Abstract
This study tested the hypothesis that controlled flow through microchannels can cause shear-induced intracellular loading of cells with molecules. The overall goal was to design a simple device to expose cells to fluid shear stress and thereby increase plasma membrane permeability. DU145 prostate cancer cells were exposed to fluid shear stress in the presence of fluorescent cell-impermeant molecules by using a cone-and-plate shearing device or high-velocity flow through microchannels. Using a syringe pump, cell suspensions were flowed through microchannels of 50 – 300 μm diameter drilled through Mylar® sheets using an excimer laser. As quantified by flow cytometry, intracellular uptake and loss of viability correlated with the average shear stress. Optimal results were observed when exposing the cells to high shear stress for short durations in conical channels, which yielded uptake to over one third of cells while maintaining viability at approximately 80%. This method was capable of loading cells with molecules including calcein (0.62 kDa), large molecule weight dextrans (150 - 2000 kDa), and bovine serum albumin (66 kDa). These results supported the hypothesis that shear-induced intracellular uptake could be generated by flow of cell suspensions through microchannels and further led to the design of a simple, inexpensive, and effective device to deliver molecules into cells. Such a device could benefit biological research and the biotechnology industry.
Keywords: Shear stress, intracellular delivery, microfluidics, MEMS
INTRODUCTION
Advances in biotechnology rely on techniques to deliver molecules into the cytoplasm and/or nucleus of cells. These techniques include cationic lipids and polymers (Goyal et al. 2005; Simoes et al. 2005), viral vectors (Young et al. 2006), electroporation (Gehl 2003), and microinjection (Jensen et al. 2003). These techniques are typically used to deliver bioactive molecules, such as genetic material or proteins, to modulate cellular behavior (Azzam and Domb 2004) or deliver diagnostic molecules in order to label organelles or cellular structures (Barber et al. 1996).
Current techniques to permeabilize cells for intracellular uptake encompass many different mechanisms to bypass the cell membrane, an impediment to molecular transport. These include chemicals to package molecules for delivery, as with liposomes or cell-penetrating proteins; exploiting the natural cell-penetrating mechanism of viral vectors (Young et al. 2006); electric means as with electroporation (Li 2004); direct injection of material by microinjection (Dice 1988); chemical fixation followed by permeablizing detergents (Melan 1994); and mechanical means to apply shear stress. Although all of these techniques have developed into effective methods, they each have disadvantages, such as requiring expensive equipment and/or chemicals and specifying complex and/or time-intensive protocols. Thus, these methods may only be effective for a small number of cells or only applicable to non-viable or fixed cells. Developing a means that is inexpensive, simple to perform, and effective at intracellular delivery of molecules into viable cells would greatly benefit biological research, biotechnology, and the medical community. Building from methods that utilize shear forces to reversibly permeabilize cells, we propose that fluid flow through microchannels can impart high shear forces upon cells to load cells with extracellular molecules and provide a low-cost, simple-to-use, and effective intracellular delivery method.
Shear permeablization of cells is commonly believed to be due to increased membrane tension and subsequent poration of the cell membrane (Lokhandwalla and Sturtevant 2001; Netz and Schick 1996). Previous studies have shown transient cell permeablization by directly applying shear forces to cells using hypodermic needles (Clarke and McNeil 1992), viscometers (LaPlaca et al. 1997), and scrape loading (McNeil et al. 1984). Additionally, studies have used methods to apply shear indirectly and load cells with molecules by generating shock waves (Kodama et al. 2002) or cavitation with ultrasound (Mitragotri 2005). Typically, indirect methods require complex equipment and have less control at the local level than direct methods of applying shear.
The mechanism of shear permeabilization of cells has also been studied. A body of literature has shown that shear permeabilization is caused by removing sections of plasma membrane, measuring up to microns in dimensions (Schlicher et al. 2006). Extracellular molecules are able to diffuse through these membrane openings and, in some cases, equilibrate intracellularly (Guzman et al. 2002). Due at least in part to the associated influx of calcium through membrane disruptions, cells respond by trafficking exocytic vesicles to the site of injury to patch it actively over a time-scale of minutes (McNeil and Steinhardt 2003; Schlicher et al. 2006). Cells can survive this process, which is believed to occur routinely in the body due to shear forces and sub-lethal wounding imposed on cells by natural physiological processes, such as the mechanical effects of intestinal motility, cardiac rhythm, and muscle contraction (Barbee 2005). Despite this mechanistic insight, there is relatively little known about the dependence of cell permeabilization as a function of imposed shear stress.
Guided by these findings, we sought to (i) systematically study the effects of different shearing environments on cellular uptake and viability, (ii) assess the breadth of applications of uptake of different-sized molecules, and (iii) design a simple and inexpensive device for applications. We hypothesize that controlled flow through microchannels can cause shear-induced loading of cells with macromolecules.
METHODS
Cell culture
Cell culture was performed as described by Guzman et al. (2001). In brief, human prostate cancer cells (DU145, American Type Culture Collection, Manassas, VA, item no. HTB-81) were cultured as monolayers in a humidified atmosphere of 95% air and 5% CO2 at 37°C in RMPI-1640 media (Cellgro, Mediatech, Herndon, VA), supplemented with 100 μg/ml penicillin-streptomycin (Cellgro) and 10% (v/v) heat-inactivated fetal bovine serum (Atlanta Biologicals, Atlanta, GA). DU145 cells were harvested by trypsin/EDTA (Cellgro) digestion, washed, and resuspended in culture media.
Cone-and-plate shearing device
DU145 cells were subjected to a high-magnitude, short-duration pulse of fluid shear stress using a custom-built, cell-shearing device capable of delivering high magnitudes of shear stress at rapid rates to cell cultures, as described in detail by Prado et al. (2005). The cell-shearing device consisted of a servo motor-driven cone (0.5°) that rotates above the cell culture surface at controlled speeds and rates of acceleration. Hank's Balanced Salt Solution (HBSS) (Sigma, St. Louis, MO) was placed between the cone and cell plate to serve as shearing buffer, transferring momentum from the rotating cone to the plate of seeded cells and producing a uniform shear stress across the culture plate.
Prior to shear exposure, cells were counted and seeded (density = 1.00-1.25 × 105 cells/cm2) onto customized culture plates. Culture plates consisted of glass plates that were briefly flamed and subsequently coated with a poly-L-lysine solution (0.0023% w/v; Sigma) for at least 12 h at 37 °C and 95% relative humidity before cell seeding. Cells were cultured as monolayers for 2-3 days with culture media and in an incubator, as described above.
Shear stress experiments were conducted by removing culture plates from the incubator and rinsing them with HBSS. Cells were then incubated at 37 °C in the buffer containing calcein (0.34 mM, Molecular Probes, Eugene, OR) for 10 min and mounted in the cell-shearing device. The cone of the cell-shearing device was lowered until its apex contacted the center of the cell plate, and then shearing buffer (HBSS) was added through a perfusion port in order to fill the gap between the cone and the plate. The applied shear stress magnitude and duration were 140 dynes/cm2 and 300 ms, respectively. The rise time, which is defined to be the length of time for the cone to reach maximum velocity, was 20 ms. Cells were then immediately removed from the cell-shearing device and analyzed by flow cytometry to determine viability and intracellular uptake of calcein, as described below. Cells exposed to shear were compared to cells in negative-control samples (i.e., cells treated exactly the same but not exposed to shear).
Microchannel fabrication
Sheets of polyethylene terephthalate (Mylar®, Dupont, Wilmington, DE) were used to make microchannels because this material is inexpensive, available in many different thicknesses, and easily cut using established laser-cutting techniques. Mylar® sheets measuring 100 μm and 250 μm in thickness were first cut into 15-mm diameter disks using a CO2 laser (LS500XL, Gravograph-New Hermes, Duluth, GA). The CO2 laser was operated at 200 Hz with a 10.6-μm wavelength and rotated in a circular pattern until the laser cut completely through the sheet. Microchannels were created in each disk as a single cylindrical channel, a single conical channel, or an array of conical channels by drilling near the center with an ultraviolet excimer laser (Resonetics Micromaster, Nashau, NH).
As shown in Fig. 1A, the relatively flat energy profile of the excimer laser allowed nearly cylindrical microchannels to be created in the Mylar® sheets with only a slight taper. Inlet and outlet diameters typically differed by less than 5 μm over 100 μm of channel length after boring from each side with the excimer laser (Davis et al. 2005). The laser was operated at 60 Hz with a 248-nm wavelength and energy density of 2.0 J/cm2. A wide range of microchannel diameters and lengths can be created using different-sized masks for the excimer laser and the many available thicknesses of the Mylar® sheets, respectively.
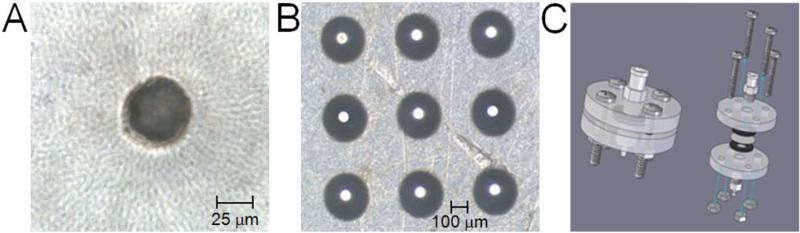
Figure 1.
Microchannels were drilled into Mylar® sheets to produce (A) cylindrical or (B) conical microchannels as (A) a single channel or (B) an array of channels. Part (A) displays a single cylindrical microchannel with a diameter of 50 μm, while part (B) displays a 3 × 3 array of conical microchannels with an inlet diameter of 300 μm and an outlet diameter of 50 μm. (C) A device was created to secure microchannel disks in place with a water-tight seal to ensure flow through microchannels and to couple a syringe and tubing for dispensing and collecting of cell solution, respectively.
To create conical channels in the Mylar® disks, the excimer laser was operated in a trepanning mode (Davis et al. 2005), as shown in Fig. 1B. During trepanning, the disk was moved in a circular motion with a diameter less than the excimer laser beam, allowing the excimer laser to ablate the Mylar® with greater intensity at the center of the circular path than at the edges, thereby creating a conical profile. Various conical profiles can be created by using different-sized masks and controlling the laser energy, speed, and radius.
Microchannel Device
To flow cell suspensions through the microchannels, a device was created to securely hold the Mylar® disk in place and allow coupling to tubing and a syringe (Fig. 1C). To secure the Mylar® disks, two flanges constructed of 0.63-cm thick polymethylmethacrylate (acrylic, McMaster-Carr, Atlanta, GA) were used. Each acrylic flange had a hole drilled in the center to allow fluid flow and four holes near the edge of the flange for securing screws. To prevent leakage within the flange, gaskets were cut from rubber gasket sheets (SBR rubber, 0.16-cm thick, McMaster-Carr) with an outer diameter of 15 mm and a hole was removed from the center with a diameter of 3 mm. Two gaskets were centered on each side of the Mylar® disk and held securely in place using the acrylic flanges and screws. Tubing and a syringe were coupled to the flange using a nozzle tip connection for 0.32-cm tubing screwed into the outlet of the flange and a male luer fitting screwed into the inlet of the flange.
Experimental protocol of microchannel exposure
Prior to fluid flow through microchannels, samples were prepared at a cell concentration of 5 × 105 cells/ml in RMPI-1640 media, supplemented with 100 μg/ml penicillin-streptomycin and 10% (v/v) heat-inactivated fetal bovine serum. To test the ability of this device to cause intracellular uptake, one of several different cell-impermeant, green-fluorescent molecules of different sizes was added to the cell suspension: calcein (10 μM, 623 Da, Molecular Probes), FITC-labeled dextrans (150, 500, and 2000 kDa, Sigma), and FITC-labeled bovine serum albumin (BSA, 66 kDa, Sigma).
A 2-ml sample solution was aliquoted into a 3-ml syringe (Becton Dickinson, Franklin Lakes, NJ) and any entrapped air was removed from the syringe. The male luer connection on the microchannel device was then filled with additional cell suspension (approximately 300 μl), which displaced any air in the fitting upstream from the microchannel, and was connected to the syringe. To accurately flow the sample through the microchannel and thereby expose cells to a controlled shear, a syringe pump (Genie, Kent Scientific Corp, Torrington, CT) was used to set the flow rate at 30, 70, or 100 ml/h. Approximately 0.5 ml of sample was flowed through the microchannel before any sample was collected, and then at least 1.0 ml of sample solution was collected in a 1.5 ml microcentrifuge tube. Cells were allowed to “recover” for 5 min at room temperature, and then microcentrifuge tubes were incubated on ice until all samples had been exposed (< 2 h) to microchannel shear flow.
After all samples had been exposed to shear, 1.0 ml of sample solution was pipetted from each collected sample and dispensed into a new microcentrifuge tube for analysis. Cells were then centrifuged (800 × g, 3 min, Eppendorf 5415C, Brinkman, Westbury, NY) and washed with phosphate buffered saline (PBS, Cellgro) three times to remove extracellular fluorescent molecules in the supernatant. The subsequent cell pellets were resuspended in a final volume of 205 μl of PBS containing 2 μg/ml of propidium iodide (Molecular Probes), a viability marker that labels non-viable cells with red fluorescence by binding to nuclear material in compromised cells.
Quantification of bioeffects
Flow cytometry was used to determine molecular uptake (i.e., fraction of cells containing intracellular fluorescent molecules) and loss of cell viability by detecting the fluorescence intensity from uptake molecules and propidium iodide, respectively, on a cell-by-cell basis. A BD LSR benchtop flow cytometer (BD Biosciences, San Jose, CA) was used to measure the green fluorescence of cells with intracellular uptake (FITC fluorescence, 530/28 nm bandpass filter) and to distinguish viable from non-viable cells by the red fluorescence of propidium iodide (Per CP fluorescence, 670 nm longpass filter). Typical analyses included approximately 30,000 cells per sample. Cell viability was determined as the rate of viable cells (i.e., not stained by propidium iodide) counted in each sample normalized to the rate of viable cells counted in a non-exposed control sample.
Statistical Analysis
A minimum of three replicates was performed for all conditions. Replicates enabled calculations of means and standard errors of the mean (SEM). Analysis of variance (ANOVA, α=0.05) was utilized to compare all parameters to determine their statistical significance in affecting molecular uptake and loss of viability.
RESULTS
Shear-induced intracellular uptake
Our hypothesis is that controlled flow through microchannels can cause shear-induced intracellular loading of cells with molecules. As a first step toward testing this hypothesis, we exposed DU145 prostate cancer cells to controlled shear stress in a cone-and-plate cell-shearing device that applied a uniform shear force to a monolayer of adherent cells in the presence of calcein (a cell-impermeant, green-fluorescent molecule). The merit of this device is that it applies a homogeneous, well-controlled shear stress to a population of cells.
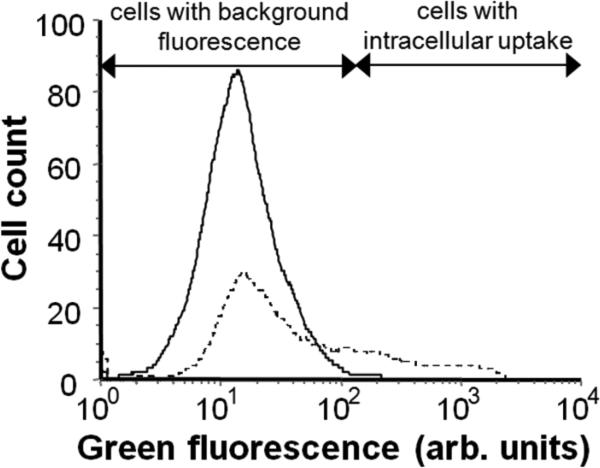
As shown in Fig. 2, flow cytometry histograms of green fluorescence display a control sample (i.e., no shear exposure) exhibiting innate autofluorescence and background noise and a sample exposed to shear (i.e., 140 dynes/cm2 for 300 ms) displaying an increase in the number of cells with green fluorescence, indicating intracellular uptake of calcein. In this representative example, 27% of cells exposed to shear exhibited increased intracellular loading with calcein. Additional experiments using different shearing conditions also caused increased intracellular uptake (data not shown). This result demonstrates that large, transient shear forces can induce cell permeablization and cause intracellular uptake of molecules.
Figure 2.
Flow cytometry histogram of cellular green fluorescence displaying a control sample with background fluorescence (solid line) and a sample exposed to shear in the cell-shearing device (dotted line), which exhibits an increase in green fluorescence indicting intracellular uptake of calcein.
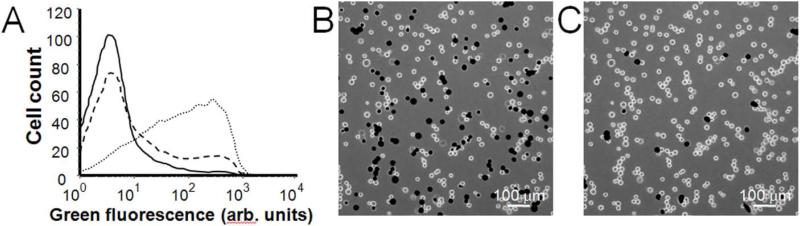
Building from these results, we exposed cells in suspension to shear by flow through our fabricated microchannel device (Fig. 1). The merit of this device is that it can apply shear stress with faster rise time and higher throughput compared to the cone-and-plate device. In Fig. 3, representative flow cytometry and fluorescent microscopy results display intracellular uptake into viable cells after cells were exposed to fluidic shear in microchannels. In Fig. 3A, the solid curve shows a control sample, the dashed curve displays heterogeneous uptake that was typical at low shear conditions, and the dotted curve shows the distribution of cells exposed to higher shear conditions that caused intracellular uptake of calcein into a larger fraction of cells in a more homogenous manner. In Fig. 3B, fluorescent microscopy displays cells with intracellular green-fluorescent calcein (dark circles), verifying intracellular delivery of calcein and not, for example, increased cell surface binding. By viewing the cells with red fluorescence, Fig. 3C shows non-viable cells stained with propidium iodide (dark circles) indicating that almost all cells remained viable after shearing.
Figure 3.
(A) Representative flow cytometry histogram of cellular green fluorescence displaying a control sample (solid curve) and samples subjected to low (dashed curve) and high (dotted curve) shear environments, which display an increase in green fluorescence indicating intracellular uptake of calcein. The samples were subjected to shear by flow of the cell suspension at 100 ml/h though channels of 100 μm in length and diameters of 60 μm for the low shear condition and 50 μm for the high shear condition (B,C) Representative fluorescent micrographs of cells after exposure to shear in microchannel flow (at the high shear condition described above), which depicts (B) viable cells with intracellular uptake of green-fluorescent calcein and (C) non-viable cells stained with red-fluorescent propidium iodide. The same view of the same cell sample is shown in parts (B) and (C) using different optics. Stained cells appear as black circles and unstained cells appear as bright circles.
Quantification of shear-induced bioeffects
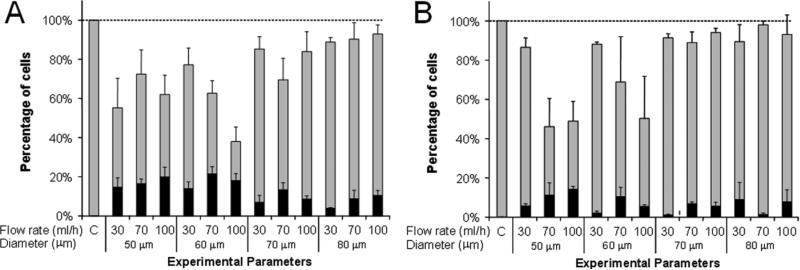
Having demonstrated that controlled flow through microchannels can cause loading of cells with macromolecules, we next sought to systematically evaluate the effects of shear environment on uptake and loss of viability by flowing DU145 cells in suspension through microchannels of various dimensions. In Fig. 4, the effects of fluid flow rate and microchannel diameter and length were examined. Fluid flow rate was varied up to 100 ml/h, which was the maximum our apparatus could accommodate, and microchannel diameter was varied down to 50 μm, which is approximately three times the DU145 cell diameter of 15-18 μm. Smaller diameters were found to clog and were therefore not used. Increased flow rate and decreased microchannel diameter should increase the fluid velocity through the microchannel and therefore increase the intensity of the shear force experienced by a cell while simultaneously decreasing its duration. Finally, microchannel length was varied between 100 μm and 250 μm, which was the maximum our apparatus could accommodate, due to the increased pressure drop associated with longer channels. For flow rates of 30, 70, and 100 ml/h, the residence time of a cell in a 50-μm diameter, 100-μm long channel was 20, 10, and 6 μs respectively, and in a 250-μm long channel was 50, 30, and 20 μs respectively.
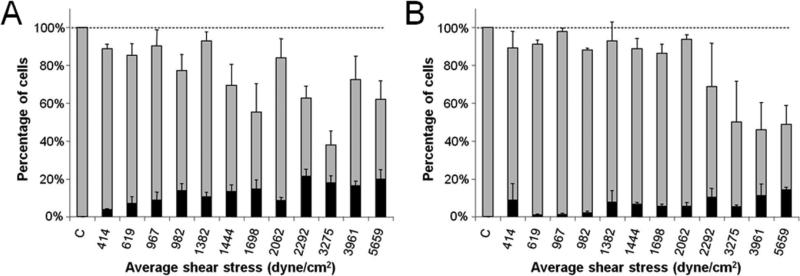
Figure 4.
Intracellular uptake and cell viability populations following shear exposure within cylindrical microchannels as a function of flow rate and of microchannel diameter and length. The total height of each bar represents the fraction of cells remaining viable, the size of the black stripe represents the fraction of cells with significant levels of intracellular uptake, and the size of the grey stripe represents the fraction of cells that were apparently unaffected. DU145 cells were subjected to shear by passage through microchannels of (A) 100 μm and (B) 250 μm channel length. Data represent the averages of n ≥ 3 replicates with SEM error bars shown.
Figure 4 shows that significant effects on intracellular uptake and cell viability occurred under the different shearing conditions. Decreasing channel diameter and channel length each increased the number of cells with intracellular uptake (p <0.01 and p <0.01, respectively) while changing the flow rate did not significantly affect the intracellular uptake (p = 0.07). Figure 4 further indicates that the viability generally decreased with decreasing channel diameter (p < 0.01), while altering the channel length or flow rate did not significantly affect the viability (p = 0.2 and p = 0.05, respectively). Overall, uptake by approximately 10% of cells was achieved with minimal viability loss and uptake in excess of 20% of cells was achieved with 20-50% viability loss. These results suggest that high shear conditions created by small diameter channels and short exposure times are favorable for affecting a large portion of the cell population and causing intracellular uptake.
To test the hypothesis that shear stress is responsible for the bioeffects observed, the average shear stress was estimated for all of the conditions tested and graphed in Fig. 5. The actual shear stress experienced by the cells was expected to vary, because the velocity profile was not fully developed over the short channel lengths, the presence of cells in the microchannel altered the local fluid dynamics, and regions of higher shear rates exised near the channel wall and lower shear stress existed in the center of the channel (Fox et al. 2004). In Fig. 5, uptake was shown to increase and viability was shown to decrease with increasing average shear stress (p > 0.01 in Fig. 5A and p = 0.02 in Fig. 5B). This result suggests that shear stress mediated the bioeffects, and that high shear forces are desirable for delivery to a larger number of cells. Differences between 100 μm (Fig. 5A) and 250 μm (Fig. 5B) channel lengths suggest that high shear for short durations is favorable to maintain high viability and delivery to a larger number of cells. Such conditions should be realized by applying high flow rates to small-diameter, short-length channels.
Figure 5.
Intracellular uptake and cell viability populations graphed versus average shear stress of exposures within (A) 100 μm and (B) 250 μm channel lengths. The total height of each bar represents the fraction of cells remaining viable, the size of the black stripe represents the fraction of cells with significant levels of intracellular uptake, and the size of the grey stripe represents the fraction of cells that were apparently unaffected. Data represent the averages of n ≥ 3 replicates with SEM error bars shown.
Intracellular delivery of molecules by shear
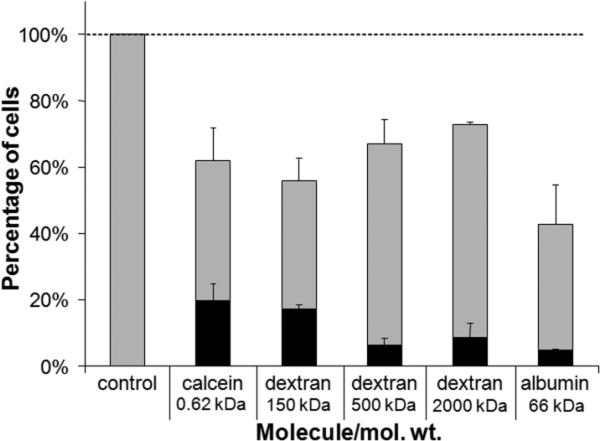
We next assessed the breadth of molecules that could be loaded into cells by testing the uptake of different-sized macromolecules. Molecules ranging in size from 0.6 to 2,000 kDa were incubated with cells and exposed to shear by flow through channels of 50 μm diameter and 100 μm length at a flow rate of 100 ml/h. As shown in Fig. 6, all five of the molecules tested, including large dextrans and bovine serum albumin, were able to be loaded intracellularly by shear exposure, although the larger molecules were delivered with lower efficiency. These findings show that large macromolecules can be delivered by transiently shearing cells, which indicates that shear-induced disruptions to the plasma membrane barrier are large enough to permit entry of large macromolecules (e.g. 2,000 kDa dextran has an effective diameter of 28 nm (Oliver et al. 1992)). Moreover, this observation suggests that shear-induced loading of cells could be developed into a useful tool for intracellular delivery of molecules with biological significance, such as intracellular stains, proteins, and genetic material.
Figure 6.
Intracellular delivery of various molecules by subjecting DU145 cells to shear in cylindrical microchannels with dimensions of 50 μm diameter and 100 μm channel length. The total height of each bar represents the fraction of cells remaining viable, the size of the black stripe represents the fraction of cells with significant levels of intracellular uptake, and the size of the grey stripe represents the fraction of cells that were apparently unaffected. Data represent the averages of n ≥ 3 replicates with SEM error bars shown.
To further assess the breadth of applicability, we carried out a small set of experiments to shear HL60 promyelocytic leukemia cells in the presence of calcein and found similar levels of uptake and viability as observed using DU145 prostate cancer cells (data not shown). This suggests that this approach to shear loading of cells may be applicable to many cell types.
Simplified device with conical microchannels
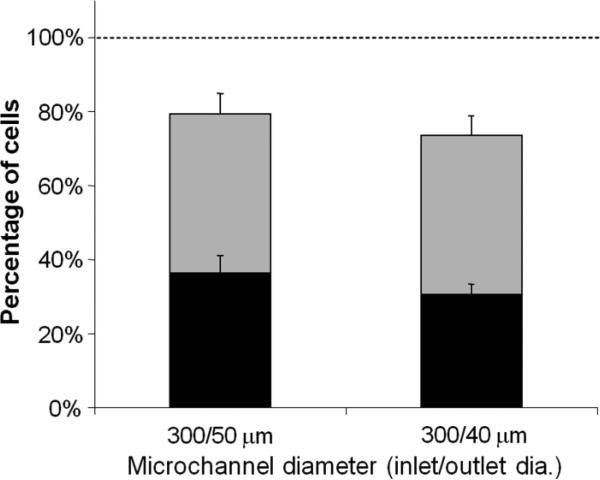
To facilitate practical use, we sought to design a simplified device that could be attached onto a syringe to manually expose cells to shear by flow through microchannels, and thereby cause intracellular loading with minimal equipment or cost. An array of nine microchannels were fabricated in a 3 × 3 configuration for higher throughput and to reduce the problem of channel clogging. Based on the observation that high stress for short durations is favorable, we created conical (i.e., tapered) microchannels that maximized the shear stress just at the channel outlets (Martanto et al. 2005) (Fig. 1B). Conical microchannels were drilled using the excimer laser in a trepanning mode to make an inlet diameter of approximately 300 μm and exit diameter of 40 and 50 μm over a 250 μm channel length. To simulate simplified use, these channels were tested by dispensing the syringe containing approximately 3 ml of cell solution in 4-5 s by hand instead of using a syringe pump.
Because we found that loading levels were relatively insensitive to flow rate (Fig. 4), we expected that dispensing by hand could be effective. As shown in Fig. 7, this approach produced significantly improved results with higher viabilities and a larger percent of cells with intracellular uptake. For example, uptake in 36% of cells was achieved while maintaining a cell viability of 80%. We believe this increased effectiveness was due primarily to the use of conical channels that achieved a very short exposure to high shear stress. This work also illustrates that a simple, hand-operated device can be used to cause intracellular loading of molecules in a large number of cells.
Figure 7.
Intracellular uptake and cell viability populations after manually dispensing cells through conical microchannels. Microchannels were fabricated as a 3 × 3 array (9 total channels) with an inlet diameter of 300 μm and an outlet diameter of (A) 50 μm and (B) 40 μm. The total height of each bar represents the fraction of cells remaining viable, the size of the black stripe represents the fraction of cells with significant levels of intracellular uptake, and the size of the grey stripe represents the fraction of cells that were apparently unaffected. Data represent the averages of n ≥ 3 replicates with SEM error bars shown.
DISCUSSION
This study sought to test the hypothesis that controlled flow through microchannels can cause shear-induced intracellular loading of cells with molecules. In support of this hypothesis, we showed that exposure of cells to shear forces generated by a cone-and plate shearing device can cause intracellular uptake (Fig. 2) consistent with previous findings (Prado et al. 2005). Flowing cells through microchannels with diameters that approached cellular dimensions showed better intracellular loading (Figs. 4 and 7) that included uptake of a range of macromolecules up to 2,000 kDa in size (Fig. 6). Consistent with a shear-based mechanism, cellular bioeffects of uptake and viability correlated with the average shear stress generated in the microchannels with different geometries and flow rates (Fig. 5). Altogether, these findings support the proposed hypothesis and suggest that a simple, inexpensive device that can load large numbers of cells with molecules for a variety of biological applications.
By demonstrating intracellular loading of compounds ranging from small molecules to large macromolecules and proteins, this study showed the potential of a shear-loading device to be useful for intracellular delivery of molecules for biological applications. Intracellular delivery is pursued by a number of techniques such as electroporation, liposomes, viral vectors, and microinjection to deliver bioactive molecules to alter cellular processes and to load diagnostic molecules into cells to label cellular structures for live cell imaging and analysis. Possible areas that could benefit from this device include in vitro studies in biological laboratories, ex vivo cell-based clinical therapies, biotechnology processes, and pharmaceutical laboratories for drug discovery and testing of intracellular targets.
This shear-based method may offer some advantages compared to other intracellular delivery techniques. It appears that cell shearing may be broadly applicable to uptake of a wide range of compounds in a variety of cell types using an inexpensive, disposable device. Chemical-based transfection methods, such as cationic lipids, and virus-based transduction methods are also relatively inexpensive and efficient, but are limited to delivery of DNA and RNA. Electroporation and ultrasound deliver molecules into cells with similar efficiency to cell shearing, but require a relatively large investment in suitable electronic equipment. Finally, microinjection is the gold standard for intracellular delivery, but its extremely slow throughput generally limits its utility to studies of small cell populations.
Evaluation of the results of different shearing environments, in terms of the microchannel geometry and flow rate, suggests that the most favorable shearing environment for high uptake and viability is large magnitude shear (e.g. > 2000 dynes/cm2) for short durations (e.g. < 1 μs), as illustrated especially by the conical microchannels (Fig. 7). Conical microchannels were created with a large inlet diameter and small outlet diameter, which was similar to the cell diameter (Fig. 1B). It is expected that the large inlet diameter (approximately 300 μm) produced insignificant shear forces on the cell, as was observed in the 70-80 μm diameter cylindrical channels (Fig. 4). However, as the conical channels tapered to smaller diameter, higher velocities were achieved that created a shear environment similar to the small cylindrical channels, such as 50 μm diameter channels, yet with much shorter durations. We believe high-magnitude shear and short exposure durations were the principle factors that led to higher viabilities and more cells with intracellular uptake using conical microchannels. Additional optimization of conditions, perhaps to increase shear stress and decrease exposure duration further could yield still better results.
A device for flowing cell suspensions through microchannels was fabricated with the following design considerations: (i) allow coupling of a syringe and tubing for cell sample dispensing and collecting, respectively, (ii) provide a water-tight seal around the microchannel disks to ensure fluid flow through the microchannels, and (iii) allow microchannel disks to be easily replaced within the device. The fabricated device, as shown in Fig. 1C, proved to be suitable for dispensing and collecting small volumes (3 - 10 ml) of cell solution. However, other designs could be envisioned for flowing larger volumes of cell suspensions for inexpensive mass production.
A number of the samples tested with single-channel disks (Fig. 2B) had the incidence of high resistance during fluid flow, which we attributed to cells or cellular debris accumulating in the channel and obstructing flow. Using micro-machining techniques, we were able to create Mylar® disks with an array of microchannels (Fig. 1B) that substantially reduced the incidence of channel clogging and allowed for higher throughput of the solutions. The ease of use of this device with an array of conical microchannels was demonstrated by manually dispensing cell solution from a hand-held syringe (Fig. 7). We expect that this device design could be applied to several experimental scenarios to load cells without the need for other specialized equipment.
In conclusion, this study indicates that controlled flow through microchannels can cause shear-induced loading of cells with macromolecules. To load cells, we have fabricated a novel device with a simple design that uses shear to deliver macromolecules into a large number of cells. The advantages of this method of delivering cells are its low-cost, ease of use, ability to be adapted for high-throughput systems, and effective intracellular delivery of small and large molecules. The disadvantages are intracellular uptake by only a fraction of cells and some loss of cell viability. Intracellular uptake and cell viability were increased by exposing cells to large shear forces (e.g., >2000 dyne/cm2) for short times (e.g., < 1 μs), which loaded more than one-third of cells with molecules and maintained viability at approximately 80%. Less heterogeneity of uptake and higher viability could be achieved by further optimization of microchannel device design and flow conditions. Altogether, this approach to shear-induced loading of cells should be a useful tool to deliver macromolecules into cells for biotechnology, biomedical, and research applications.
ACKNOWLEDEMENTS
We thank Dr. Mark Allen for the use of his micro-machining lab and Richard Schafer for his technical assistance with the CO2 and excimer lasers. We also thank Wijaya Martanto, Harvinder Gill, Jung-Hwan Park, Josh Hutchenson, Kristy Rostad, Prerona Chakravarty, and Ying Liu for their helpful discussions and technical assistance in the laboratory. This work was supported in part by the U.S. National Institutes of Health, The Georgia Tech/Emory Center (GTEC) for the Engineering of Living Tissues URS Program and a fellowship to DM Hallow from the U.S. Department of Education Graduate Assistance in Areas of National Need program. MRP is the Emerson-Lewis Faculty Fellow. This work took place in the Institute for Bioengineering and Biosciences and the Center for Drug Design, Development and Delivery at Georgia Tech. DM Hallow carried out these studies with the assistance of RA Seeger and under the supervision of MR Prausnitz. PP Kamaev, GR Prado, and MC LaPlaca participated in the studies involving cone-and-plate shearing of cells.
REFERENCES
- Azzam T, Domb AJ. Current developments in gene transfection agents. Curr Drug Deliv. 2004;1(2):165–93. doi: 10.2174/1567201043479902. [DOI] [PubMed] [Google Scholar]
- Barbee KA. Mechanical cell injury. Ann N Y Acad Sci. 2005;1066:67–84. doi: 10.1196/annals.1363.006. [DOI] [PubMed] [Google Scholar]
- Barber K, Mala RR, Lambert MP, Qiu R, MacDonald RC, Klein WL. Delivery of membrane-impermeant fluorescent probes into living neural cell populations by lipotransfer. Neurosci Lett. 1996;207(1):17–20. doi: 10.1016/0304-3940(96)12497-6. [DOI] [PubMed] [Google Scholar]
- Clarke MS, McNeil PL. Syringe loading introduces macromolecules into living mammalian cell cytosol. J Cell Sci. 1992;102(Pt 3):533–41. doi: 10.1242/jcs.102.3.533. [DOI] [PubMed] [Google Scholar]
- Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52(5):909–15. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- Dice JF. Microinjected ribonuclease A as a probe for lysosomal pathways of intracellular protein degradation. J Protein Chem. 1988;7(2):115–27. doi: 10.1007/BF01025241. [DOI] [PubMed] [Google Scholar]
- Fox RW, McDonald AT, Pritchard PJ. Introduction to fluid mechanics. Wiley; Hoboken, N.J.: 2004. [Google Scholar]
- Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177(4):437–447. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- Goyal P, Goyal K, Vijaya Kumar SG, Singh A, Katare OP, Mishra DN. Liposomal drug delivery systems--clinical applications. Acta Pharm. 2005;55(1):1–25. [PubMed] [Google Scholar]
- Guzman HR, Nguyen DX, Khan S, Prausnitz MR. Ultrasound-mediated disruption of cell membranes. I. Quantification of molecular uptake and cell viability. J Acoust Soc Am. 2001;110(1):588–596. doi: 10.1121/1.1376131. [DOI] [PubMed] [Google Scholar]
- Guzman HR, Nguyen DX, McNamara AJ, Prausnitz MR. Equilibrium loading of cells with macromolecules by ultrasound: effects of molecular size and acoustic energy. J Pharm Sci. 2002;91(7):1693–1701. doi: 10.1002/jps.10156. [DOI] [PubMed] [Google Scholar]
- Jensen KD, Nori A, Tijerina M, Kopeckova P, Kopecek J. Cytoplasmic delivery and nuclear targeting of synthetic macromolecules. J Control Release. 2003;87(1-3):89–105. doi: 10.1016/s0168-3659(02)00352-8. [DOI] [PubMed] [Google Scholar]
- Kodama T, Doukas AG, Hamblin MR. Shock wave-mediated molecular delivery into cells. Biochim Biophys Acta. 2002;1542(1-3):186–194. doi: 10.1016/s0167-4889(01)00177-x. [DOI] [PubMed] [Google Scholar]
- LaPlaca MC, Lee VM, Thibault LE. An in vitro model of traumatic neuronal injury: loading rate-dependent changes in acute cytosolic calcium and lactate dehydrogenase release. J Neurotrauma. 1997;14(6):355–368. doi: 10.1089/neu.1997.14.355. [DOI] [PubMed] [Google Scholar]
- Li S. Electroporation gene therapy: new developments in vivo and in vitro. Curr Gene Ther. 2004;4(3):309–316. doi: 10.2174/1566523043346336. [DOI] [PubMed] [Google Scholar]
- Lokhandwalla M, Sturtevant B. Mechanical haemolysis in shock wave lithotripsy (SWL): I. Analysis of cell deformation due to SWL flow-fields. Phys Med Biol. 2001;46(2):413–437. doi: 10.1088/0031-9155/46/2/310. [DOI] [PubMed] [Google Scholar]
- Martanto W, Baisch SM, Costner EA, Prausnitz MR, Smith MK. Fluid dynamics in conically tapered microneedles. Aiche Journal. 2005;51(6):1599–1607. [Google Scholar]
- McNeil PL, Murphy RF, Lanni F, Taylor DL. A method for incorporating macromolecules into adherent cells. J Cell Biol. 1984;98(4):1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- Melan MA. Overview of cell fixation and permeabilization. Methods Mol Biol. 1994;34:55–66. doi: 10.1385/0-89603285-x:55. [DOI] [PubMed] [Google Scholar]
- Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4(3):255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- Netz RR, Schick M. Pore formation and rupture in fluid bilayers. Physical Review. E. Statistical Physics, Plasmas, Fluids, And Related Interdisciplinary Topics. 1996;53(4):3875–3885. doi: 10.1103/physreve.53.3875. [DOI] [PubMed] [Google Scholar]
- Oliver JD, Anderson S, Troy JL, Brenner BM, Deen WM. Determination of Glomerular Size-Selectivity in the Normal Rat with Ficoll. Journal of the American Society of Nephrology. 1992;3(2):214–228. doi: 10.1681/ASN.V32214. [DOI] [PubMed] [Google Scholar]
- Prado GR, Ross JD, DeWeerth SP, LaPlaca MC. Mechanical trauma induces immediate changes in neuronal network activity. J Neural Eng. 2005;2(4):148–158. doi: 10.1088/1741-2560/2/4/011. [DOI] [PubMed] [Google Scholar]
- Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Zarnitsyn V, Prausnitz MR. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med Biol. 2006;32(6):915–924. doi: 10.1016/j.ultrasmedbio.2006.02.1416. [DOI] [PubMed] [Google Scholar]
- Simoes S, Filipe A, Faneca H, Mano M, Penacho N, Duzgunes N, de Lima MP. Cationic liposomes for gene delivery. Expert Opin Drug Deliv. 2005;2(2):237–254. doi: 10.1517/17425247.2.2.237. [DOI] [PubMed] [Google Scholar]
- Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006;208(2):299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]