Abstract
Chondrocytes provide the framework for the developing skeleton and regulate long-bone growththrough the activity of the growth plate. Chondrocytes in the articular cartilage, found at the ends of bones in diarthroidial joints, are responsible for maintenance of the tissue through synthesis and degradation of the extracellular matrix. The processes of growth, differentiation, cell death and matrix remodeling are regulated by a network of cell signaling pathways in response to a variety of extracellular stimuli. These stimuli consist of soluble ligands, including growth factors and cytokines, extracellular matrix proteins, and mechanical factors that act in concert to regulate chondrocyte function through a variety of canonical and non-canonical signaling pathways. Key chondrocyte signaling pathways include, but are not limited to, the p38, JNK and ERK MAP kinases, the PI-3 kinase-Akt pathway, the Jak-STAT pathway, Rho GTPases and Wnt-β-catenin and Smad pathways. Modulation of the activity of any of these pathways has been associated with various pathological states in cartilage. This review focuses on the Rho GTPases, the PI-3 kinase-Akt pathway, and some selected aspects of MAP kinase signaling. Most studies to date have examined these pathways in isolation but it is becoming clear that there is significant cross talk among the pathways and that the overall effects on chondrocyte function depend on the balance in activity of multiple signaling proteins.
Keywords: CELL SIGNALING, CHONDROCYTES, KINASES
Chondrocytes are the cells present in various types of cartilage and are responsible for the growth and maintenance of the tissue. Chondrocytes are unique cells in that they are often asked to perform multiple functions, including matrix synthesis and matrix degradation, that in other tissues are performed by more than one cell type (for example bone formation by osteoblasts and bone resorption by osteoclasts). The formation and removal (i.e. remodeling) of cartilaginous tissues requires regulated cell proliferation, growth, synthesis of extracellular matrix proteins, production and activation of matrix degrading enzymes, and in some cases matrix calcification and cell death. Two types of chondrocytes that have received the most attention in terms of how these processes are regulated are growth plate and articular chondrocytes and it has been found that a large number of signaling pathways control their activity. A PubMed search of chondrocytes and cell signaling reveals over 2000 publications with over 200 papers published in 2009 alone. Space limitations do not allow us to discuss all of the relevant chondrocyte signaling pathways and several have been covered in recent reviews [Bobick and Kulyk, 2008; Luyten et al., 2009; Teixeira et al., 2008; Yoon and Lyons, 2004]. Here we focus on signaling pathways in growth plate and adult articular chondrocytes that include the Rho GTPases, the PI-3 kinase-Akt pathway and selected aspects of MAP kinase signaling.
RHO GTPases IN CARTILAGE DEVELOPMENT AND ARTICULAR CARTILAGE
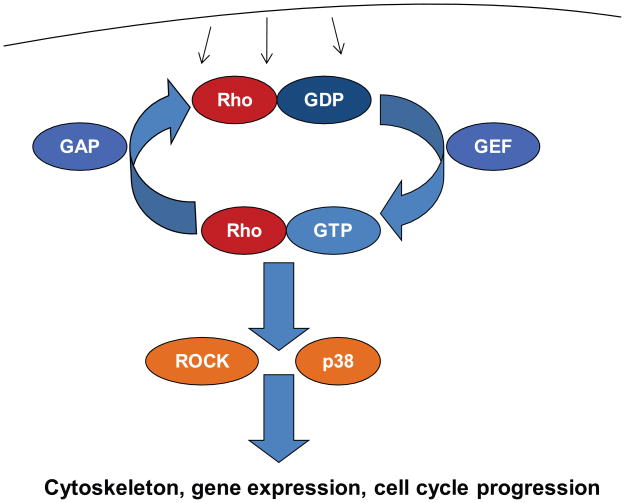
Recent years have seen a number of studies implicating small GTPases of the Rho family in both chondrocyte differentiation and the physiology of articular chondrocytes. These proteins act as molecular switches, cycling between an active, GTP-bound and an inactive, GDP-bound form [Heasman and Ridley, 2008; Vega and Ridley, 2008]. Cycling between the two forms is regulated by dozens of other proteins, in particular guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) (Fig. 1). GEFs activate Rho GTPases by replacing GDP with GTP while GAPs increase the intrinsic GTPase activity of Rho proteins and thereby accelerate their inactivation. Activity of GEFs and GAPs in turn is controlled by many cell surface receptors, including tyrosine and serine/threonine kinase receptors, G protein-coupled receptors and integrins. Rho GTPases are of particular interest in chondrocytes because of their role in connecting signals from the extracellular matrix to the actin cytoskeleton and cellular morphology, which in turn appear to control cellular activities such as cell cycle progression, gene expression and apoptosis [Woods et al., 2007a]. RhoA, Rac1 and Cdc42 are the prototypes of the three main families of Rho GTPases, and their roles in chondrocytes have been studied to some degree, though not exhaustively.
Fig. 1.
Rho GTPase signaling. Numerous cell surface receptors regulate the activity of guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) which in turn control activity of Rho GTPases. GTP-bound Rho GTPases are the active forms and bind to downstream effectors, including ROCK1/2 and p38 kinases. These in turn control cellular events such as actin and microtubule dynamics, gene expression and cell cycle progression.
RhoA promotes fibroblastoid cell shape and the formation of actin stress fibers transversing the cell. Normally chondrocytes are rounded or polygonal and display characteristic cortical actin organization while the formation of actin stress fibers, at least in vitro, has been associated with de-differentiation of chondrocytes to a fibroblast-like phenotype. Therefore, it was not unexpected to find that overexpression of RhoA inhibits both early chondrogenesis and hypertrophic chondrocyte differentiation, while inhibition of RhoA, or the immediate downstream kinases ROCK1/2, promotes chondrocyte maturation [Kumar and Lassar, 2009; Wang et al., 2004a; Woods and Beier, 2006; Woods et al., 2005]. The effects of RhoA/ROCK signaling on chondrogenesis appear to be mediated by regulation of both expression and activity (e.g. phosphorylation) of Sox9, the central transcriptional regulator of chondrogenesis. Regulation of Sox9 expression by Rho signaling appears to occur, at least in part, at the level of Sox9 transcription. However, the specific transcription factors connecting ROCK to the Sox9 promoter have not been identified. In addition, Rho signaling also controls the expression of Sox5 and Sox6, which act in concert with Sox9 to control chondrogenesis.
In contrast to RhoA, Rac1 and Cdc42 promote early and late chondrocyte differentiation in vitro, partially through activation of p38 MAP kinases [Kerr et al., 2008; Wang and Beier, 2005; Woods et al., 2007b]. Cartilage-specific inactivation of the Rac1 gene in mice using a Col2Cre driver line resulted in increased perinatal lethality, dwarfism, skeletal deformities such as kyphosis, and disorganized growth plates [Wang et al., 2007]. A similar reduction in the size of long bones was seen when Rac1 was inactivated using a limb-specific Cre driver line [Suzuki et al., 2009]. At the cellular level, the main phenotype of Rac1-deficient chondrocytes was a reduction in cellular proliferation, likely due (at least in part) to lower expression of the cell cycle promoter cyclin D1 [Wang et al., 2007]. Preliminary data suggest that the reduction in cyclin D1 expression is secondary to upregulation of ATF3 (G. Wang and F. Beier, in prep.), a transcription factor that suppresses activity of the cyclin D1 promoter in chondrocytes[James et al., 2006]. Hypertrophic differentiation appeared to be less affected, and expression of some hypertrophic markers was upregulated, which was somewhat unexpected in light of the published in vitro data and highlights the need for in vivo loss-of-function studies to determine the role of these proteins in the physiological context of intact cartilage. In addition, cartilage-specific ablation of Rac1 resulted in increased apoptosis. In contrast to Rac1, no in vivo models for the study of RhoA or Cdc42 function in cartilage have been published.
Not surprisingly, several roles of the Rho/ROCK pathway in articular cartilage have been described, but studies on the other family members in the mature joint are still lacking. Recent manuscripts demonstrated activation of chondrocyte RhoA by diverse stimuli such as transforming growth factor α [Appleton et al., 2010], the cytokine interleukin 1α [Novakofski et al., 2009] and dynamic compression [Haudenschild et al., 2008], while insulin-like growth factor 1 was reported to repress RhoA activity [Novakofski et al., 2009]. ROCK inhibition by the pharmacological inhibitor Y27632 increased Sox9 expression in articular chondrocytes [Tew and Hardingham, 2006], similar to the effects observed during chondrogenesis. In agreement with these data, inhibition of RhoA by the toxin C3 transferase also increased expression of Sox9 and its target genes collagen II and aggrecan in articular chondrocytes [Novakofski et al., 2009]. However, in other studies RhoA/ROCK activation by dynamic compression resulted in direct phosphorylation and activation of Sox9 by ROCK [Haudenschild et al., 2010; Haudenschild et al., 2008]. In yet another study, we recently demonstrated that transforming growth factor alpha (TGFα) activates RhoA and downstream pathways in chondrocytes, both in monolayer culture and three-dimensional explant culture. Inhibition of Rho or ROCK blocked the induction of stress fiber formation and blocked the increase in cell number in response to TGFα, but did not affect the TGFα repression of Sox9 expression [Appleton et al., 2010]. Most interestingly, the ROCK inhibitor Y27632 blocked the collagen II and aggrecan degradation mediated by TGFα in explant cultures, suggesting the potential therapeutic application of this inhibitor in osteoarthritis. This occurred despite upregulation of MMP-13 expression in response to Y27632 both in this study and in embryonic micromass cultures [Wang et al., 2004a], suggesting the involvement of additional targets of ROCK signaling. For example, it will be of interest to examine whether Rho signaling controls the expression, localization or activity of tissue inhibitors of metalloproteinases (TIMPs) or additional proteins involved in the activation of MMP13 and other proteases in cartilage.
All together, the somewhat contradictory results from the various studies summarized here clearly indicate the need for a deeper understanding of the function of the Rho/ROCK pathway in articular cartilage. One potential explanation for the discrepancy in results is that the role of Rho/ROCK in articular cartilage is context-dependent, both with regards to culture conditions (e.g. two- versus three-dimensional cultures) and the presence of specific growth factors and cytokines. We have described similar context-dependency during chondrogenesis [Woods and Beier, 2006]. Overall, available data clearly point to the need for more in vivo studies, where cells embedded in an authentic extracellular matrix are exposed to appropriate growth factors and experience physiological biomechanical loads.
A possible role of Rho signaling in articular cartilage and osteoarthritis also emerged from a completely different line of research. RhoB is a close relative to RhoA, inducing similar biological responses. Interestingly, two manuscripts suggest an association of a single nucleotide polymorphism in the promoter region of the human RhoB gene with osteoarthritis in some patient populations [Mahr et al., 2006; Shi et al., 2008], while another study could not replicate this association [Loughlin et al., 2007]. These findings raise the possibility that altered expression of RhoB could contribute to OA, at least in some subsets of patients, but further studies are required to resolve this issue.
Numerous open questions on the role of Rho GTPases in cartilage development and osteoarthritis remain. There is an urgent need to define the downstream pathways connecting Rho GTPases to their various cellular responses, as well as the upstream events and players (e.g. receptors, GEFs and GAPs) linking GTPases to specific extracellular signals. The role of additional Rho family members in cartilage have to be addressed, in particular since microarray data show that several of them undergo changes in expression during chondrocyte differentiation in vitro and in vivo [James et al., 2005; James et al., 2010]. Maybe most importantly, in vivo models (e.g. tissue-specific knockout mice) for genes other than Rac1 have to be described, ideally including specific gene inactivation in adult articular cartilage and arthritis models. Finally, the value of drugs targeting Rho pathways in the treatment of OA or other forms of arthritis needs to be studied. Based on results in explant culture [Appleton et al., 2010] and the fact that ROCK inhibitors are under active investigation for a plethora of other diseases [Olson, 2008], Y27632 and related compounds are strong candidates for these purposes. However, in light of the caveats described above and the discussed discrepancies in the literature, more research is clearly required before any such agents can be seriously considered for the treatment of cartilage disorders or osteoarthritis.
INTEGRIN LINKED KINASE: AN ADDITIONAL MEDIATOR OF EXTRACELLULAR MATRIX SIGNALING IN CHONDROCYTES?
The consequences of cartilage-specific Rac1 deletion [Wang et al., 2007] resembled those of inactivation of the gene for β1 integrin in chondrocytes [Aszodi et al., 2003], further supporting the model that Rho GTPases are important mediators of extracellular matrix signaling in chondrocytes. Two groups reported the phenotypes of cartilage-specific deletion of another gene involved in integrin signaling, integrin-linked kinase or ILK [Grashoff et al., 2003; Terpstra et al., 2003]. These mice display similar phenotypes as the two lines described above, including dwarfism, disorganized growth plates, reduced chondrocyte proliferation and lower levels of cyclin D1. Therefore, it seems plausible that β1 integrin, Rac1 and ILK act in the same pathway in cartilage, but this model has not been proven by either biochemical or genetic means. Moreover, despite the obvious importance of ILK in cartilage development, no follow-up studies on this role or on a possible function of ILK in articular cartilage have been published. Finally, it should be mentioned that it is a matter of ongoing debate whether ILK exerts its biological roles through its kinase activity or through other, kinase-independent mechanisms [Wickstrom et al., 2010].
THE PI-3 KINASE-AKT PATHWAY
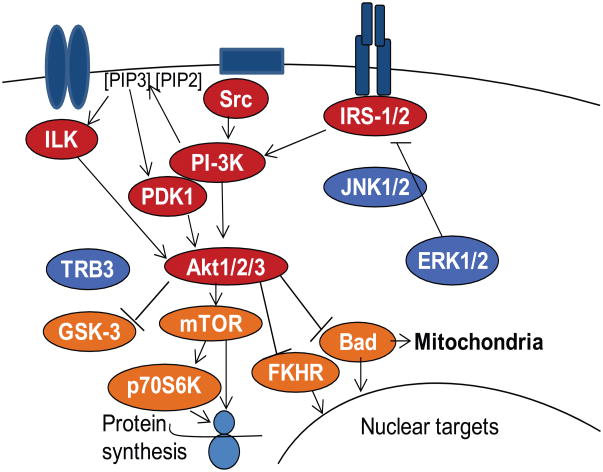
ILK has, however, been implicated in another signaling pathway whose importance in both developing and articular cartilage is becoming increasingly evident, the phosphoinositide-3 (PI-3) kinase-Akt pathway [Delcommenne et al., 1998]. PI-3 kinase and its downstream kinases Akt1-3 are activated by a large number of receptors, but most notably by tyrosine receptor kinases such as the insulin-like growth factor 1 receptor (IGF1R) (Fig. 2). They control a large number of cellular functions including proliferation, differentiation, matrix synthesis and survival in a cell type-specific manner.
Fig. 2.
Signaling through the PI-3 Kinase-Akt pathway. Several different types of receptors can activate PI-3 kinase-Akt signaling through upstream mediators including ILK, Src, and IRS1/2. PI-3 kinase can directly activate Akt or indirectly through activation of PDK1 via the conversion of PIP2 to PIP3 which is mediated by PI-3 kinase. Akt phosphorylates numerous downstream signaling proteins resulting in positive regulation of mTOR and negative regulation of GSK-3, FKHR, and Bad among other targets. Inhibition of PI-3 kinase-Akt signaling includes ERK1/2 and JNK1/2 inhibition of IRS-1 as well as Akt inhibition by TRB3. Not shown is the regulation of these pathways by phosphatases which include PTEN, SHP-2, and PP2A, among others.
During cartilage development, the majority of published studies suggest that PI-3 kinase signaling is required for normal hypertrophic cell differentiation and endochondral bone growth. For example, the PI-3 kinase inhibitor LY294002 blocks both basal growth and the anabolic effects of parathyroid hormone, C-type natriuretic peptide and IGF1, respectively, in explant cultures [Harrington et al., 2010; MacRae et al., 2007; Ulici et al., 2008]. In tibial explants, this growth retardation is accompanied by a significant shortening of the hypertrophic zone of the growth plate [Ulici et al., 2008], indicating that PI-3 kinase is required both for cellular growth and hypertrophy and potentially for survival of hypertrophic chondrocytes. In cell systems, PI-3 kinase has been shown to act both upstream and downstream of the transcription factor Runx2 to promote chondrocyte hypertrophy [Fujita et al., 2004], providing a molecular basis for the observed effects in explant culture. Genome-wide gene expression analyses of the effects of LY294002 on embryonic chondrocytes further supports the role of PI-3 kinase signaling in chondrocyte hypertrophy [Ulici et al., 2010]. However, at least one study found the opposite effect where PI-3 kinase-Akt blocked terminal differentiation [Keisuke et al., 2008].
The reasons for this discrepancy in findings are unknown. However, in vivo results support the differentiation-promoting activities of the PI-3 kinase pathway in developing cartilage. Most notably, overexpression of an activated form of Akt in the cartilage of transgenic mice promoted chondrocyte differentiation whereas overexpression of a dominant-negative form delayed this process [Rokutanda et al., 2009]. Determination of the specific roles of the three Akt genes in cartilage has been less successful. While Akt1-deficient mice are smaller than controls, they only show very subtle changes in their growth plates [Kawamura et al., 2007; Ulici et al., 2009], suggesting that Akt2 and/or Akt3 partially compensate for Akt1 in chondrocyte differentiation. The main difference found in one study [Ulici et al., 2009] was a delay in formation of secondary ossification centers, possibly secondary to reduced levels of MMP14. On the other hand, cartilage-specific inactivation of PTEN, the main phosphatase counteracting PI-3 kinase activity, leads to profound defects in skeletal development as shown by three independent studies [Ford-Hutchinson et al., 2007; Hsieh et al., 2009; Yang et al., 2008]. These mice showed skeletal overgrowth, consistent with over-activation of the PI-3 kinase pathway. Growth plates were disorganized and showed accelerated hypertrophic differentiation of chondrocytes. Strikingly, the primary and secondary ossification centers of these mice fused, an event that does not normally occur in wild type mice (in contrast to humans). In addition, cartilage-specific loss of PTEN, which is an important tumor suppressor in many tissues, also resulted in formation of lipoma and enchondroma-like features. Overall, the phenotype of these mice supports a model where PI-3 kinase-Akt signaling promotes chondrocyte hypertrophy and endochondral bone growth while PTEN is a physiological antagonist or break to these activities.
In adult articular cartilage, PI-3 kinase-Akt signaling promotes matrix synthesis and chondrocyte survival. Expression of constitutively active Akt in human articular chondrocytes resulted in significant increases in proteoglycan synthesis, type II collagen synthesis and expression, as well as Sox9 expression [Yin et al., 2009]. Inhibition of PI-3 kinase-Akt signaling has been shown to inhibit chondrocyte proteoglycan synthesis and reduce chondrocyte survival [Cravero et al., 2009; Oh and Chun, 2003; Starkman et al., 2005; Yin et al., 2009]. Recently, we found evidence for increased expression of an Akt inhibitor called TRB3 (tribbles homolog) in human osteoarthritic cartilage [Cravero et al., 2009]. TRB3 is a pseudokinase that contains a truncated kinase domain and binding of TRB3 to Akt inhibits Akt phosphorylation and activation, resulting in reduced chondrocyte proteoglycan synthesis and survival [Cravero et al., 2009]. Endoplasmatic Reticulum stress was shown to stimulate TRB3 expression, providing a mechanism for reduced PI-3 kinase-Akt signaling and subsequent matrix synthesis under stress conditions.
In chondrocytes, a key activator of the PI-3 Kinase-Akt pathway is IGF-I. With increasing age and during the development of osteoarthritis, chondrocytes produce less matrix in response to IGF-I and display a reduced survival response to IGF-I, suggesting a state of IGF resistance [Loeser, 2009]. Compared to cells from young animals, aged equine chondrocytes demonstrated less Akt phosphorylation in response to IGF-I while ERK phosphorylation did not differ [Boehm et al., 2007]. When comparing chondrocytes isolated from normal adult cartilage to those from OA tissue, we noted significantly less activation of the PI-3 kinase-Akt pathway in OA cells in response to IGF-I. In contrast, ERK was transiently phosphorylated in normal cells after IGF-I treatment and constitutively phosphorylated in OA cells [Yin et al., 2009]. Inducing oxidative stress in normal cells resulted in changes in signaling that mirrored that seen in untreated OA cells, suggesting that oxidative stress in OA chondrocytes was responsible for inhibiting IGF-I activation of the PI-3 kinase-Akt pathway. In support of this, treatment of OA chondrocytes with anti-oxidants restored the activation of Akt and the subsequent increase in proteoglycan production.
Another mechanism for altered PI-3 kinase-Akt signaling in OA chondrocytes may be a reduced level of the protein deacetylase SirT1 in OA [Gagarina et al., 2010]. Restoration of SirT1in OA chondrocytes by transfection was shown to activate the IGF type I receptor and subsequent downstream phosphorylation of PI-3 kinase, PDK1, and Akt as well as mTOR, a downstream target of Akt. The ability of SirT1 to activate this pathway appeared to be related to down-regulation of the phosphatase PTP1B which was found to be increased in OA chondrocytes. Repression of PTP1B by SirT1 improved chondrocyte survival consistent with the survival promoting activity of the PI-3 kinase-Akt pathway in chondrocytes.
The PI-3 kinase-Akt pathway has been shown to regulate the response of articular chondrocytes to ligands in addition to IGF-I. TGF-β was shown to increase the phosphorylation of Akt, but unlike IGF-I, where Akt phosphorylation is noted within minutes of stimulation, TGF-β induced phosphorylation of Akt was not detected until about 4 hours [Qureshi et al., 2007] consistent with an indirect mechanism of activation, perhaps through induction of an autocrine loop. Inhibition of PI-3 kinase, Akt, or mTOR was found to block the ability of TGF-β to stimulate expression of the tissue inhibitor of metalloproteinase-3. In another study, the cytokines oncostatin M (OSM) and IL-6 (combined with sIL-6R) where shown to stimulate Akt phosphorylation within 20 minutes of treatment and this was blocked with the PI-3 kinase inhibitor LY294002 [Litherland et al., 2008]. Blocking PI-3 kinase also inhibited the ability of IL-1+OSM to induce collagenase activity through inhibition of MMP-1 and MMP-13 expression. A role for the p110α subunit of PI-3 kinase and for Akt1 in this process was suggested by the results of siRNA experiments. These studies demonstrate that activation of the PI-3 kinase-Akt pathway can have either anabolic or catabolic effects that are ligand-dependent and indicate that additional studies are needed to identify the pathways that cross-talk with the PI-3 kinase-Akt pathway resulting in these differential effects. In addition, TGFα induced Akt phosphorylation in chondrocytes; inhibition of PI-3 kinase activity by LY294002 blocked TGFα-induced chondrocyte proliferation, but further increased the stimulation of MMP13 mRNA levels by this growth factor [Appleton et al., 2010]. These data provide further support for the concept of context-specific activity of the PI3-kinase-Akt pathway in cartilage.
A recent study [Fukai et al., 2010] has examined the development of OA-like changes in Akt −/− mice. As noted above, the Akt1 deficient mice exhibited dwarfism which appeared to be related to reduced calcification in the growth plates. When OA was induced in 8-week old mice by medial collateral ligament transection with removal of the medial meniscus, no difference in cartilage degradation was noted between wild-type and Akt1deficient mice at 8 weeks after surgery. However, the Akt1−/− mice formed fewer osteophytes suggesting a role for Akt1 in the endochondral ossification process that results in osteophytes formation. Since OA was induced in very young dwarf mice the relevance of these findings to adult human OA is not clear and suggest further work is needed including inactivation of Akt in adult mice after skeletal development has been completed.
The downstream effects of Akt activation are mediated by several Akt substrates. One of them is the kinase mTOR (mammalian target of rapamycin). Inhibition of mTOR resulted in decreased bone growth and hypertrophy in rat metatarsal cultures as well as in vivo, possibly due to reduced expression of indian hedgehog, a key regulator of chondrocyte differentiation [Chanika et al., 2008]. Thus, mTOR inhibition resulted in similar effects as blockade of PI-3 kinase or Akt signaling, which was also supported by another publication [Rokutanda et al., 2009]. However, a third in vivo study using application of the mTOR inhibitor rapamycin in rats also showed reduced bone growth, but in this case through a reduction in chondrocyte proliferation, without effects on the hypertrophic zone and with increased expression of indian hedgehog [Sanchez and He, 2009]. Reasons for these seemingly contradictory findings could include dose-specific effects of rapamycin or different maturation stages of the employed models. In adult articular chondrocytes, inhibition of mTOR resulted in significant inhibition of IGF-I mediated proteoglycan synthesis [Starkman et al., 2005]. Therefore, the available data support a critical role of mTOR downstream of PI-3 kinase-Akt in cartilage development and likely in adult cartilage homeostasis as well.
PI-3 kinase-Akt also signal through numerous other substrates that could contribute to their effects on cartilage, including glycogen synthase kinase 3 (GSK3), FoxO transcription factors and others. While many of these have been implicated in cartilage development, they are often also involved in other pathways (for example, GSK3 in Wnt signaling), and their specific contributions to PI-3 kinase signaling are therefore less well defined. Elucidation of the exact connections between these pathways and identification of critical target genes are urgent tasks for the future. In addition, and similar to the Rho GTPases discussed above, in-depth analyses of appropriate in vivo models (including double- or triple KO mice and tissue-/stage-specific KO models) will also be required to fully understand these pathways and to potentially utilize this information therapeutically.
MITOGEN ACTIVATED PROTEIN KINASES
MAP kinases including ERK, JNK, and p38 have been implicated in the regulation of chondrocyte signaling in developing and adult articular cartilage. They are activated by a diverse array of receptors that include various receptor tyrosine kinases, G-protein coupled receptors, cytokine receptors, and integrins. Depending on the ligand initiating the signaling and the context in which activation occurs, the MAP kinases regulate multiple processes that include chondrocyte proliferation, cartilage matrix remodeling (synthesis and/or degradation), and cell death. There is evidence for activity of all three MAP kinases in the pathological state of osteoarthritis. Levels of phosphorylated JNK [Clancy et al., 2001; Fan et al., 2007] and p38 [Fan et al., 2007] were higher in human OA cartilage compared to normal while phosphorylated ERK was found in both OA and normal cartilage. Importantly, each of the MAP kinases has more than one isoform expressed by chondrocytes but there has been little investigation of isoform specific functions or a determination of which isoforms might be more important in pathological states.
Like most cell types, chondrocytes express both ERK1 and ERK2. Knockout studies in mice have not been very informative with regards to function of individual ERK genes in cartilage. While ERK1-deficient mice have a subtle phenotype with no obvious skeletal or growth abnormalities [Pag et al., 1999], ERK2 null mice die early in development because of trophoblast deficiencies[Saba-El-Leil et al., 2003]. Activation of ERK1/2 through expression of constitutively active MEK1 resulted in achondroplasia-like dwarfism in mice [Murakami et al., 2004].
Chondrocytes express the MAP kinases JNK1 and JNK2 and deletion of either isoform has not been reported to be associated with a skeletal phenotype. Overexpression of activated MKK6, an upstream activator of p38, in cartilage of transgenic mice resulted in dwarfism, inhibition of both chondrocyte proliferation and differentiation, and a delay in primary and secondary ossification[Zhang et al., 2006]. Inhibition of p38 by cartilage-specific expression of a dominant-negative construct that interferes with p38 activation (type IIA collagen-p38 DN, isoform specificity not known) resulted in death shortly after birth in homozygotes due to deficient endochondral bone formation while heterozygotes had reduced limb length but lived a normal lifespan [Namdari et al., 2008]. When the DN p38 heterozygotes were examined at 1 year of age, they were found to have articular cartilage defects in the knee joints not seen in wild-type controls, indicating that life-long p38 deficiency was detrimental to articular cartilage.
In adult articular chondrocytes, MAP kinase activation has been associated with increased expression of matrix metalloproteinases (MMPs) and aggrecanases. Induction of matrix degrading enzymes by various cytokines [Fan et al., 2007; Mengshol et al., 2000; Sondergaard et al., 2010], by type II collagen binding to the discoidin domain receptor [Xu et al., 2007] and by fibronectin and collagen fragments [Loeser et al., 2003] requires MAP kinase activation. The majority of stimuli that mediate cartilage matrix destruction activate more than one specific MAP kinase, but inhibition of a single MAP kinase is often sufficient to inhibit the increased MMP or aggrecanase expression. For example, stimulation of the chondrocyte α5β1 integrin by fibronectin fragments activates all three major MAP kinases (ERK1/2, p38α, and JNK1/2) while inhibition of any one of the three is sufficient to inhibit fibronectin fragment stimulation of MMP-13 expression [Forsyth et al., 2002; Loeser et al., 2003]. Similar to fibronectin fragments, stimulation of MMP-13 by bFGF required activity of ERK, p38, and JNK [Im et al., 2007; Wang et al., 2004b], while IL-1 stimulation of MMP-13 required p38 and JNK but not ERK [Mengshol et al., 2000]. These findings are consistent with the hypothesis that expression of matrix degrading enzymes is under tight control such that multiple switches (signaling proteins) must be on in order to induce MMP expression. This would explain why a stimulus that only activates one MAP kinase, such as ERK activation by IGF-I, is not sufficient to induce MMP expression. In addition to the regulation of catabolic signaling, certain MAP kinases are also involved in the regulation of chondrocyte anabolic activity, although most often this is a negative regulation. IGF-I stimulates transient ERK1/2 phosphorylation which in normal chondrocytes declines at around 30 minutes after stimulation when IL-1 stimulation of ERK phosphorylation is still active [Starkman et al., 2005]. Inhibition of ERK phosphorylation using MEK inhibitors can increase chondrocyte proteoglycan synthesis [Starkman et al., 2005], while activation of ERK by constitutively active MEK significantly decreases proteoglycan synthesis, aggrecan expression, and Sox9 expression [Yin et al., 2009]. LPS activation of toll-like receptor 4 can also inhibit chondrocyte proteoglycan synthesis, and under these conditions inhibition of p38, but not ERK, partially restored it [Bobacz et al., 2007]. In contrast, the down-regulation of type II collagen and aggrecan expression by IL-6 plus sIL-6R was blocked by JAK/STAT but not ERK inhibition [Legendre et al., 2003]. The latter finding should be interpreted with some caution, however, since the use of PD098059 only partially inhibited IL-6/sIL-6R activation of ERK.
Importantly, none of the described pathways (or any of the pathways not covered here) acts in isolation. Numerous pathways are activated in a cell at any given time, and crosstalk between pathways, positive and negative feedback loops and integration of multiple signals all contribute to ultimately determine the behavior of the cell. In addition, many signaling proteins that are regulated by phosphorylation have multiple serine/threonine and/or tyrosine sites which control the activity of the protein and which can be activated by different pathways.
An example of pathway cross-talk is the IGF-I regulation of matrix synthesis by adult articular chondrocytes. IGF-I stimulation of normal articular chondrocytes results in rapid (within minutes) and prolonged (at least 60 minutes) Akt phosphorylation and transient (5–30 minute) ERK phosphorylation [Yin et al., 2009]. Activation of PI-3 kinase-Akt signaling by IGF-I requires upstream activation of IRS-1 at activating tyrosine sites. Sustained ERK phosphorylation seen in OA cells or in normal cells under conditions of oxidative stress, inhibits Akt phosphorylation through phosphorylation of IRS-1 at inhibitory serine residues (S312 and S616) [Yin et al., 2009]. The balance of PI-3 kinase-Akt to ERK MAP kinase activation can control the chondrocyte’s production of matrix proteins such as proteoglycans. Conditions which favor PI-3 kinase-Akt activation will promote matrix synthesis while those favoring ERK activation over PI-3 kinase-Akt will be inhibitory.
We are far from a comprehensive understanding of the complex interactions among cell signaling pathways and their spatial and temporal dynamics, making further research into this area a high priority. Because of the complexities involved, a systems biology approach will be needed in order to integrate large data sets of cell signaling proteins and their activities under various conditions. This approach is just beginning to be used to study the interactions of multiple signaling pathways involved in cancer and the knowledge gained from such work, in particular the computational methods developed, may be applicable to the study of signaling ways in chondrocytes. The eventual goal will be to discover key nodes in cell signaling pathways that could be targeted with small molecule inhibitors to restore the balance in signaling that is disrupted in pathological conditions of cartilage such as osteoarthritis.
Acknowledgments
This work was supported by NIH grants AG16697 and AR49003 and by funds from the Canadian Institutes of Health Research, The Arthritis Society and the Canada Research Chairs Program.
References
- Appleton CT, Usmani SE, Mort JS, Beier F. Rho/ROCK and MEK/ERK activation by transforming growth factor-alpha induces articular cartilage degradation. Lab Invest. 2010;90:20–30. doi: 10.1038/labinvest.2009.111. [DOI] [PubMed] [Google Scholar]
- Aszodi A, Hunziker EB, Brakebusch C, Fassler R. Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 2003;17:2465–79. doi: 10.1101/gad.277003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobacz K, Sunk IG, Hofstaetter JG, Amoyo L, Toma CD, Akira S, Weichhart T, Saemann M, Smolen JS. Toll-like receptors and chondrocytes: the lipopolysaccharide-induced decrease in cartilage matrix synthesis is dependent on the presence of toll-like receptor 4 and antagonized by bone morphogenetic protein 7. Arthritis Rheum. 2007;56:1880–93. doi: 10.1002/art.22637. [DOI] [PubMed] [Google Scholar]
- Bobick BE, Kulyk WM. Regulation of cartilage formation and maturation by mitogen-activated protein kinase signaling. Birth Defects Research Part C: Embryo Today: Reviews. 2008;84:131–154. doi: 10.1002/bdrc.20126. [DOI] [PubMed] [Google Scholar]
- Boehm AK, Seth M, Mayr KG, Fortier LA. Hsp90 mediates insulin-like growth factor 1 and interleukin-1beta signaling in an age-dependent manner in equine articular chondrocytes. Arthritis Rheum. 2007;56:2335–43. doi: 10.1002/art.22664. [DOI] [PubMed] [Google Scholar]
- Chanika P, Ke-Ying W, Valerie A, Qian C, Philip AG. mTOR signaling contributes to chondrocyte differentiation. Developmental Dynamics. 2008;237:702–712. doi: 10.1002/dvdy.21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy R, Rediske J, Koehne C, Stoyanovsky D, Amin A, Attur M, Iyama K, Abramson SB. Activation of stress-activated protein kinase in osteoarthritic cartilage: evidence for nitric oxide dependence. Osteoarthritis Cartilage. 2001;9:294–9. doi: 10.1053/joca.2000.0388. [DOI] [PubMed] [Google Scholar]
- Cravero JD, Carlson CS, Im HJ, Yammani RR, Long D, Loeser RF. Increased expression of the Akt/PKB inhibitor TRB3 in osteoarthritic chondrocytes inhibits insulin-like growth factor 1-mediated cell survival and proteoglycan synthesis. Arthritis Rheum. 2009;60:492–500. doi: 10.1002/art.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Soder S, Oehler S, Fundel K, Aigner T. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am J Pathol. 2007;171:938–46. doi: 10.2353/ajpath.2007.061083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Hutchinson AF, Ali Z, Lines SE, Hallgrimsson B, Boyd SK, Jirik FR. Inactivation of Pten in osteo-chondroprogenitor cells leads to epiphyseal growth plate abnormalities and skeletal overgrowth. J Bone Miner Res. 2007;22:1245–59. doi: 10.1359/jbmr.070420. [DOI] [PubMed] [Google Scholar]
- Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–76. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- Fujita T, Azuma Y, Fukuyama R, Hattori Y, Yoshida C, Koida M, Ogita K, Komori T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004;166:85–95. doi: 10.1083/jcb.200401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai A, Kawamura N, Saito T, Oshima Y, Ikeda T, Kugimiya F, Higashikawa A, Yano F, Ogata N, Nakamura K, Chung UI, Kawaguchi H. Akt1 in murine chondrocytes controls cartilage calcification during endochondral ossification under physiologic and pathologic conditions. Arthritis Rheum. 2010;62:826–836. doi: 10.1002/art.27296. [DOI] [PubMed] [Google Scholar]
- Gagarina V, Gabay O, Dvir-Ginzberg M, Lee EJ, Brady JK, Quon MJ, Hall DJ. SirT1 enhances survival of human osteoarthritic chondrocytes by repressing PTP1B and activating the IGF receptor pathway. Arthritis Rheum. 2010 doi: 10.1002/art.27369. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C, Aszodi A, Sakai T, Hunziker EB, Fassler R. Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep. 2003;4:432–8. doi: 10.1038/sj.embor.embor801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EK, Coon DJ, Kern MF, Svoboda KK. PTH stimulated growth and decreased Col-X deposition are phosphotidylinositol-3,4,5 triphosphate kinase and mitogen activating protein kinase dependent in avian sterna. Anat Rec (Hoboken) 2010;293:225–34. doi: 10.1002/ar.21072. [DOI] [PubMed] [Google Scholar]
- Haudenschild DR, Chen J, Pang N, Lotz MK, D’Lima DD. Rho kinase-dependent activation of SOX9 in chondrocytes. Arthritis Rheum. 2010;62:191–200. doi: 10.1002/art.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild DR, Nguyen B, Chen J, D’Lima DD, Lotz MK. Rho kinase-dependent CCL20 induced by dynamic compression of human chondrocytes. Arthritis Rheum. 2008;58:2735–42. doi: 10.1002/art.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hsieh SC, Chen NT, Lo SH. Conditional loss of PTEN leads to skeletal abnormalities and lipoma formation. Mol Carcinog. 2009;48:545–52. doi: 10.1002/mc.20491. [DOI] [PubMed] [Google Scholar]
- Im H-J, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, van Wijnen AJ, Loeser RF. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J Biol Chem. 2007;282:11110–21. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CG, Appleton CT, Ulici V, Underhill TM, Beier F. Microarray analyses of gene expression during chondrocyte differentiation identifies novel regulators of hypertrophy. Mol Biol Cell. 2005;16:5316–33. doi: 10.1091/mbc.E05-01-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CG, Stanton LA, Agoston H, Ulici V, Underhill TM, Beier F. Genome-wide analyses of gene expression during mouse endochondral ossification. PLoS One. 2010;5:e8693. doi: 10.1371/journal.pone.0008693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CG, Woods A, Underhill TM, Beier F. The transcription factor ATF3 is upregulated during chondrocyte differentiation and represses cyclin D1 and A gene transcription. BMC Mol Biol. 2006;7:30. doi: 10.1186/1471-2199-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura N, Kugimiya F, Oshima Y, Ohba S, Ikeda T, Saito T, Shinoda Y, Kawasaki Y, Ogata N, Hoshi K, Akiyama T, Chen WS, Hay N, Tobe K, Kadowaki T, Azuma Y, Tanaka S, Nakamura K, Chung U-i, Kawaguchi H. Akt1 in Osteoblasts and Osteoclasts Controls Bone Remodeling. PLoS One. 2007;2:e1058. doi: 10.1371/journal.pone.0001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keisuke K, Tohru K, Norimasa N, Hideki Y, Toru N. PI3K/Akt signaling as a key regulatory pathway for chondrocyte terminal differentiation. Genes to Cells. 2008;13:839–850. doi: 10.1111/j.1365-2443.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- Kerr BA, Otani T, Koyama E, Freeman TA, Enomoto-Iwamoto M. Small GTPase protein Rac-1 is activated with maturation and regulates cell morphology and function in chondrocytes. Exp Cell Res. 2008;314:1301–12. doi: 10.1016/j.yexcr.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Lassar AB. The transcriptional activity of Sox9 in chondrocytes is regulated by RhoA signaling and actin polymerization. Mol Cell Biol. 2009;29:4262–73. doi: 10.1128/MCB.01779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre F, Dudhia J, Pujol JP, Bogdanowicz P. JAK/STAT but not ERK1/ERK2 pathway mediates interleukin (IL)-6/soluble IL-6R down-regulation of Type II collagen, aggrecan core, and link protein transcription in articular chondrocytes. Association with a down-regulation of SOX9 expression. J Biol Chem. 2003;278:2903–12. doi: 10.1074/jbc.M110773200. [DOI] [PubMed] [Google Scholar]
- Litherland GJ, Dixon C, Lakey RL, Robson T, Jones D, Young DA, Cawston TE, Rowan AD. Synergistic Collagenase Expression and Cartilage Collagenolysis Are Phosphatidylinositol 3-Kinase/Akt Signaling-dependent. J Biol Chem. 2008;283:14221–9. doi: 10.1074/jbc.M710136200. [DOI] [PubMed] [Google Scholar]
- Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971–9. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem. 2003;278:24577–85. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin J, Meulenbelt I, Min J, Mustafa Z, Sinsheimer JS, Carr A, Slagboom PE. Genetic association analysis of RHOB and TXNDC3 in osteoarthritis. Am J Hum Genet. 2007;80:383–6. doi: 10.1086/511443. author reply 386–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten FP, Tylzanowski P, Lories RJ. Wnt signaling and osteoarthritis. Bone. 2009;44:522–7. doi: 10.1016/j.bone.2008.12.006. [DOI] [PubMed] [Google Scholar]
- MacRae VE, Ahmed SF, Mushtaq T, Farquharson C. IGF-I signalling in bone growth: Inhibitory actions of dexamethasone and IL-1[beta] Growth Hormone IGF Res. 2007;17:435–439. doi: 10.1016/j.ghir.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Mahr S, Burmester GR, Hilke D, Gobel U, Grutzkau A, Haupl T, Hauschild M, Koczan D, Krenn V, Neidel J, Perka C, Radbruch A, Thiesen HJ, Muller B. Cis- and trans-acting gene regulation is associated with osteoarthritis. Am J Hum Genet. 2006;78:793–803. doi: 10.1086/503849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–11. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Murakami S, Balmes G, McKinney S, Zhang Z, Givol D, de Crombrugghe B. Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype. Genes Dev. 2004;18:290–305. doi: 10.1101/gad.1179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namdari S, Wei L, Moore D, Chen Q. Reduced limb length and worsened osteoarthritis in adult mice after genetic inhibition of p38 MAP kinase activity in cartilage. Arthritis Rheum. 2008;58:3520–9. doi: 10.1002/art.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakofski K, Boehm A, Fortier L. The small GTPase Rho mediates articular chondrocyte phenotype and morphology in response to interleukin-1alpha and insulin-like growth factor-I. J Orthop Res. 2009;27:58–64. doi: 10.1002/jor.20717. [DOI] [PubMed] [Google Scholar]
- Oh CD, Chun JS. Signaling mechanisms leading to the regulation of differentiation and apoptosis of articular chondrocytes by insulin-like growth factor-1. J Biol Chem. 2003;278:36563–71. doi: 10.1074/jbc.M304857200. [DOI] [PubMed] [Google Scholar]
- Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20:242–8. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès G, Guérin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouyssegur J. Defective Thymocyte Maturation in p44 MAP Kinase (Erk 1) Knockout Mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- Qureshi HY, Ahmad R, Sylvester J, Zafarullah M. Requirement of phosphatidylinositol 3-kinase/Akt signaling pathway for regulation of tissue inhibitor of metalloproteinases-3 gene expression by TGF-beta in human chondrocytes. Cell Signal. 2007;19:1643–51. doi: 10.1016/j.cellsig.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Rokutanda S, Fujita T, Kanatani N, Yoshida CA, Komori H, Liu W, Mizuno A, Komori T. Akt regulates skeletal development through GSK3, mTOR, and FoxOs. Dev Biol. 2009;328:78–93. doi: 10.1016/j.ydbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Saba-El-Leil MK, Vella FD, Vernay B, Voisin L, Chen L, Labrecque N, Ang SL, Meloche S. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4:964–8. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, He Y-Z. Bone growth during rapamycin therapy in young rats. BMC Pediatrics. 2009;9:3. doi: 10.1186/1471-2431-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Nakamura T, Nakajima M, Dai J, Qin J, Ni H, Xu Y, Yao C, Wei J, Liu B, Ikegawa S, Jiang Q. Association of single-nucleotide polymorphisms in RHOB and TXNDC3 with knee osteoarthritis susceptibility: two case-control studies in East Asian populations and a meta-analysis. Arthritis Res Ther. 2008;10:R54. doi: 10.1186/ar2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondergaard BC, Schultz N, Madsen SH, Bay-Jensen AC, Kassem M, Karsdal MA. MAPKs are essential upstream signaling pathways in proteolytic cartilage degradation -divergence in pathways leading to aggrecanase and MMP-mediated articular cartilage degradation. Osteoarthritis Cartilage. 2010;18:279–288. doi: 10.1016/j.joca.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Starkman BG, Cravero JD, Delcarlo M, Loeser RF. IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem J. 2005;389:723–9. doi: 10.1042/BJ20041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki D, Yamada A, Amano T, Yasuhara R, Kimura A, Sakahara M, Tsumaki N, Takeda S, Tamura M, Nakamura M, Wada N, Nohno T, Shiroishi T, Aiba A, Kamijo R. Essential mesenchymal role of small GTPase Rac1 in interdigital programmed cell death during limb development. Dev Biol. 2009;335:396–406. doi: 10.1016/j.ydbio.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Teixeira CC, Agoston H, Beier F. Nitric oxide, C-type natriuretic peptide and cGMP as regulators of endochondral ossification. Dev Biol. 2008;319:171–8. doi: 10.1016/j.ydbio.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra L, Prud’homme J, Arabian A, Takeda S, Karsenty G, Dedhar S, St-Arnaud R. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J Cell Biol. 2003;162:139–48. doi: 10.1083/jcb.200302066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew SR, Hardingham TE. Regulation of SOX9 mRNA in human articular chondrocytes involving p38 MAPK activation and mRNA stabilization. J Biol Chem. 2006;281:39471–9. doi: 10.1074/jbc.M604322200. [DOI] [PubMed] [Google Scholar]
- Ulici V, Hoenselaar KD, Agoston H, McErlain DD, Umoh J, Chakrabarti S, Holdsworth DW, Beier F. The role of Akt1 in terminal stages of endochondral bone formation: angiogenesis and ossification. Bone. 2009;45:1133–45. doi: 10.1016/j.bone.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Ulici V, Hoenselaar KD, Gillespie JR, Beier F. The PI3K pathway regulates endochondral bone growth through control of hypertrophic chondrocyte differentiation. BMC Dev Biol. 2008;8:40. doi: 10.1186/1471-213X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulici V, James CG, Hoenselaar KD, Beier F. Regulation of Gene Expression by PI3K in Mouse Growth Plate Chondrocytes. PLoS One. 2010;5:e8866. doi: 10.1371/journal.pone.0008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Wang G, Beier F. Rac1/Cdc42 and RhoA GTPases antagonistically regulate chondrocyte proliferation, hypertrophy, and apoptosis. J Bone Miner Res. 2005;20:1022–31. doi: 10.1359/JBMR.050113. [DOI] [PubMed] [Google Scholar]
- Wang G, Woods A, Agoston H, Ulici V, Glogauer M, Beier F. Genetic ablation of Rac1 in cartilage results in chondrodysplasia. Dev Biol. 2007;306:612–23. doi: 10.1016/j.ydbio.2007.03.520. [DOI] [PubMed] [Google Scholar]
- Wang G, Woods A, Sabari S, Pagnotta L, Stanton LA, Beier F. RhoA/ROCK signaling suppresses hypertrophic chondrocyte differentiation. J Biol Chem. 2004a;279:13205–14. doi: 10.1074/jbc.M311427200. [DOI] [PubMed] [Google Scholar]
- Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004b;12:963–73. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wickstrom SA, Lange A, Montanez E, Fassler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 2010;29:281–91. doi: 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Beier F. RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J Biol Chem. 2006;281:13134–40. doi: 10.1074/jbc.M509433200. [DOI] [PubMed] [Google Scholar]
- Woods A, Wang G, Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem. 2005;280:11626–34. doi: 10.1074/jbc.M409158200. [DOI] [PubMed] [Google Scholar]
- Woods A, Wang G, Beier F. Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J Cell Physiol. 2007a;213:1–8. doi: 10.1002/jcp.21110. [DOI] [PubMed] [Google Scholar]
- Woods A, Wang G, Dupuis H, Shao Z, Beier F. Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J Biol Chem. 2007b;282:23500–8. doi: 10.1074/jbc.M700680200. [DOI] [PubMed] [Google Scholar]
- Xu L, Peng H, Glasson S, Lee PL, Hu K, Ijiri K, Olsen BR, Goldring MB, Li Y. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56:2663–73. doi: 10.1002/art.22761. [DOI] [PubMed] [Google Scholar]
- Yang G, Sun Q, Teng Y, Li F, Weng T, Yang X. PTEN deficiency causes dyschondroplasia in mice by enhanced hypoxia-inducible factor 1alpha signaling and endoplasmic reticulum stress. Development. 2008;135:3587–97. doi: 10.1242/dev.028118. [DOI] [PubMed] [Google Scholar]
- Yin W, Park JI, Loeser RF. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways. J Biol Chem. 2009;284:31972–81. doi: 10.1074/jbc.M109.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BS, Lyons KM. Multiple functions of BMPs in chondrogenesis. J Cell Biochem. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]
- Zhang R, Murakami S, Coustry F, Wang Y, de Crombrugghe B. Constitutive activation of MKK6 in chondrocytes of transgenic mice inhibits proliferation and delays endochondral bone formation. Proc Natl Acad Sci U S A. 2006;103:365–70. doi: 10.1073/pnas.0507979103. [DOI] [PMC free article] [PubMed] [Google Scholar]