Here, all groups were significantly conservative for the WP condition. This is particularly surprising for patients with AD, who are known to have a fairly consistent liberal response bias for pictures and words (Beth et al., 2009; Budson et al., 2006). In the current study, patients with AD are liberal for both of the within format conditions compared to healthy controls. As discussed previously by Budson et al. (2006), this is likely because perceptual or conceptual overlap between studied items and unstudied items on within format conditions drives an aberrant sense of familiarity in AD patients (see also Lekeu et al., 2003). However, in the current study, patients with AD become conservative in the form change conditions (near neutral for the PW condition, but more conservative than healthy controls). On these conditions, patients likely shift to a more conservative bias because the ability to use perceptual fluency driven familiarity during memory judgments is drastically reduced. On the WP condition, patients not only have significantly reduced ability to use perceptual fluency, but also possible impairment in the conceptual processing of words (Chertkow & Bub, 1990). If patients have disrupted semantic networks or cannot recall the meaning of a word, the pictures at test may not generate a sense of familiarity.
As briefly mentioned above, one limitation to this hypothesis that conceptual driven familiarity is a major force behind superior memory for pictures compared to words in AD is the possibility that patients with aMCI and AD could possibly have been using recollection to help support recognition on the form change conditions. If this were true, the fact that performance was better for the PW condition compared to the WP condition certainly fits well with previous literature in healthy older adults. Ally, Waring, Beth, McKeever, Milberg, and Budson (2008) used event-related potentials to show that pictures at study enhance recollection compared to words in healthy older adults. Perhaps pictures worked to increase the ability to use recollection in our patient groups for the PW condition compared to the WP condition in the current study. At the very least, pictures could work to increase the amount recollection experienced by the patients, giving support to their increased sense of familiarity or increasing confidence in their memories. In addition to examining the possibility of using recollection to increase performance for pictures in our patient populations, we should also acknowledge that given multiple directed comparisons between the three groups on our four conditions invites the possibility of Type I error and warrants independent replication. Follow-up studies are encouraged to use larger sample sizes and potentially include both objective and subjective measures of recollection and familiarity to further understand the form change paradigm in patients with aMCI and AD.
The results of the current investigation raise several potential questions worthy of future investigation. One potential query, with relevance to the robust picture superiority effect reported in patients with aMCI and AD (Ally, Gold, & Budson, 2009b), is whether patients with aMCI and AD demonstrate enhanced conceptual processing of pictures, or diminished conceptual processing of words? If, as Voss and Paller (2006, 2007, 2008) speculate, the early frontal ERP old/new effect actually reflects conceptual processing and not memorial familiarity specifically, then the findings of Ally, McKeever et al., (2009) in combination with the current data suggest that conceptual processing of words is likely impaired. This is consistent with previous research reporting degraded semantic networks and possibly weakened conceptual fluency in patients with very mild AD (Chertkow & Bub, 1990). As it relates to the sense of familiarity, it is possible that intact conceptual processing of pictures leads to an enhanced sense of familiarity, whereas impaired conceptual processing of words does not. Some support for this hypothesis comes from an analysis of studies of familiarity in aMCI and mild AD. Methodologies using words as stimuli generally find impaired familiarity (Algarabel et al., 2009; Ally et al., 2009a; Wolk et al, 2008), whereas the majority of studies demonstrating relatively preserved familiarity have used pictures as stimuli (Ally, McKeever, et al., 2009; Anderson et al., 2008; Westerberg et al., 2006).
In summary, the results of the current study suggest that patients with aMCI and mild AD can successfully use increased perceptual and conceptual based familiarity to perform significantly better on within format conditions compared to the across format conditions. Further, these patient groups demonstrated intact conceptual processing of pictures that helped them to overcome the deleterious effects of form change on recognition performance in a similar manner as healthy older adults. However, the question still remains as to whether patients demonstrate relatively intact, or even enhanced conceptual processing of pictures or alternatively, whether these patients have impaired conceptual processing of words. Indirect evidence suggests that conceptual processing of words is likely impaired due to degraded semantic networks. Future research should be aimed at understanding how these processes interact with familiarity based recognition memory in patients with aMCI and AD. This understanding of which memorial processes are impaired and which remain relatively intact in patients is critical to our understanding of the disease process as well as our work as clinicians. Perhaps future behavioral strategies and interventions, can be aimed at boosting this relatively preserved picture memory system with the goal of helping patients lead more independent and engaged lives.
Acknowledgements
This research was supported by National Institute on Aging grants K23 AG031925 and P30 AG13846 to BAA. This material is also the result of work supported with resources and the use of facilities at the Edith Nourse Rogers Memorial Veterans Hospital in Bedford, MA. The authors would like to thank Andrew Budson, M.D. for his invaluable input on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjutant General’s Office. Army Individual Test Battery: Manual of Directions and Scoring. Washington, D.C.: War Department; 1944. [Google Scholar]
- Algarabel S, Escudero J, Mazon JF, Pitarque A, Fuentes M, Peset V, et al. Familiarity-based recognition in the young, healthy elderly, mild cognitive impaired and Alzheimer's patients. Neuropsychologia. 2009;47:2056–2064. doi: 10.1016/j.neuropsychologia.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Ally BA, Budson AE. The worth of pictures: Using high density event-related potentials to understand the memorial power of pictures and the dynamics of recognition memory. NeuroImage. 2007;35:378–395. doi: 10.1016/j.neuroimage.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer's disease and mild cognitive impairment using receiver operating characteristics. Brain Cogn. 2009a;69:504–513. doi: 10.1016/j.bandc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. The picture superiority effect in patients with Alzheimer's disease and mild cognitive impairment. Neuropsychologia. 2009b;47:595–598. doi: 10.1016/j.neuropsychologia.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, McKeever JD, Waring JD, Budson AE. Preserved frontal memorial processing for pictures in patients with mild cognitive impairment. Neuropsychologia. 2009;47:2044–2055. doi: 10.1016/j.neuropsychologia.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Waring JD, Beth EH, McKeever JD, Milberg WP, Budson AE. Aging memory for pictures: Using high-density event-related potentials to understand the effect of aging on the picture superiority effect. Neuropsychologia. 2008;46:287–297. doi: 10.1016/j.neuropsychologia.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. Recollection- and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology. 2008;22:177–187. doi: 10.1037/0894-4105.22.2.177. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Reales JM, Mayas J. Picture priming in normal aging and Alzheimer's disease. Psicothema. 2007;19:239–244. [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: A study of the effects of test format and aging. Neuropsychology. 2003;17:14–24. [PubMed] [Google Scholar]
- Beth EH, Budson AE, Waring JD, Ally BA. Response bias for picture recognition in patients with Alzheimer disease. Cogn Behav. Neurol. 2009;22:229–235. doi: 10.1097/WNN.0b013e3181b7f3b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: Gist-based memory distortion in Alzheimer's disease. Neuropsychology. 2000;14:277–287. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Sitarski J, Daffner KR, Schacter DL. False recognition of pictures versus words in Alzheimer's disease: The distinctiveness heuristic. Neuropsychology. 2002;16:163–173. doi: 10.1037//0894-4105.16.2.163. [DOI] [PubMed] [Google Scholar]
- Budson AE, Wolk DA, Chong H, Waring JD. Episodic memory in Alzheimer's disease: Separating response bias from discrimination. Neuropsychologia. 2006;44:2222–2232. doi: 10.1016/j.neuropsychologia.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D. Semantic memory loss in dementia of Alzheimer's type. What do various measures measure? Brain. 1990;113(Pt 2):397–417. doi: 10.1093/brain/113.2.397. [DOI] [PubMed] [Google Scholar]
- Christensen H, Kopelman MD, Stanhope N, Lorentz L, Owen P. Rates of forgetting in Alzheimer dementia. Neuropsychologia. 1998;36:547–557. doi: 10.1016/s0028-3932(97)00116-4. [DOI] [PubMed] [Google Scholar]
- Dalla Barba G. Recognition memory and recollective experience in Alzheimer's disease. Memory. 1997;5:657–672. doi: 10.1080/741941546. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Gabrieli JD. Repetition priming in normal aging and Alzheimer's disease: a review of findings and theories. Psychol. Aging. 1998;13:88–119. doi: 10.1037//0882-7974.13.1.88. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Shahid KR, Olson MA, Solomon TM, Schacter DL, Budson AE. Overdependence on degraded gist memory in Alzheimer's disease. Neuropsychology. 2006;20:625–632. doi: 10.1037/0894-4105.20.6.625. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Sullivan AL, Daffner KR, Schacter DL, Budson AE. Associative recognition in Alzheimer's disease: Evidence for impaired recall-to-reject. Neuropsychology. 2004;18:556–563. doi: 10.1037/0894-4105.18.3.556. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Java RL, Richardson-Klavehn A. Experiences of remembering, knowing, and guessing. Consciousness and Cognition. 1998;7:1–26. doi: 10.1006/ccog.1997.0321. [DOI] [PubMed] [Google Scholar]
- Gold CA, Marchant NL, Koutstaal W, Schacter DL, Budson AE. Conceptual fluency increases false recognition of ambiguous images in Alzheimer's disease. Neuropsychologia. 2007;45:2791–2801. doi: 10.1016/j.neuropsychologia.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, Geraci L. The picture superiority effect in conceptual implicit memory: a conceptual distinctiveness hypothesis. Am. J. Psychol. 2006;119:1–20. [PubMed] [Google Scholar]
- Hudon C, Belleville S, Gauthier S. The assessment of recognition memory using the Remember/Know procedure in amnestic mild cognitive impairment and probable Alzheimer's disease. Brain Cogn. 2009;70:171–179. doi: 10.1016/j.bandc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process-dissociation framework: separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Jacoby JJ, Dallas M. On the relationship between autobiographical memory and perceptual learning. J Exp Psychol Gen. 1981;110:306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Whitehouse K. An illusion of memory: False recognition influenced by unconscious perception. Journal of Experimental Psychology: General. 1989;118:126–135. [Google Scholar]
- Kelley CM, Jacoby LL. Recollection and familiarity: Process-Dissociation. In: Craik FIM, Tulving E, editors. The Oxford Handbook of Memory. New York: Oxford University Press; 2000. pp. 215–228. [Google Scholar]
- Kelley CM, Rhodes MG. Making sense and nonsense of experience: Attributions in memory and judgment. In: Ross BH, editor. Psychology of Learning and Motivation: Advances in Theory and Research Vol. 41. 2002. pp. 293–320. [Google Scholar]
- Knight RG. Controlled and automatic memory process in Alzheimer's disease. Cortex. 1998;34:427–435. doi: 10.1016/s0010-9452(08)70765-2. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Portin R, Seinela A, Rinne J. Automatic influences of memory in Alzheimer's disease. Cortex. 1998;34:209–219. doi: 10.1016/s0010-9452(08)70748-2. [DOI] [PubMed] [Google Scholar]
- Lekeu F, Van der Linden M, Chicherio C, Collette F, Degueldre C, Franck G, Moonen G, Salmon E. Brain correlates of performance in a free/cued recall task with semantic encoding in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2003;17:35–45. doi: 10.1097/00002093-200301000-00005. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston naming test: Shortened versions for use in alzheimer's disease. J Gerontol. 1992;47:154–158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- Martins CA, Lloyd-Jones TJ. Preserved conceptual priming in Alzheimer's disease. Cortex. 2006;42:995–1004. doi: 10.1016/s0010-9452(08)70205-3. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:285–297. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Snodgrass JG. The picture superiority effect: support for the distinctiveness model. Am. J. Psychol. 1999;112:113–146. [PubMed] [Google Scholar]
- Mitchell JP, Sullivan AL, Schacter DL, Budson AE. Mis-attribution errors in Alzheimer's disease: the illusory truth effect. Neuropsychology. 2006;20:185–192. doi: 10.1037/0894-4105.20.2.185. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Ward J, Squires EJ, Furbear H, Clark A, Townshend J. Data-driven recognition memory: a new technique and some data on age differences. Psychon. Bull. Rev. 2001;8:812–819. doi: 10.3758/bf03196222. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment. Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Rajaram S. Remembering and knowing: two means of access to the personal past. Mem. Cognit. 1993;21:89–102. doi: 10.3758/bf03211168. [DOI] [PubMed] [Google Scholar]
- Rajaram S, Geraci L. Conceptual fluency selectively influences knowing. J. Exp. Psychol. Learn. Mem. Cogn. 2000;26:1070–1074. doi: 10.1037//0278-7393.26.4.1070. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Piolino P, Mezenge F, Landeau B, Lalevee C, Pelerin A, Viader F, de, l.S. V, Eustache F, Desgranges B. Autonoetic consciousness in Alzheimer's disease: neuropsychological and PET findings using an episodic learning and recognition task. Neurobiol. Aging. 2007;28:1410–1420. doi: 10.1016/j.neurobiolaging.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Schloerscheidt AM, Rugg MD. The impact of change in stimulus format on the electrophysiological indices of recognition. Neuropsychologia. 2004;42:451–466. doi: 10.1016/j.neuropsychologia.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Smith JA, Knight RG. Memory processing in Alzheimer's disease. Neuropsychologia. 2002;46:666–682. doi: 10.1016/s0028-3932(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Stenberg G, Radeborg K, Hedman LR. The picture superiority effect in a cross-modality recognition task. Mem. Cognit. 1995;23:425–441. doi: 10.3758/bf03197244. [DOI] [PubMed] [Google Scholar]
- Thapar A, Westerman DL. Aging and fluency-based illusions in recognition memory. Psychol. Aging. 2009;24:595–603. doi: 10.1037/a0016575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Fluent conceptual processing and explicit memory for faces are electrophysiologically distinct. J. Neurosci. 2006;26:926–933. doi: 10.1523/JNEUROSCI.3931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Neural correlates of conceptual implicit memory and their contamination of putative neural correlates of explicit memory. Learn. Mem. 2007;14:259–267. doi: 10.1101/lm.529807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Brain substrates of implicit and explicit memory: the importance of concurrently acquired neural signals of both memory types. Neuropsychologia. 2008;46:3021–3029. doi: 10.1016/j.neuropsychologia.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon MS, Roediger HL., III Altering retrieval demands reverses the picture superiority effect. Mem. Cognit. 1987;15:269–280. doi: 10.3758/bf03197030. [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, Reber PJ. When memory does not fail: Familiarity-based recognition in mild cognitive impairment and Alzheimer's disease. Neuropsychology. 2006;20:193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Westerman DL. The role of familiarity in item recognition, associative recognition, and plurality recognition on self-paced and speeded tests. J. Exp. Psychol. Learn. Mem. Cogn. 2001;27:723–732. [PubMed] [Google Scholar]
- Whittlesea BW, Williams LD. The source of feelings of familiarity: the discrepancy-attribution hypothesis. J. Exp. Psychol. Learn. Mem. Cogn. 2000;26:547–565. doi: 10.1037//0278-7393.26.3.547. [DOI] [PubMed] [Google Scholar]
- Whittlesea BW, Williams LD. The discrepancy-attribution hypothesis: I. The heuristic basis of feelings of familiarity. J. Exp. Psychol. Learn. Mem. Cogn. 2001;27:3–13. [PubMed] [Google Scholar]
- Whittlesea BW, Williams LD. The discrepancy-attribution hypothesis: II. Expectation, uncertainty, surprise, and feelings of familiarity. J. Exp. Psychol. Learn. Mem. Cogn. 2001;27:14–33. [PubMed] [Google Scholar]
- Whittlesea BWA. Illusions of familiarity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19:1235–1253. [Google Scholar]
- Willems S, Germain S, Salmon E, Van der LM. Patients with Alzheimer's disease use metamemory to attenuate the Jacoby-Whitehouse illusion. Neuropsychologia. 2009;47:2672–2676. doi: 10.1016/j.neuropsychologia.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Gold CA, Signoff ED, Budson AE. Discrimination and reliance on conceptual fluency cues are inversely related in patients with mild Alzheimer's disease. Neuropsychologia. 2009;47:1865–1872. doi: 10.1016/j.neuropsychologia.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Schacter DL, Berman AR, Holcomb PJ, Daffner KR, Budson AE. Patients with Alzheimer's Disease attribute conceptual fluency to prior experience. Neuropsychologia. 2005;43:1662–1672. doi: 10.1016/j.neuropsychologia.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Signoff ED, DeKosky ST. Recollection and familiarity in Amnestic Mild Cognitive Impairment: A global decline in recognition memory. Neuropsychologia. 2008;4:1965–1978. doi: 10.1016/j.neuropsychologia.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]