Abstract
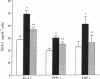
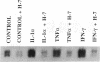
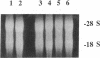
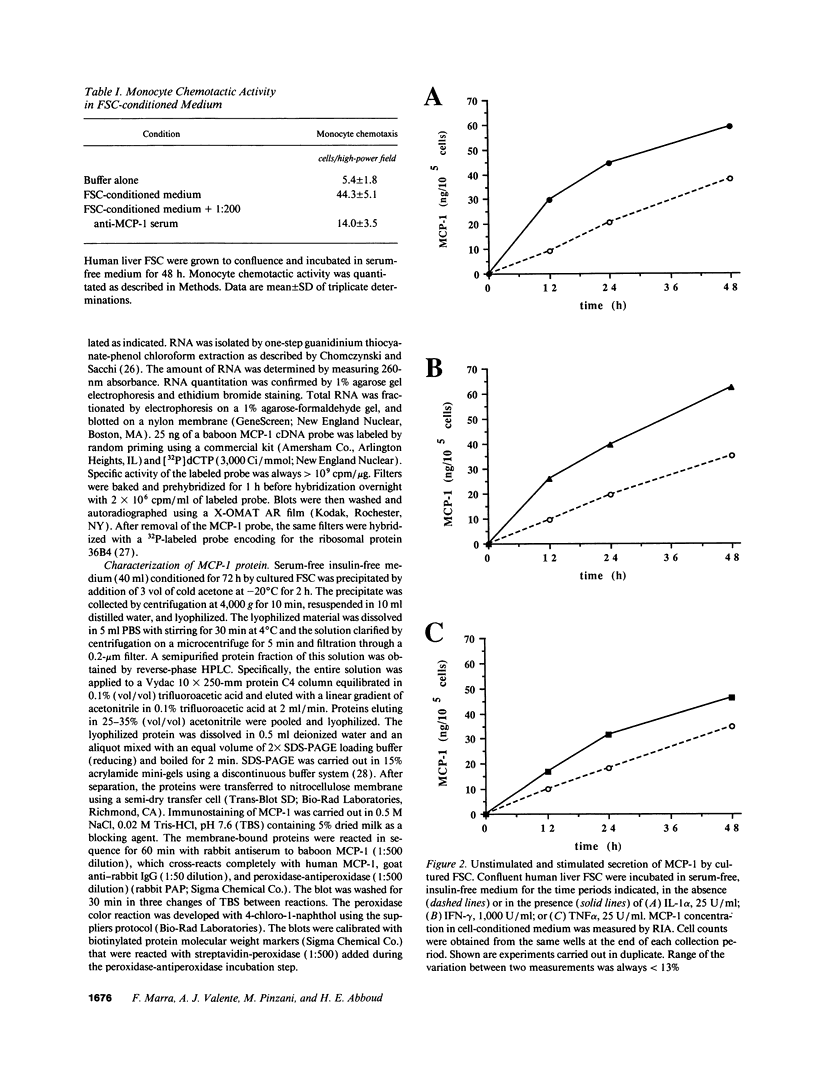
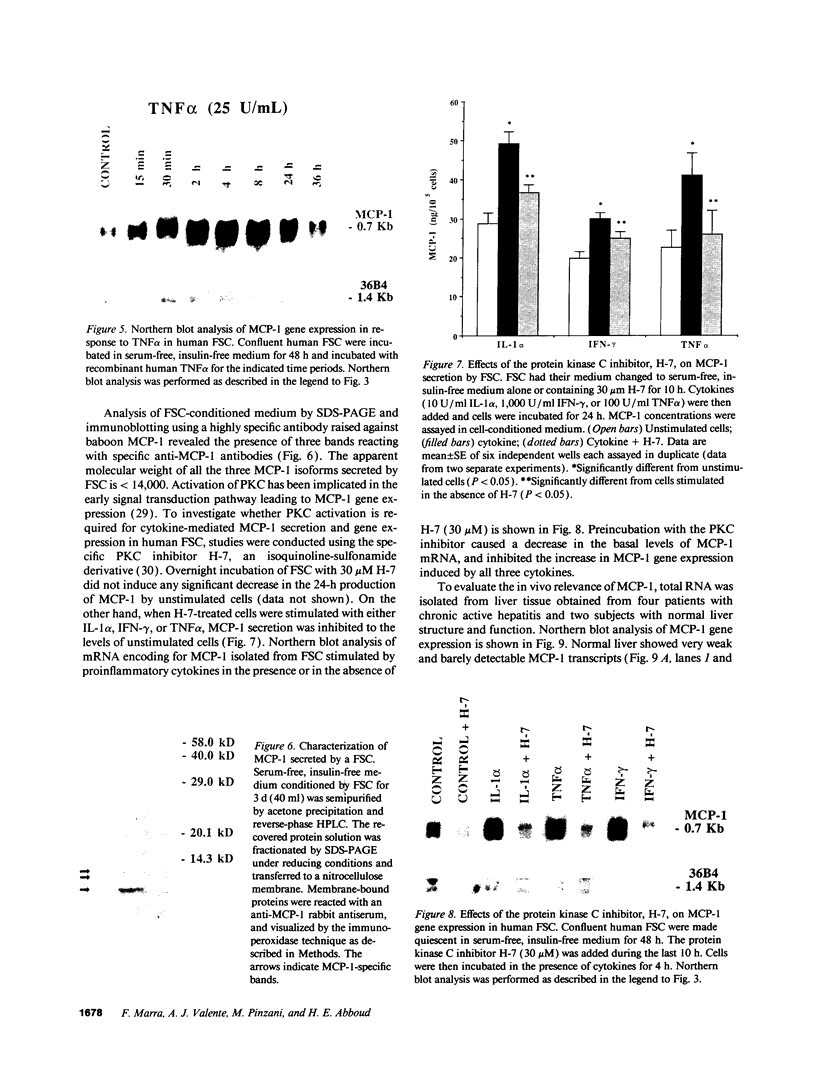
Monocytes infiltrate the portal space during chronic liver inflammation. Monocyte chemotactic protein-1 (MCP-1) is a cytokine that induces monocyte chemotaxis and activation. We investigated if human liver fat-storing cells (FSC) secrete MCP-1, and the mechanisms that regulate MCP-1 production. Unstimulated FSC secrete MCP-1 as measured by radioimmunoassay as well as a chemotactic assay and express mRNA that encodes for this cytokine. A two- to threefold increase in MCP-1 secretion was observed when FSC were treated with either interleukin-1 alpha (IL-1 alpha) or interferon-gamma (IFN-gamma). Tumor necrosis factor-alpha (TNF alpha) also increased MCP-1 secretion, although to a lesser extent (1.6-fold). Northern blot analysis showed that IL-1 alpha and IFN-gamma strongly increase the levels of mRNA that encodes for MCP-1, whereas TNF alpha appears to be a weaker stimulus. Analysis of FSC-conditioned medium by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting revealed three bands of MCP-1 that most likely represent isoforms of different apparent molecular weights. Pretreatment of FSC with H-7, a protein kinase C inhibitor, blocked cytokine-induced increase in both MCP-1 gene expression and secretion. To determine the potential role of MCP-1 in vivo, we also analyzed normal and pathologic human liver tissue. Northern blot analysis showed that MCP-1 mRNA expression is more abundant in liver tissue obtained from patients with chronic active hepatitis compared with normal liver tissue. These studies indicate that MCP-1 secreted by FSC is stimulated by proinflammatory cytokines and that MCP-1 gene expression is upregulated in chronic inflammatory liver disease. MCP-1 released by FSC may participate in the recruitment and activation of monocytes at sites of liver injury.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. N., Jones M. L., Swenson C. L., Sarma V., Mitra R. S., Ward P. A., Johnson K. J., Fantone J. C., Dixit V. M., Nickoloff B. J. Monocyte chemotaxis and activating factor production by keratinocytes in response to IFN-gamma. J Immunol. 1991 Feb 15;146(4):1192–1197. [PubMed] [Google Scholar]
- Basset P., Bellocq J. P., Wolf C., Stoll I., Hutin P., Limacher J. M., Podhajcer O. L., Chenard M. P., Rio M. C., Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990 Dec 20;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Bernuau D., Rogier E., Feldmann G. A quantitative ultrastructural analysis of the leukocytes in contact with hepatocytes in chronic active hepatitis, with a cytochemical detection of mononuclear phagocytes. Am J Pathol. 1982 Dec;109(3):310–320. [PMC free article] [PubMed] [Google Scholar]
- Bernuau D., Rogier E., Feldmann G. In situ ultrastructural detection and quantitation of liver mononuclear phagocytes in contact with hepatocytes in chronic type B hepatitis. Lab Invest. 1984 Dec;51(6):667–674. [PubMed] [Google Scholar]
- Bioulac-Sage P., Balabaud C. Proliferation and phenotypic expression of perisinusoidal cells. J Hepatol. 1992 Jul;15(3):284–287. doi: 10.1016/0168-8278(92)90057-v. [DOI] [PubMed] [Google Scholar]
- Bissell D. M. Lipocyte activation and hepatic fibrosis. Gastroenterology. 1992 May;102(5):1803–1805. doi: 10.1016/0016-5085(92)91747-r. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991 Mar 1;5(3):271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis B. H., Pratt B. M., Madri J. A. Retinol and extracellular collagen matrices modulate hepatic Ito cell collagen phenotype and cellular retinol binding protein levels. J Biol Chem. 1987 Jul 25;262(21):10280–10286. [PubMed] [Google Scholar]
- Friedman S. L. Cellular sources of collagen and regulation of collagen production in liver. Semin Liver Dis. 1990 Feb;10(1):20–29. doi: 10.1055/s-2008-1040454. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Bissell D. M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts A., Vrijsen R., Rauterberg J., Burt A., Schellinck P., Wisse E. In vitro differentiation of fat-storing cells parallels marked increase of collagen synthesis and secretion. J Hepatol. 1989 Jul;9(1):59–68. doi: 10.1016/0168-8278(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Jiang Y. L., Williamson M. J., Valente A. J. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989 Sep 29;245(4925):1490–1493. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maher J. J., McGuire R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa H., Nagy P., Evarts R. P., Hsia C. C., Marsden E., Thorgeirsson S. S. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990 Jun;85(6):1833–1843. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Abboud H. E., Gesualdo L., Abboud S. L. Regulation of macrophage colony-stimulating factor in liver fat-storing cells by peptide growth factors. Am J Physiol. 1992 Apr;262(4 Pt 1):C876–C881. doi: 10.1152/ajpcell.1992.262.4.C876. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Failli P., Ruocco C., Casini A., Milani S., Baldi E., Giotti A., Gentilini P. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992 Aug;90(2):642–646. doi: 10.1172/JCI115905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Rieder H., Knittel T., Dienes H. P., Meyer zum Büschenfelde K. H. Fat storing cells (FSC) of rat liver synthesize and secrete fibronectin. Comparison with hepatocytes. J Hepatol. 1987 Apr;4(2):190–197. doi: 10.1016/s0168-8278(87)80079-x. [DOI] [PubMed] [Google Scholar]
- Ramadori G. The stellate cell (Ito-cell, fat-storing cell, lipocyte, perisinusoidal cell) of the liver. New insights into pathophysiology of an intriguing cell. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;61(3):147–158. doi: 10.1007/BF02890417. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Walz A., Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991 Aug 15;78(4):1112–1116. [PubMed] [Google Scholar]
- Schäfer S., Zerbe O., Gressner A. M. The synthesis of proteoglycans in fat-storing cells of rat liver. Hepatology. 1987 Jul-Aug;7(4):680–687. doi: 10.1002/hep.1840070411. [DOI] [PubMed] [Google Scholar]
- Shyy Y. J., Li Y. S., Kolattukudy P. E. Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem Biophys Res Commun. 1990 Jun 15;169(2):346–351. doi: 10.1016/0006-291x(90)90338-n. [DOI] [PubMed] [Google Scholar]
- Valente A. J., Graves D. T., Vialle-Valentin C. E., Delgado R., Schwartz C. J. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988 May 31;27(11):4162–4168. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- Valente A. J., Rozek M. M., Schwartz C. J., Graves D. T. Characterization of monocyte chemotactic protein-1 binding to human monocytes. Biochem Biophys Res Commun. 1991 Apr 15;176(1):309–314. doi: 10.1016/0006-291x(91)90925-w. [DOI] [PubMed] [Google Scholar]
- Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol. 1980;66:303–353. doi: 10.1016/s0074-7696(08)61977-4. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Leonard E. J. Identification of high affinity receptors for human monocyte chemoattractant protein-1 on human monocytes. J Immunol. 1990 Jul 1;145(1):292–297. [PubMed] [Google Scholar]
- Yoshimura T., Leonard E. J. Secretion by human fibroblasts of monocyte chemoattractant protein-1, the product of gene JE. J Immunol. 1990 Mar 15;144(6):2377–2383. [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Moore S. K., Appella E., Lerman M. I., Leonard E. J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989 Feb 27;244(2):487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- de Leeuw A. M., McCarthy S. P., Geerts A., Knook D. L. Purified rat liver fat-storing cells in culture divide and contain collagen. Hepatology. 1984 May-Jun;4(3):392–403. doi: 10.1002/hep.1840040307. [DOI] [PubMed] [Google Scholar]