Abstract
In the 2003–2004 influenza season, the predominant circulating influenza A (H3N2) virus in the United States was similar antigenically to A/Fujian/411/2002 (H3N2), a drift variant of A/Panama/2007/99 (H3N2), the vaccine strain. That year, a field study of trivalent live-attenuated influenza vaccine (LAIV-T) was conducted in Temple-Belton, Texas, as part of a larger community-based, non-randomized, open-label study in three communities that began in August 1998 [1, 2, 3]. Participants were healthy children aged 5 – 18 years. The analysis here concerns 6,403 children in the Scott & White Health Plan (SWHP) database living within zip codes of the Temple-Belton area, of whom 1,706 received LAIV-T and 548 received trivalent inactivated vaccine (TIV) in 2003, 983 had been previously vaccinated in 1998–2001, but not in 2002–2003 or 2003, and 3,166 had never been vaccinated. The main outcome measure was medically-attended acute respiratory illness (MAARI). Surveillance culture results were incorporated into the analysis to estimate efficacy against culture-confirmed influenza illness. Vaccine effectiveness of LAIV-T against MAARI was 26% (95% confidence interval (CI) 11,39). Vaccine efficacy of LAIV-T against culture-confirmed influenza illness including surveillance cultures of children in the SWHP database in the validation calculation was 56% (95% CI 24,84). LAIV-T was cross-protective with a drift variant strain in 2003–2004, evidence that such vaccines could be important for preparing for a pandemic and for annual influenza.
Keywords: efficacy, influenza, surveillance, vaccine
Introduction
In the 2003–04 influenza season, the predominant circulating influenza A (H3N2) virus in the United States was similar antigenically to A/Fujian/411/2002 (H3N2), a drift variant of A/Panama/2007/99 (H3N2), the vaccine strain. The efficacy of live-attenuated vaccines against drift variants is highly relevant in view of the widespread concern about how to prepare vaccine in advance for pandemic influenza, where a new virulent strain is likely be drifted from the avian precursor. Previous studies have demonstrated a high efficacy of LAIV-T against drifted strains. In particular, in the 1997–1998 season when the vaccine antigen was an A/Wuhan-like (H3N2) and the circulating strain was the drift variant A/Sydney-like (H3N2), the efficacy against culture-confirmed influenza in a double-blinded placebo controlled trial was 0.89 (95% confidence interval (CI) 0.81–0.94)[4]. A field study of live-attenuated, cold-adapted, trivalent influenza virus vaccine (LAIV-T) was being conducted in Temple-Belton, Texas, during the 2003–04 influenza season [1, 2, 3], making prospective evaluation of the vaccine possible. The influenza season started early in Texas, so vaccination occurred during the influenza season.
The primary outcome was the nonspecific outcome medically-attended acute respiratory illness (MAARI). Surveillance cultures were obtained on some of the children presenting with MAARI. Vaccine effectiveness estimates against a nonspecific outcome like MAARI are lower than compared to the efficacy estimates based on culture-positive influenza because the outcome includes respiratory syncytial virus illness and other acute respiratory illness against which the vaccine has no effect. Previous research has shown that incorporating surveillance culture results into the analysis yields estimates of vaccine efficacy against influenza that are closer to the estimates one gets in randomized studies based on culture-confirmed influenza illness [5, 6, 7].
In this paper, we report on an analysis incorporating surveillance cultures to estimate the protective efficacy of LAIV-T against the drift variant influenza in the 2003–2004 season in Temple-Belton, Texas. The analysis allows a child’s vaccination status to change during the influenza season.
Materials and Methods
Field study
A community-based, non-randomized, open-label field study began in August 1998 in Temple-Belton, Texas, as well as two other communities to evaluate the indirect effectiveness of LAIV-T vaccination of healthy children [1,2,3]. Temple-Belton was the intervention community. Comparison of the vaccinated with the unvaccinated children within Temple-Belton allows prospective evaluation of the direct protective effects of LAIV-T against the drift variant during the 2003–2004 influenza season. Comparison with the other non-intervention communities allows estimation of indirect effects of increased herd immunity and is considered elsewhere[2,8].
Healthy children aged 5–18 years were offered LAIV-T vaccination at public schools, the Temple Mall, churches, and Scott & White (S&W) Clinics during the 2003–2004 influenza season. A mobile vaccination unit advertised and offered vaccination. Advertisement was community-wide. During the influenza seasons 1998–99 through 2001–02, healthy children aged 18months–18years were offered LAIV-T, at that time an investigational product. Children were not vaccinated in the 2002–2003 season. Vaccination began again in the fall of 2003, by which time the vaccine was licensed but not approved for children under 5 years. Children received a single dose of LAIV-T each year that they enrolled. In the 2003–2004 season, healthy children and adolescents aged 5–18 years who were not pregnant and were not planning a pregnancy within 6 weeks were eligible to enroll. Other exclusionary criteria are detailed in [1]. Children who were contra indicated to receive LAIV-T, such as history of asthma, were offered trivalent in activated vaccine(TIV). Thus, there were three main vaccinated groups of interest: 1)those receiving LAIV-Tin 2003, whether or not they had received LAIV-T before, 2) those having received LAIV-T in the seasons 1998–99 through 2001–02, but not in 2002–03 or in the fall of 2003, 3)those receiving TIV in the fall of 2003. A signed informed consent was obtained from a parent or legal guardian, and an assent was obtained from children ≥7years old who were capable. The protocol was approved each year by the institutional review boards of S & W Clinic, Baylor College of Medicine, and the Texas Department of Health.
The Temple-Belton area has approximately 22,000 children aged 5–18years. S & W is the major health care provider, covering about 80% of the population. The Scott & White Health Plan (SWHP) database was the source of the information about cases used in this analysis. Age-eligible members of the SWHP on October 10, 2003 were considered for inclusion in the analysis. The final inclusion was restricted to children living within zip codes in the Temple-Belton area.
Case definition
The clinical outcome medically-attended acute respiratory infection (MAARI) included all International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)diagnoses codes(Codes381–383,460–487)for upper and lower respiratory tract infections, otitis media, and sinusitis. MAARI outcomes and demographic data were extracted from the SWHP administrative database. Each visit had one or two ICD-9-CM diagnosis codes.
Influenza surveillance
Central Texas influenza surveillance was performed as previously described [1,2,5]. In brief, any individual presenting with history of febrile respiratory illness at S & W Clinics was eligible to have a throat swab for influenza virus culture obtained after informed verbal consent as a standard of care. The decision to obtain specimens was made irrespective of the vaccination status of the patient. Throat cultures obtained from the S & W Clinic surveillance sites were processed at the viral diagnostic laboratory of Scott & White Hospital in Temple, Texas. Some of the children who had surveillance cultures done were in the SWHP, and others were not. Those in the SWHP were included in the SWHP administrative database. The non-SWHP children were not in the SWHP database, though their culture results, age, and vaccination status were available.
The primary influenza season was defined as the weeks with the most intense influenza activity accounting for 80–85% of all positive influenza cultures [2,9]. The primary influenza season occurred during the 10-week period from October 10–December 20, 2003. We considered MAARI cases and cultures within the 10-week period of interest. The first day of the study period, October 10,2003, was used to compute age. Vaccination occurring in 2003 before the 10 week period was taken into account. Children were considered vaccinated on day of vaccination. Evidence for protection was sought immediately after vaccine administration for both vaccines because of the public health considerations such as pandemic preparedness. We consider only the first 2003 LAIV-T or TIV. The time-dependent analysis allowed children to change their vaccination status during the study period.
Vaccine
LAIV-T was supplied by MedImmune Vaccines, frozen in single-dose nasal spray applicators and is administered as a nasal spray. The vaccine contained 107 median tissue culture infective dose(TCID50) of each of the three attenuated strains that matched the antigens recommended for TIV by the Food and Drug Administration for the influenza season 2003–2004. Table 1 shows the strains in the LAIV-T in the 2003–2004 season and the previous seasons 1998–2002 when vaccination took place. The vaccine was stored frozen at−20°C and was thawed to room temperature by holding in the palm for < 5 minutes prior to use. The TIV contained vaccine strains similar to those in LAIV-T.
Table 1.
LAIV-T Vaccine Strains in the 1998–2002 and 2003–2004 Seasons in this Study
| A(H3N2) | A(H1N1) | B | |
|---|---|---|---|
| Current | |||
| 2003–2004 | A/Panama/2007/99 | A/New Caledonia/20/99 | B/Hong Kong/330/2001 |
| Previous | |||
| 2001–2002 | A/Panama/2007/99 | A/New Caledonia/20/99 | B/Sichuan/379/99-like |
| 2000–2001 | A/Sydney/5/97 | A/New Caledonia/20/99 | B/Beijing/184/93-like (B/Ann Arbor/1/94) |
| 1998–2000 | A/Sydney/5/97 | A/Beijing/262/95 | B/Beijing/184/93-like (B/Ann Arbor/1/94) |
Statistical analysis
The effectiveness of protection against MAARI and the efficacy of protection against culture-confirmed influenza were computed using the basic equation V E = 1−RR, where RR is the ratio of the number of MAARI (estimated influenza) cases/child-days in the vaccinated compared to the unvaccinated group. Our main interest was in the efficacy of LAIV-T, but estimates were also obtained for TIV and previously vaccinated in 1998–2001 (PREV), all three being compared to the unvaccinated group. Age-group specific values were computed for the two age groups 5–9 years and 10–18 years. Overall efficacy was computed by weighting the contributions of the age groups by the combined number of child-days at risk in the vaccinated and unvaccinated groups in each age group.
A child who began the season as either unvaccinated or previously vaccinated could be switched to the LAIV-T group or the TIV group once they got vaccinated in 2003. The child-days contributed by each child to each group was calculated taking the switch into account. To take the changing vaccine status into account and to integrate the surveillance cultures into the analysis, we grouped the data by week over the ten week period. If vaccination occurred before the day of MAARI, the child was counted as a vaccinated MAARI case. Otherwise, the child was counted as a previously vaccinated or unvaccinated MAARI case. Similarly, a child contributed child-days at risk to either the unvaccinated or the previously vaccinated group up until and if they received LAIV-T or TIV, after which they contributed child-days at risk to the appropriate vaccinated group. We assumed that multiple visits in a week were not independent. Only the first MAARI case in the week was included if a child had more than one MAARI presentation in that week. We denote vaccine effectiveness against MAARI as VEa.
To estimate the efficacy against culture-confirmed influenza illness, the expected number of influenza cases in each week for each age and vaccine group was estimated by multiplying the proportion of positive surveillance cultures in each age and vaccine group by the number of MAARI cases in that group [6]. For each week, the child day at risk contribution was computed by subtracting the expected number of influenza cases times half the time interval from the number at risk at the beginning of the interval to adjust the child-days at risk. Children who had positive cultures were considered to be no longer at risk for influenza and did not contribute further child-days at risk for the rest of the ten week period.
We computed two different estimates using the surveillance cultures. The first, denoted VEin, uses just the surveillance cultures from the SWHP members in the database. The second, denoted VEex, uses the surveillances cultures from both the SWHP members and the non-SWHP children. The surveillance cultures from the SWHP members are called the internal validation set because we also have the MAARI data on these children, while the others are the external validation set. Confidence intervals were obtained using 2000 bootstrap samples [10]. The details are in the technical appendix.
Results
Vaccination, MAARI cases, and child-days at risk
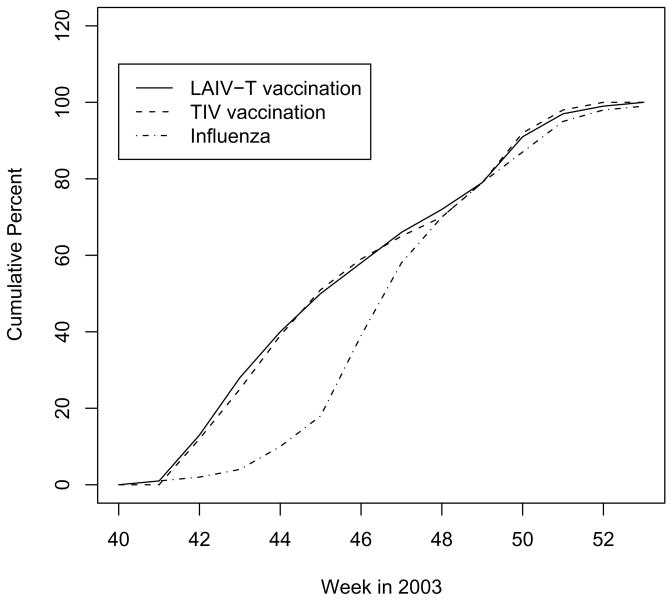
A total of 6,403 age-eligible children in the SWHP database living in the zip codes in the Temple-Belton are included in the analysis, of whom 1,706 received LAIV-T and 548 received TIV in 2003 before the end of the study period. Of the remaining children, 983 had been previously vaccinated in 1998–2001 and 3,166 had never been vaccinated by the end of the study period. Figure 1 shows the cumulative distributions of vaccination and positive cultures during the 2003 influenza season. About four weeks into the period, by November 8, 2003, 50% of the vaccinees had been vaccinated with either LAIV-T or TIV. About 82% of the influenza culture positive MAARI occurred in the six weeks following November 8, 2003.
Figure 1.
Cumulative distributions of the proportion of LAIV-T and TIV vaccinations administered and the cumulative distribution of the positive influenza surveillance cultures in the fall of 2003. The peak influenza season was during the period of weeks 45 to 47.
Table 2 shows the covariate distribution by vaccine status at the end of the study period. The potential confounder Group 1 is composed of diagnoses of chronic obstructive pulmonary disease (COPD) and allied diseases, including asthma (ICD-9-CM Codes 490–496). Group 2 consists of several dozen chronic and congenital conditions, including HIV infection. The distributions of COPD and other diseases were similar in the LAIV-T, the previously vaccinated, and the unvaccinated groups. The TIV group has a much higher percentage of COPD and other diseases than the other groups, so that the comparison with the unvaccinated group is not valid, because one would expect that a valid comparison group with risk factors contraindicating LAIV-T would likely have a higher baseline MAARI rate. We present the data on the TIV vaccinees here for completeness, with the results in the text, not the tables. Differences in the age group distribution by vaccine status is adjusted for by weighting the age groups in the estimates of overall efficacy as described in the methods.
Table 2.
Covariates by Vaccine Group
| Vaccine status at end of period |
|||||
|---|---|---|---|---|---|
| LAIV-T no. (%) | TIV no. (%) | PREV no. (%) | UNVAC no. (%) | Total | |
| Total | 1706 | 548 | 983 | 3166 | 6403 |
| 5–9 years | 757(44) | 225(41) | 224(23) | 739(23) | 1945(30) |
| 10–18 years | 949(56) | 323(59) | 759(77) | 2427 (77) | 4458(70) |
| Male | 820(48) | 296(54) | 516(52) | 1637(52) | 3269 (51) |
| Female | 886(52) | 252(46) | 467 (48) | 1529 (48) | 3134(49) |
| Group 1* | 82(5) | 190(35) | 60(6) | 227(7) | 618(10) |
| Group 2† | 73(4) | 37(7) | 52(5) | 158 (5) | 379(6) |
| Both‡ | 10(0.3) | 13(2) | 8(0.8) | 28(0.9) | 59(1) |
| Either§ | 165(10) | 240(44) | 120(12) | 413(13) | 938(15) |
Chronic obstructive pulmonary disease (COPD), including asthma (ICD-9-CM codes 490–496)
Numerous other chronic underlying conditions, including HIV
Having conditions from both categories
Total number with COPD or other chronic condition
The total number of MAARI cases during the study period was 1,807. After counting just one MAARI event per child in a week, the total number of MAARI cases was 1,702, with 5,144 children having no MAARI event, 1,011 having just one, 165 having just two, and 48 having just three. Table 3 contains the number of MAARI events, child-days at risk, and rate per 1,000 by age and vaccine status used in the analysis.
Table 3.
MAARI events, child-days at risk and rate per 1,000 child-days at risk by age group and vaccine status.
| Age (years) | Vaccination status | MAARI Events | Child-days at Risk | Rate/1,000 Child-days at risk |
|---|---|---|---|---|
| 5–9 | ||||
| LAIV-T | 105 | 35,886 | 2.93 | |
| TIV | 80 | 10,598 | 7.55 | |
| PREV | 143 | 26,902 | 5.32 | |
| UNVAC | 261 | 61,522 | 4.24 | |
| 10–18 | ||||
| LAIV-T | 117 | 42,991 | 2.72 | |
| TIV | 82 | 13,741 | 5.97 | |
| PREV | 273 | 71,424 | 3.82 | |
| UNVAC | 641 | 179,828 | 3.56 | |
| Combined | ||||
| LAIV-T | 222 | 78,883 | 2.81 | |
| TIV | 162 | 24,383 | 6.64 | |
| PREV | 416 | 98,297 | 4.23 | |
| UNVAC | 902 | 241,331 | 3.74 | |
| Totals | ||||
| 5–9 | 589 | 134,908 | 4.37 | |
| 10–18 | 1113 | 307,984 | 3.61 | |
| Combined | 1702 | 442,896 | 3.84 |
Cultures and positive cultures
Table 4 shows the influenza surveillance data and proportion of cultures positive by age and vaccine status at the time of culture. We checked whether all cultures in the SWHP main dataset were associated with MAARI cases. There are 157 cultures in the study period in the main dataset. For 140 of them there was a MAARI admit-day that exactly matched the culture-day. There were 17 children with cultures for whom the automatically compiled data set had no MAARI visits on the day of culture. The records reviewed by P.A. Piedra established that 14 of the children had clinic visits on the day of culture. Of these 14, 10 had visits associated with MAARI illness, and 4 did not. For the 10 children with MAARI visits, we recorded a MAARI event in the data set on the day of culture. For the four children whose visit was not a MAARI event and the three children with no recorded visit on the day of culture, we excluded the culture. Thus, for the analysis, there are 150 cultures, representing 8.8% of the MAARI cases in the main SWHP data set.
Table 4.
Influenza Surveillance Data (Number positive/Number cultured (proportion)), Temple-Belton, Texas, 2003–2004.
| Age Group (years) | SWHP |
non-SWHP |
Combined |
|||
|---|---|---|---|---|---|---|
| Unvaccinated | LAIV-T | Unvaccinated | LAIV-T | Unvaccinated | LAIV-T | |
| 5–9 | 8/20 (0.40) | 3/15 (0.20) | 19/34 (0.56) | 4/9 (0.44) | 27/54 (0.50) | 7/24 (0.29) |
| 10–18 | 35/56 (0.63) | 5/13 (0.38) | 30/49 (0.61) | 4/11 (0.36) | 65/105 (0.62) | 9/24 (0.38) |
| Total | 43/76 (0.57) | 8/28 (0.29) | 49/83 (0.59) | 8/20 (0.40) | 92/159 (0.58) | 16/48 (0.33) |
|
|
||||||
| TIV | PREV | TIV | PREV | TIV | PREV | |
|
| ||||||
| 5–9 | 2/5 (0.40) | 3/9 (0.33) | 0/3 (0.33) | 7/21 (0.33) | 2/8 (0.25) | 10/30 (0.33) |
| 10–18 | 3/3 (1.0) | 15/29 (0.52) | 5/6 (0.83) | 8/15 (0.53) | 8/9 (0.89) | 23/44 (0.52) |
| Total | 5/8 (0.63) | 18/38 (0.47) | 5/9 (0.56) | 15/36 (0.42) | 10/17 (0.59) | 33/74 (0.44) |
There are 177 non-SWHP children with surveillance cultures, 147 of whom had cultures within the study period. Only one child had two cultures within the study period, the first of which was negative for influenza. We created a second record and include the second culture as an independent data point. Within the 10 week period of interest, there were then 148 cultures in the non-SWHP children.
Vaccine effectiveness against MAARI and influenza
The estimates of VEa, VEin, and VEex are given in Table 5. Overall effectiveness of LAIV-T against MAARI VEa is 0.26 (95% CI: 0.11,0.39). Overall efficacy against culture-confirmed influenza using just surveillance cultures from children in the SWHP database, VEin, is 0.56 (95% CI 0.24,0.84). Using surveillance cultures from both children in the SWHP database and those from the S & W clinic surveillance sites who were not in the SWHP, overall efficacy against culture-confirmed influenza is 0.56 (95% CI 0.32,0.75). The point estimates for VEin and VEex are quite similar, but the confidence intervals using all of the surveillance cultures are narrower than those using just the surveillance cultures from SWHP, reflecting the higher precision conferred by the larger number of cultures. There is no evidence that being previously vaccinated two to five years earlier in 1998–2001, but not in 2002 or 2003, provided any protection. The results for TIV are not contained in Table 5 because of the concern that the unvaccinated group was not comparable to the group receiving TIV on important risk factors. However, for completeness, we present the overall estimates here in the text. The estimate of the overall effectivenees of TIV against MAARI is −0.71, (95% CI −1.2, −0.25) and against culture-confirmed influenza using surveillance cultures from just children in the SWHP database is −1.5,(95% CI −2.7, −0.53).
Table 5.
Vaccine effectiveness of LAIV-T: VEa against MAARI (95% CI), against culture-confirmed influenza using just SWHP surveillance cultures VEin (95% CI), and against culture-confirmed influenza using surveillance cultures from the children in the SWHP database and children not in the SWHP database, VEex (95% CI).
| Vaccine status | Age group (years) | VEa (95% CI)‡ MAARI | VEin (95% CI) influenza | VEex (95% CI) influenza |
|---|---|---|---|---|
| LAIV-T* | ||||
| 5–9 | 0.31 (0.11,0.47) | 0.66 (−0.03,1.0) | 0.60 (0.25,0.84) | |
| 10–18 | 0.24 (0.03,0.40) | 0.53 (0.12,0.86) | 0.54 (0.23,0.78) | |
| All | 0.26 (0.11,0.39) | 0.56 (0.24,0.84) | 0.56 (0.32,0.75) | |
| PREV† | ||||
| 5–9 | −0.25 (−0.61,0.05) | −0.04 (−1.9,1.0) | 0.17 (−0.50,0.61) | |
| 10–18 | −0.07 (−0.28,0.10) | 0.11 (−0.37,0.46) | 0.09 (−0.28, 0.39) | |
| All | −0.13 (−0.30,0.03) | 0.08 (−0.38,0.44) | 0.11 (−0.19,0.37) |
vaccinated with LAIV-T in 2003, regardless previously vaccinated or not
previously vaccinated in 1998–2001, but not in the 2002–2003 season or in 2003
Percentile bootstrap confidence intervals based on 2000 bootstrap samples.
Discussion
This analysis shows that the live-attenuated influenza vaccine was cross-protective against a drift variant in children aged 5 to 18 years in 2003. By incorporating the surveillance cultures, the estimates of protection by LAIV-T against influenza were over twice as high as the effectiveness against non-specific MAARI alone. Although this is not a randomized study, these are the best estimates available. Previous vaccination in the 1998–2001 seasons with LAIV-T, but not in 2002or2003, did not show evidence of protection in the fall of 2003. No vaccination had taken place in the previous 2002–2003 season, so we did not expect the previously vaccinated children to be protected in the fall of 2003 against the drift variant.
The new H3N2 variant began spreading coincident with the beginning of administration of vaccine in mid-October. Under these circumstances it was not possible to administer second doses to the children aged 5–9 years who were receiving vaccine for the first time. We anticipated that most of these children would have been primed by natural infection in prior years negating the need for a second dose. Over 95% of the children received only one dose of LAIV-T or TIV.
Our unvaccinated group was not comparable to the TIV group and the numbers were small. Analysis of the experience with TIV in Colorado in 2003–2004 [11] in a retrospective cohort study of children aged 6–23 months and a case-control study in persons aged 50–74 years are not comparable to our observations in school aged children.
Use of the surveillance cultures to estimate efficacy assumes that the decision to obtain a culture on a child is not related to whether he actually had true influenza. If this assumption is violated, then the estimates could be subject to selection bias [5,7]. However, Scharfstein et al [7] showed that even if there were substantial and differential selection bias in the vaccinated and unvaccinated groups, the bias in the efficacy estimates using the surveillance cultures is minimal.
Live-attenuated vaccine would be a good candidate for stockpiling vaccines for a pandemic where one would expect a mismatch with the anticipated strain [12,13,14]. In the case of a pandemic, it will be important to be able to evaluate the efficacy of a mismatched vaccine. The use of the well-planned surveillance cultures combined with rapid tests could be an important method for field evaluation in the case of a pandemic.
Acknowledgments
The field study was supported by National Institute of Allergy and Infectious Diseases grant UO1-AI41050. MedImmune Vaccines Inc. provided the intranasal influenza vaccine free of charge. Drs. Halloran and Longini were partially supported by National Institute of Allergy and Infectious Diseases grant R01-AI32042 and National Institute of General Medical Sciences MIDAS grant U01-GM070749. We thank Nadine Zimmerman for SWHP data extraction and Hope Gonzalez and Dianne Harvey for helping to coordinate the respiratory virus surveillance.
Technical Appendix
We use a method to estimate the number of influenza cases and child-days at risk in each group within discrete time intervals, similar to [6]. We grouped the data within one-week time intervals τ, (tτ−1, tτ], τ = 1,…, T, T = 10. Let k, k = 1,…, K, indicate the relevant strata, in our case age groups, and K = 2. Let nντ, ν = 0, 1, be the number of participants in the unvaccinated and vaccinated group at risk of influenza at the beginning of each time interval, with nkντ, ν = 0, 1, k = 1,…, K, the corresponding number in each stratum. For each stratum k, k = 1,…, K, and vaccine status ν, ν = 0, 1, let the number of MAARI cases ascertained in each time interval be wkντ, the number of surveillance cultures be rkντ, and the number of positive cultures be ckντ. For each τ, estimate the proportion ρkντ of true influenza cases among the MAARI cases in each age and vaccine group by ρ̂kντ = ckντ/rkντ. We multiply wkντ by {ρ̂kντ} to obtain an estimate of the number of influenza cases in each interval. Summing over the weekly estimates of the number of true influenza cases, we estimate the total number of influenza cases in each age and vaccine group during the study. We make the assumption that the outcome of interest, the result of a culture, is missing at random [15].
To compute child-days at risk, we assumed everyone was at risk at the beginning of the study period. For each time interval t, the child-days at risk in each stratum, dkντ, was computed as 7 × (nkντ − 0.5ρ̂kντwkντ), ν = 0, 1, k = 1,…, K. That is, we subtracted the expected number of influenza cases times half the time interval from the number at risk at the beginning of the interval to adjust the child-days at risk. Children who had positive cultures were considered to be no longer at risk for influenza and did not contribute further child-days at risk for the rest of the ten week period.
From this, we estimate the incidence rate of true influenza in each vaccine group, and from that, the group specific vaccine efficacy, VEk,v, based on the validation set as
| (1) |
Overall VEv is computed by weighting the contributions of the age groups by the combined number of child-days at risk in the vaccinated and unvaccinated groups in each age group. Confidence intervals were based on the bootstrap [10].
To estimate vaccine efficacy based on the SWHP surveillance cultures, VEin, we used only the SWHP surveillance cultures to estimate ρkντ in equation (1). To estimate VEex, we added the non-SWHP culture data to the SWHP culture data to estimate ρkντ in equation (1). To get confidence intervals for VEex, we bootstrap the external culture data separately, then add each external bootstrap dataset to the corresponding bootstrap dataset from the main SWHP data set to get the bootstrap estimate of the proportion of cultures that are positive. We then compute VEex for the bootstrap data set. In using the external cultures, we make the assumption that the population producing the non-SWHP cultures is similar to the population producing the SWHP data. This would result in the proportion positive in the non-SWHP cultures being the same as the proportion positive in the MAARI cases in the SWHP data. A chi-square test did not reject the hypothesis of equality of the proportions of positive cultures of the SWHP and non-SWHP surveillance cultures across the age groups in the four vaccine groups (p = 0.63).
Sensitivity analysis on assumption about positive cultures
When spread over a ten week period, the culture data were too sparse to obtain a weekly estimate of ρkντ for use in equation (1). So we used the overall estimated proportion positive in Table 4 in each group as the weekly estimate for the proportion positive in equation (1). A sensitivity analysis explored the effect of assuming that the proportion of positive cultures was constant throughout the season. The data were too sparse to use any nonparametric or parametric smoothing method. We assumed a form for the change in the proportion of cultures over the ten week period that had a peak in the middle of the season and was lower on both ends, so that the total proportion of the positive cultures over the season equaled the observed overall proportion positve. We multiplied the vector (.70,.75,1,1.25,1.3,1.3,1.25,1,.75,.70) (which sums to 10) by the proportion positive in each age and vaccination group to obtain the estimated weekly proportion positive, {ρ̂kντ}. Then equation (1) was used to compute VEin or VEex. Inference was based on bootstrap confidence intervals. The results assuming that the proportion positive varied over time were essentially identical to those in Table 5. For example, the overall VEex for LAIV-T in the sensitivity analysis was 0.56 (95% CI 0.33,0.75), the same as in Table 5. Thus, assuming that the proportion of cultures that were positive was constant did not seem to influence the efficacy estimates VEin and VEex.
Footnotes
Contribution to the paper: M. Elizabeth Halloran: planned and directed the statistical analysis and wrote the paper.
Pedro A. Piedra: conducted the field study that provided the data and contributed to the paper.
Ira M. Longini, Jr: provided input to the analysis and the writing of the paper.
Manjusha J. Gaglani: conducted the field study that provided the data and contributed to the paper.
Brian Schmotzer: programmed the software for the analysis and provided summaries of the data.
Charles Fewlass: collected, summarized, and extracted the data for analysis.
Gayla B. Herschler: conducted the field study that provided the data.
W. Paul Glezen: conducted the field study that provided the data and contributed to the paper.
Conflicts of Interest
IML, PAP, MJG, GBH and WPG have received small amounts of money from Med Immune for ad hoc consulting, talks, or small grants. MEH, BS, and CF have no conflict of interest.
MedImmune did not try to influence the analysis in any way.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaglani MJ, Piedra PA, Herschler GB, Griffith ME, Kozinetz CA, Riggs MW, Fewlass C, Halloran ME, Longini IM, Glezen P. Direct effectiveness of the trivalent, cold-adapted, influenza virus vaccine (CAIV-T) against the 2000–2001 influenza A (H1N1) and B epidemic in healthy children. Arch Pediatr Adolesc Med. 2004;158:65–73. doi: 10.1001/archpedi.158.1.65. [DOI] [PubMed] [Google Scholar]
- 2.Piedra PA, Gaglani MJ, Kozinetz CA, Herschler G, Riggs M, Griffith M, Fewlass C, Watts M, Hessel C, Cordova J, Glezen WP. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine. 2005;23:1540–8. doi: 10.1016/j.vaccine.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Piedra PA, Gaglani MJ, Riggs M, Herschler G, Fewlass C, Watts M, Kosinetz C, Hessel C, Glezen WP. Live attenuated influenza vaccine, trivalen, is safe in healthy children 18 months to 4years, 5 to 9years, and 10 to 18 years of age in a community-based, nonrandomized, open-label trial. Pediatrics. 2005;116:397–407. doi: 10.1542/peds.2004-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longini IM, Halloran ME, Nizam A, Wolff M, Mendelman PM, Fast P, Belshe RB. Estimation of the efficacy of live, attenuated influenza vaccine from a two-year, multi-center vaccine trial: implications for influenza epidemic control. Vaccine. 2000;18:1902–9. doi: 10.1016/s0264-410x(99)00419-3. [DOI] [PubMed] [Google Scholar]
- 5.Halloran ME, Longini IM, Gaglani MJ, Piedra PA, Chu H, Herschler GB, Glezen WP. Estimating efficacy of trivalent, cold-adapted, influenza virus vaccine (CAIV-T) against influenza A (H1N1) and B using surveillance cultures. American Journal of Epidemiology. 2003;158:305–311. doi: 10.1093/aje/kwg163. [DOI] [PubMed] [Google Scholar]
- 6.Halloran ME, Longini IM. Using validation sets for outcomes and exposure to infection in vaccine field studies. Am J Epidemiol. 2001;154:391–398. doi: 10.1093/aje/154.5.391. [DOI] [PubMed] [Google Scholar]
- 7.Scharfstein DO, Halloran ME, Chu H, Daniels MJ. On estimation of vaccine efficacy using validation samples with selection bias. Biostatistics. 2006;7:615–629. doi: 10.1093/biostatistics/kxj031. Biostatistics Advanced Access published on March 23, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piedra PA, Gaglani MJ, Kozinetz CA, Herschler GB, Fewlass C, Harvey D, Zimmerman N, Glezen WP. Live attenuated intranasal influenza vaccine-trivalent (LAIV-T) administered during the 2003–04 influenza type A (H3N2) out break provided immediate, direct and indirect protection in children. [accepted, 2007];Pediatrics. doi: 10.1542/peds.2006-2836. [DOI] [PubMed] [Google Scholar]
- 9.Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, Glezen WP, Wittes J. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy working adults: a randomized controlled trial. J Am Med Assoc. 1999;282:137–144. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- 10.Efron Bradley, Tibshirani Robert J. An Introduction to the Bootstrap. Chapman and Hall; New York: 1993. [Google Scholar]
- 11.CDC. Assessment of the effectiveness of the 2003–04 influenza vaccine among children and adults – Colorado, 2003. MMWR. 2004;53:707–710. [PubMed] [Google Scholar]
- 12.Longini IM, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DAT, Halloran ME. Containing pandemic influenza at the source. Science. 2005;309:1083–87. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 13.Germann TC, Kadau K, Longini IM, Macken CA. Mitigation strategies for pandemic influenza in the United States. PNAS. 2006;103:5935–40. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson NM, Cummings DAT, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–252. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little RJA, Rubin DA. Statistical Analysis with Missing Data. 2. John Wiley, Hoboken; New Jersey: 2002. [Google Scholar]