Abstract
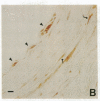
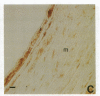
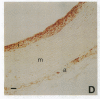
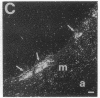
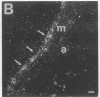
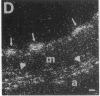
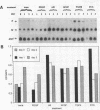
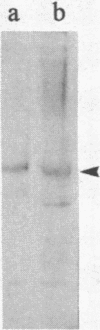
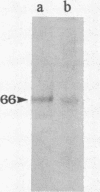
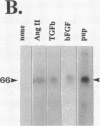
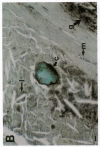
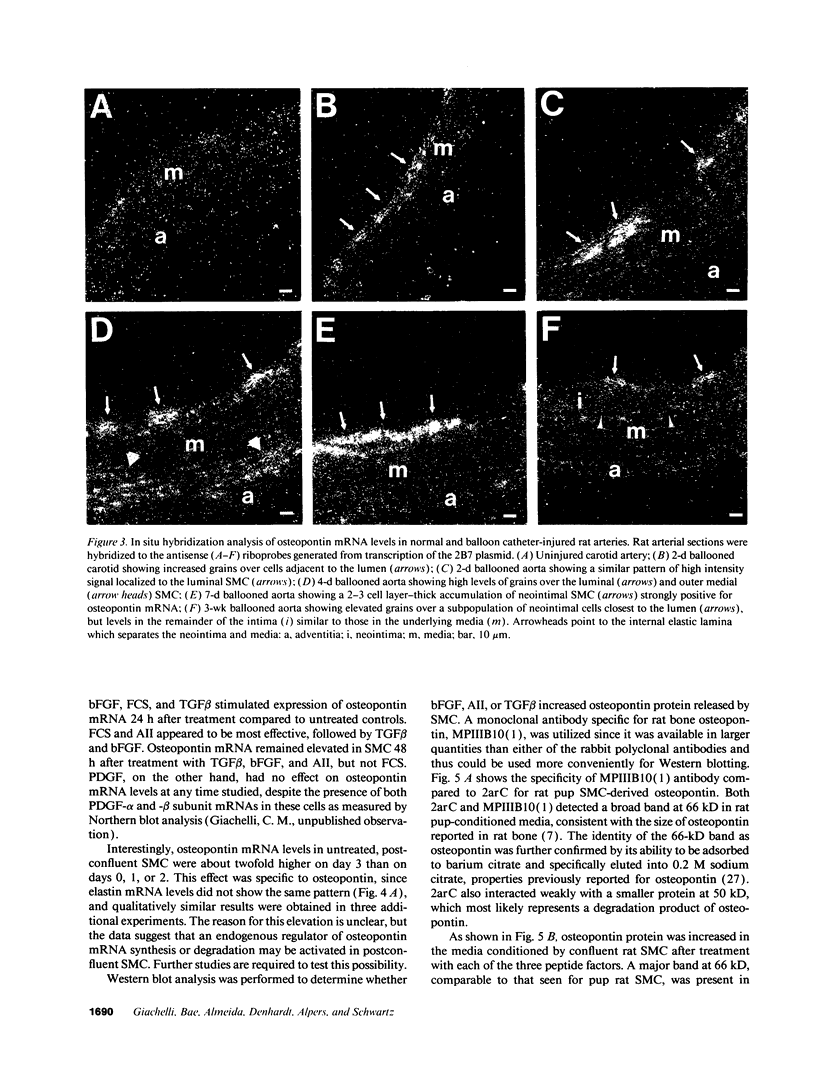
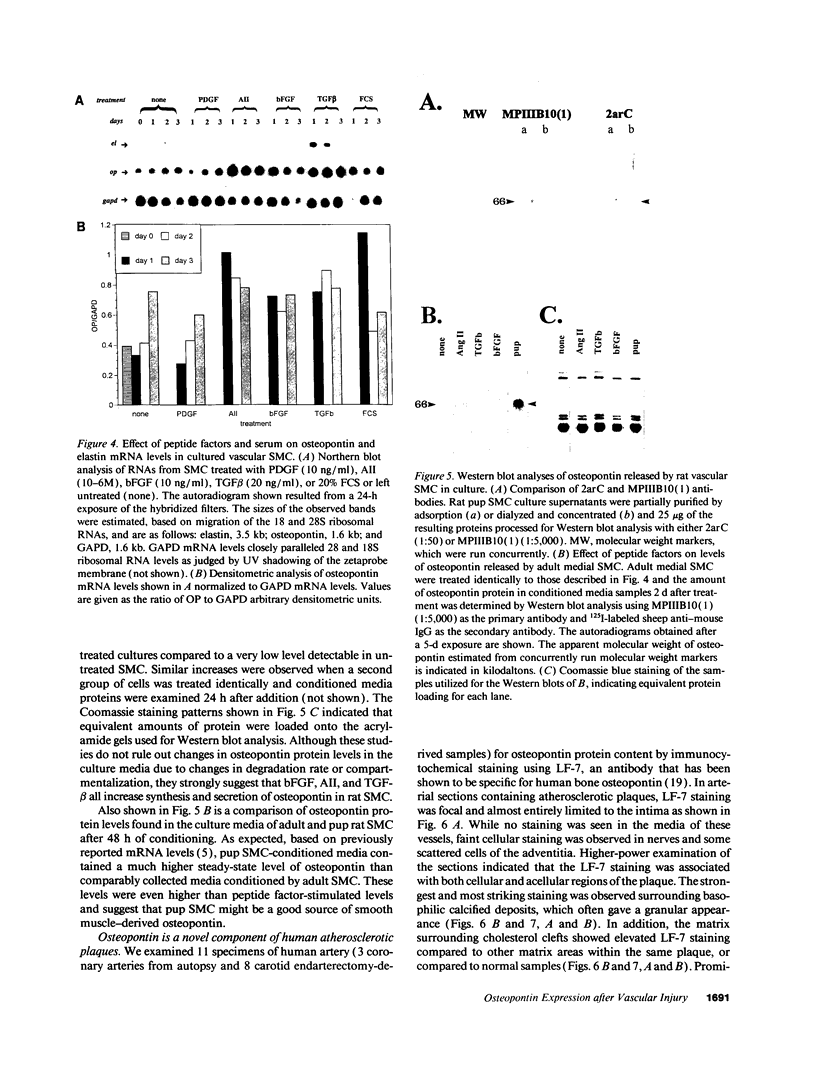
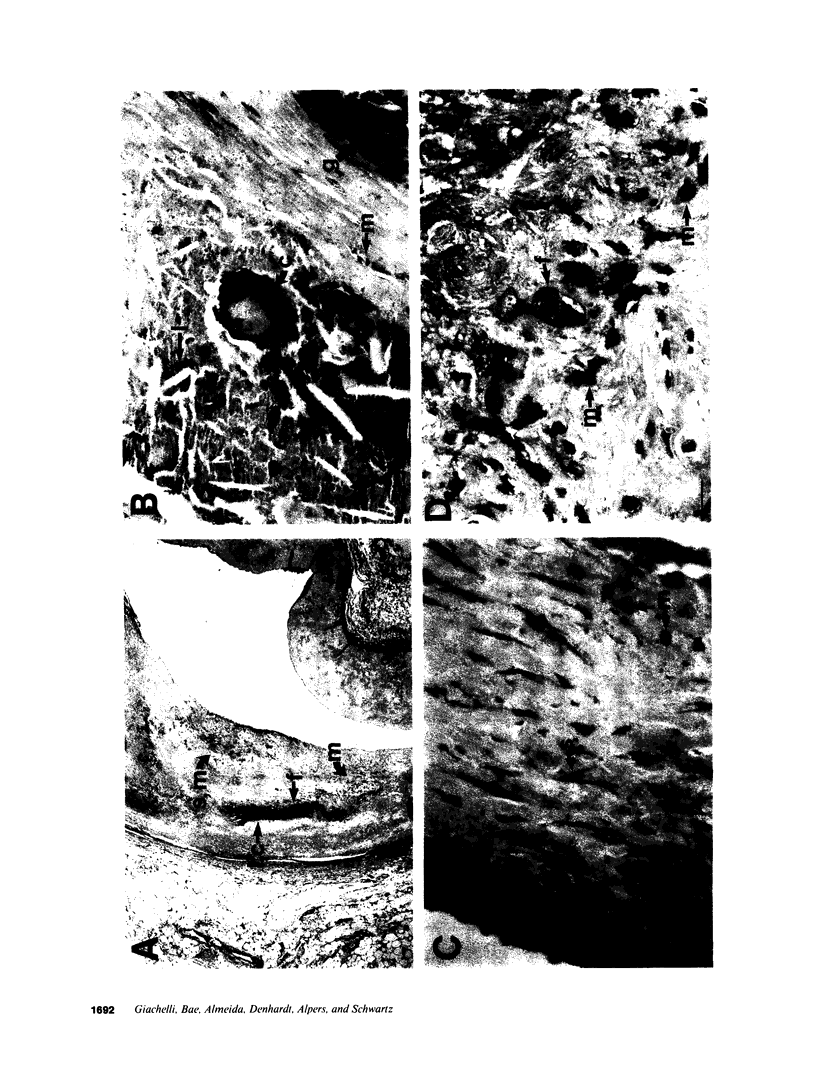
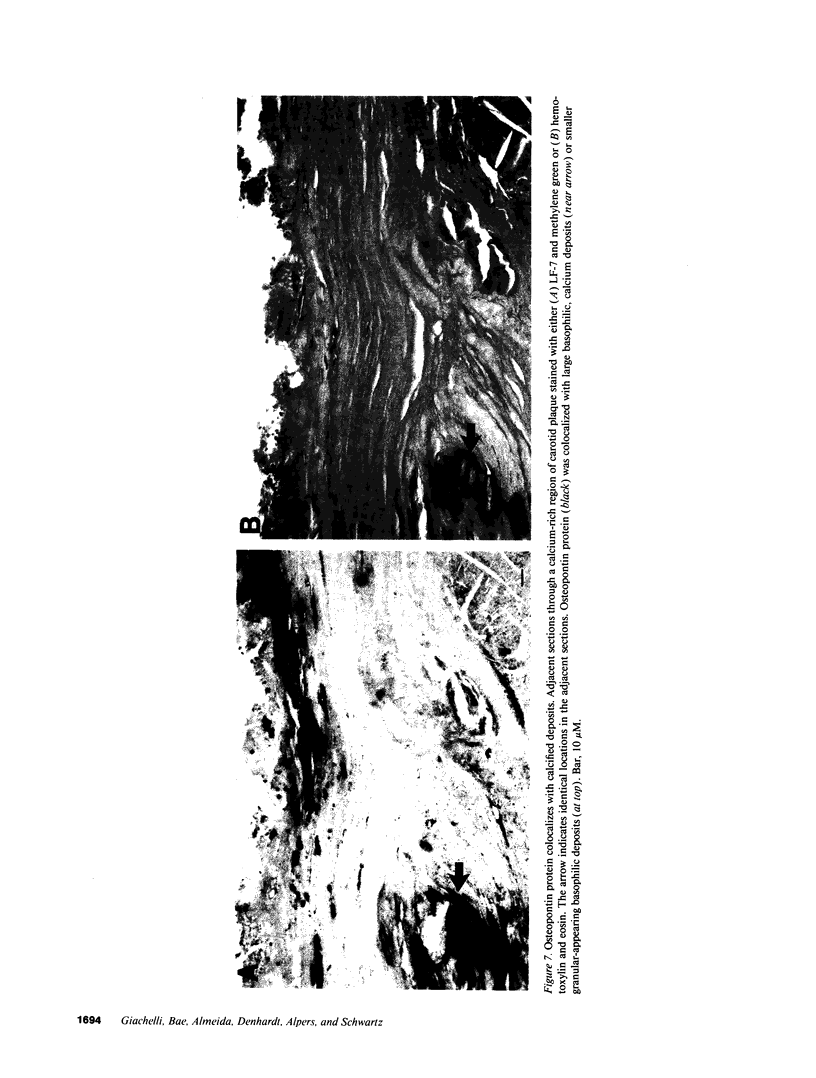
In an earlier report, we used differential cloning to identify genes that might be critical in controlling arterial neointima formation (Giachelli, C., N. Bae, D. Lombardi, M. Majesky, and S. Schwartz. 1991. Biochem. Biophys. Res. Commun. 177:867-873). In this study, we sequenced the complete cDNA and conclusively identified one of these genes, 2B7, as rat osteopontin. Using immunochemistry and in situ hybridization, we found that medial smooth muscle cells (SMC) in uninjured arteries contained very low levels of osteopontin protein and mRNA. Injury to either the adult rat aorta or carotid artery using a balloon catheter initiated a qualitatively similar time-dependent increase in both osteopontin protein and mRNA in arterial SMC. Expression was transient and highly localized to neointimal SMC during the proliferative and migratory phases of arterial injury, suggesting a possible role for osteopontin in these processes. In vitro, basic fibroblast growth factor (bFGF), transforming growth factor-beta (TGF-beta), and angiotensin II (AII), all proteins implicated in the rat arterial injury response, elevated osteopontin expression in confluent vascular SMC. Finally, we found that osteopontin was a novel component of the human atherosclerotic plaque found most strikingly associated with calcified deposits. These data implicate osteopontin as a potentially important mediator of arterial neointima formation as well as dystrophic calcification that often accompanies this process.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. T. The nature and significance of osteopontin. Connect Tissue Res. 1989;23(2-3):123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- Chen Y., Bal B. S., Gorski J. P. Calcium and collagen binding properties of osteopontin, bone sialoprotein, and bone acidic glycoprotein-75 from bone. J Biol Chem. 1992 Dec 5;267(34):24871–24878. [PubMed] [Google Scholar]
- Craig A. M., Nemir M., Mukherjee B. B., Chambers A. F., Denhardt D. T. Identification of the major phosphoprotein secreted by many rodent cell lines as 2ar/osteopontin: enhanced expression in H-ras-transformed 3T3 cells. Biochem Biophys Res Commun. 1988 Nov 30;157(1):166–173. doi: 10.1016/s0006-291x(88)80028-7. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Smith J. H., Denhardt D. T. Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate in mouse epidermis. J Biol Chem. 1989 Jun 5;264(16):9682–9689. [PubMed] [Google Scholar]
- Daemen M. J., Lombardi D. M., Bosman F. T., Schwartz S. M. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res. 1991 Feb;68(2):450–456. doi: 10.1161/01.res.68.2.450. [DOI] [PubMed] [Google Scholar]
- Fet V., Dickinson M. E., Hogan B. L. Localization of the mouse gene for secreted phosphoprotein 1 (Spp-1) (2ar, osteopontin, bone sialoprotein 1, 44-kDa bone phosphoprotein, tumor-secreted phosphoprotein) to chromosome 5, closely linked to Ric (Rickettsia resistance). Genomics. 1989 Aug;5(2):375–377. doi: 10.1016/0888-7543(89)90074-8. [DOI] [PubMed] [Google Scholar]
- Fleckenstein-Grün G., Fleckenstein A. Calcium--a neglected key factor in arteriosclerosis. The pathogenic role of arterial calcium overload and its prevention by calcium antagonists. Ann Med. 1991;23(5):589–599. doi: 10.3109/07853899109150522. [DOI] [PubMed] [Google Scholar]
- Gadeau A. P., Campan M., Millet D., Candresse T., Desgranges C. Osteopontin overexpression is associated with arterial smooth muscle cell proliferation in vitro. Arterioscler Thromb. 1993 Jan;13(1):120–125. doi: 10.1161/01.atv.13.1.120. [DOI] [PubMed] [Google Scholar]
- Giachelli C. M., Majesky M. W., Schwartz S. M. Developmentally regulated cytochrome P-450IA1 expression in cultured rat vascular smooth muscle cells. J Biol Chem. 1991 Feb 25;266(6):3981–3986. [PubMed] [Google Scholar]
- Giachelli C., Bae N., Lombardi D., Majesky M., Schwartz S. Molecular cloning and characterization of 2B7, a rat mRNA which distinguishes smooth muscle cell phenotypes in vitro and is identical to osteopontin (secreted phosphoprotein I, 2aR). Biochem Biophys Res Commun. 1991 Jun 14;177(2):867–873. doi: 10.1016/0006-291x(91)91870-i. [DOI] [PubMed] [Google Scholar]
- Gorski J. P. Acidic phosphoproteins from bone matrix: a structural rationalization of their role in biomineralization. Calcif Tissue Int. 1992 May;50(5):391–396. doi: 10.1007/BF00296767. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Hultenby K., Oldberg A., Reinholt F., Wendel M. Macromolecules in bone matrix. Connect Tissue Res. 1989;21(1-4):3–14. doi: 10.3109/03008208909049990. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Jawien A., Bowen-Pope D. F., Lindner V., Schwartz S. M., Clowes A. W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992 Feb;89(2):507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. M., Fisher L. W., Termine J. D., Young M. F. The cDNA cloning and RNA distribution of bovine osteopontin. Gene. 1991 Dec 15;108(2):237–243. doi: 10.1016/0378-1119(91)90439-i. [DOI] [PubMed] [Google Scholar]
- Lampe M. A., Patarca R., Iregui M. V., Cantor H. Polyclonal B cell activation by the Eta-1 cytokine and the development of systemic autoimmune disease. J Immunol. 1991 Nov 1;147(9):2902–2906. [PubMed] [Google Scholar]
- Lindner V., Majack R. A., Reidy M. A. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990 Jun;85(6):2004–2008. doi: 10.1172/JCI114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Schwartz S. M. Smooth muscle diversity in arterial wound repair. Toxicol Pathol. 1990;18(4 Pt 1):554–559. [PubMed] [Google Scholar]
- Noda M., Rodan G. A. Type beta transforming growth factor regulates expression of genes encoding bone matrix proteins. Connect Tissue Res. 1989;21(1-4):71–75. doi: 10.3109/03008208909049997. [DOI] [PubMed] [Google Scholar]
- O'Brien K. D., Gordon D., Deeb S., Ferguson M., Chait A. Lipoprotein lipase is synthesized by macrophage-derived foam cells in human coronary atherosclerotic plaques. J Clin Invest. 1992 May;89(5):1544–1550. doi: 10.1172/JCI115747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D., Pierschbacher M., Ruoslahti E. Identification of a bone sialoprotein receptor in osteosarcoma cells. J Biol Chem. 1988 Dec 25;263(36):19433–19436. [PubMed] [Google Scholar]
- Patarca R., Freeman G. J., Singh R. P., Wei F. Y., Durfee T., Blattner F., Regnier D. C., Kozak C. A., Mock B. A., Morse H. C., 3rd Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med. 1989 Jul 1;170(1):145–161. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince C. W. Secondary structure predictions for rat osteopontin. Connect Tissue Res. 1989;21(1-4):15–20. doi: 10.3109/03008208909049991. [DOI] [PubMed] [Google Scholar]
- Reinholt F. P., Hultenby K., Oldberg A., Heinegård D. Osteopontin--a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Wesolowski G., Yoon K., Rodan G. A. Opposing effects of fibroblast growth factor and pertussis toxin on alkaline phosphatase, osteopontin, osteocalcin, and type I collagen mRNA levels in ROS 17/2.8 cells. J Biol Chem. 1989 Nov 25;264(33):19934–19941. [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A. Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res. 1989 Sep-Oct;9(5):1291–1299. [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A., Tenen D. G. Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim Biophys Acta. 1989 Jun 13;996(1-2):43–48. doi: 10.1016/0167-4838(89)90092-7. [DOI] [PubMed] [Google Scholar]
- Singh R. P., Patarca R., Schwartz J., Singh P., Cantor H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J Exp Med. 1990 Jun 1;171(6):1931–1942. doi: 10.1084/jem.171.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. F., Kerr J. M., Termine J. D., Wewer U. M., Wang M. G., McBride O. W., Fisher L. W. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN). Genomics. 1990 Aug;7(4):491–502. doi: 10.1016/0888-7543(90)90191-v. [DOI] [PubMed] [Google Scholar]