Abstract

Olefinic pyrazines are found to react with benzene in CF3SO3H and give anti-Markovnikov-type addition products. We propose that this is caused by two effects: destabilization of the carbocationic intermediates that would lead to Markovnikov-type products and the generation of a considerable amount of positive charge at the terminal carbon of the olefinic groups. This suggests that acid-catalyzed addition reactions can give anti-Markovnikov-type products when a multiply charged (i.e. superelectrophilic) group is adjacent to the olefinic site.
Superelectrophilic activation was first proposed by Olah and co-workers to explain the high reactivities of electrophilic species in superacidic media.1 When dissolved in superacids, electrophilic reagents like nitronium salts (NO2+X−) and acetylium salts (CH3CO+X−) are capable of reacting with exceptionally weak nucleophiles (i.e. alkanes and deactivated arenes). Despite having a formal positive charge, these electrophilic reagents can interact with the superacid through their non-bonding electron pairs and the superelectrophilic species are formed (HNO22+ and CH3COH2+ or partially protonated forms). There have been several recent studies in which olefinic superelectrophiles undergo Michael addition with very weak nucleophiles like benzene.2 In two of these studies, conjugate addition leads to products in which

nucleophilic attack has occurred at the less-substituted position of an olefinic group (eq 1-2), leading to anti-Markovnikov addition in one case.2a,b Anti-Markovnikov addition chemistry has been of general interest since the concept was first proposed, and most known cases of anti-Markovnikov addition involve free-radical,3 photochemical,4 or transition metal-promoted reactions.5 In the following manuscript, we report the superacid-catalyzed reactions of olefinic pyrazines with benzene and the formation of anti-Markovnikov addition products. We also propose a general route towards anti-Markovnikov addition products involving olefinic superelectrophiles.
When vinylpyrazine (1a, R = H) is reacted with the Brønsted superacid CF3SO3H (triflic acid) and benzene, the anti-Markovnikov product (2a) is formed as the only major product (Scheme 1). In contrast, 2-vinylpyridine, 1-vinylimidazole, and 5-vinylthiazole react under similar conditions to give exclusively the (Markovnikov) addition products having the 1-phenylethyl group (eq 3).6 Remarkably, the 2-isopropenyl and the α-sytryl substituted pyrazines (1b and 1c) likewise give the anti-Markovnikov addition products (2b and 2c, respectively). Although protonation at the terminal carbon of compound 1c would generate a benzylic carbocation center and lead to Markovnikov addition, compound 2c is the only major product.
Scheme 1.

Reactions of olefinic pyrazines.
In order to further probe this chemistry, an isotopically labelled derivative (3) was prepared and reacted with CF3SO3H and C6H6 (eq 4). Analysis of the product (4) reveals that the 13C label is only at the benzylic position. When 2-isopropenyl



pyrazine (1b) is reacted with CF3SO3D and C6D6, the product 5 is found to have deuterium incorporation at the methine carbon and on the pyrazine ring at the 5-position (as well as on the phenyl ring; eq 5).7 Using alcohol substrates as precursors to cationic intermediates, the pyrazines give both the expected substitution products as well as rearrangement products (Scheme 2). In the case of the 1-hydroxyethyl pyrazine (6a), the substitution product (7a) is formed exclusively, while the methyl and phenyl-substituted pyrazines (6b-c) give mixtures of the possible products. If product 7c is isolated and redissolved in CF3SO3H and C6H6, it does not isomerize to product 2c.
Scheme 2.

Reactions of alcohols 6a-c.
The above results are consistent with the formation of tricationic species involving diprotonated and triprotonated pyrazines. The pKa values for methylpyrazine have been estimated to be 1.45 and −5.25,8 so it is reasonable to assume that the olefinic pyrazines are completely diprotonated in the excess superacid (CF3SO3H,9 H0 −14.1). In order for deuterium incorporation to occur in the reactions of 1b with CF3SO3D, the pyrazine must be deuterated at the two nitrogens and at a ring carbon (forming a trication), since deuteration at just one ring nitrogen and a ring carbon is highly unlikely. This suggests a reaction mechanism in which the diprotonated olefinic pyrazines (8a-c, Scheme 3) are in equilibrium with a ring-protonated tricationic species. Triprotonation of the pyrazine rings generate the superelectrophiles (9a-c) and this leads to the formation of positive charge on the less-substituted position of the olefinic groups. The incoming nucleophile (C6H6) then reacts at the less-substituted position and subsequent protonation gives the anti-Markovnikov addition products (2a-c). In the addition reaction between 1b and CF3SO3D with C6D6 (eq 5), there is incomplete deuterium incorporation onto the pyrazine ring, suggesting that the addition reaction is occuring at a faster rate than protonation of the pyrazine ring. This leaves open the possibility of an alternative mechanism involving protosolvation of the olefin group with concominent nucleophilic attack by the arene nucleophile, an AdE3 type mechanism (eq 6).10 Deuterium incorporation could then occur in a secondary reaction at the


pyrazine ring. The alcohol substrates give two types of products: direct substitution products (7a-c) and the addition-type products (2a-c; Scheme 2). These results can be explained by assuming that the pyrazine rings are doubly protonated and an oxonium ion is formed by protonation of the hydroxy group (eq 7). The resulting trications (10a-c) can either undergo direct nucleophilic attack by benzene, or dehydration leading to a highly unstable carbocation (11a-c). Deprotonation then gives intermediates 8a-c leading to the anti-Markovnikov addition products. Despite having the favorable resonance stabilization, the phenyl-substituted carbocation (11c) rapidly undergoes deprotonation leading to the olefin (8c) and the addition product (2c). This suggests that the doubly-protonated pyrazine ring exerts a powerful destabilizing effect on the adjacent carbocationic center. We believe that this is the basis for the anti-Markovnikov addition involving the olefinic pyrazines.
Scheme 3.

Proposed mechanism of addition.
There has been a recent suggestion that some reactions of superelectrophiles may occur by single electron transfer (SET) pathways, based on the results of quantum mechanical calculations.11 These computational studies showed that the lowest unoccupied molecular orbitals (LUMOs) of several dicationic electrophilies were energetically below that of the highest occupied molecular orbitals (HOMOs) of benzene and cyclohexane. Free radical chemistry is well known to produce anti-Markovnikov addition products, so to determine if radical cations are involved in the chemistry of the vinyl pyrazines, CIDNP experiments were done. For example, 4-(3-phenylpropyl)pyridine (12) was reacted with vinylpyrazine (1a) in triflic acid (eq 8) as a completely homogenous liquid phase.12 When the reaction is followed by 1H NMR at 25°C, no CIDNP signal enhancements or absorptions are observed. The reaction between 12 and 1a however, gives the expected

addition product (13) in good yield. While the failure to observe CIDNP effects cannot rigorously exclude the possiblity of SET mechanisms and radical intermediates, it should be noted that a SET mechanism between a trication (like 9a) and the protonated form of 12 would produce a pair of radical dications from a SET pathway. Although dimerizations of radical cations have been reported previously,13 there are no known examples of dimerizations involving radical dications. The present results suggest that long-lived radical intermediates are not present in the reaction of 1a and 12 and consequently SET mechanisms are not involved in the reactions of the olefinic pyrazines (1a-c).
Based on the preliminary results described above, it is clear that the doubly charged pyrazine ring plays an important role in the protonation equilibria and the regiochemistry of nucleophilic attack. Moreover, this chemistry provides further evidence that superelectrophilic activation can be the basis for Michael addition leading to anti-Markovnikov-type products. When the earlier studies involving acrylic acid and


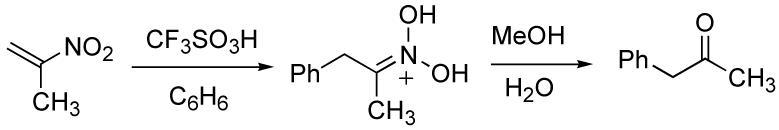
2-nitropropene are also considered, these systems all have a considerable amount of positive charge generated at the terminal (less-substituted) carbon (eq 9-10).14 This causes nucleophilic attack to occur at the terminal carbon leading to formal anti-Markovnikov addition in the case of acrylic acid.
In summary, olefinic pyrazines are found to react with benzene in CF3SO3H and give anti-Markovnikov-type addition products. We propose that this is caused by two effects: destabilization of the carbocationic intermediates that would lead to Markovnikov-type products and the generation of a considerable amount of positive charge at the terminal carbon of the olefinic groups. This suggests that acid-catalyzed addition reactions can give anti-Markovnikov-type products when a multiply charged (i.e. superelectrophilic) group is adjacent to the olefinic site. Not only can superelectrophilic activation enable chemistry with very weak nucleophiles, but our results show that it can also be the basis for unusual regiochemistry. Further studies are in progress to explore the scope of this type electrophilic activation and Michael addition.
Acknowledgment
The financial support of the NIH-NIGMS (GM071368-01 and SO6GM53933-0251), Merck-UNCF, and Northern Illinois University is greatly appreciated.
Supporting Information Available: Experimental procedures and characterization data, inlcuding 1H and 13C NMR data spectra and low and high resolution mass spectra data. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- 1.(a) Olah GA. Angew. Chem., Int. Ed. Engl. 1993;32:767. [Google Scholar]; (b) Olah GA, Klumpp DA. Acct. Chem. Res. 2004 doi: 10.1021/ar020102p. [DOI] [PubMed] [Google Scholar]
- 2.(a) Prakash GKS, Yan P, Torok B, Olah GA. Catal. Lett. 2003;87(3-4):109. [Google Scholar]; (b) Ohwada T, Okabe K, Ohta T, Shudo K. Tetrahedron. 1990;46:7539. [Google Scholar]; (c) Klumpp DA, Rendy R, Zhang Y, Gomez A, McElrea A. Org. Lett. 2004;6:1789. doi: 10.1021/ol049512z. [DOI] [PubMed] [Google Scholar]; (d) Rendy R, Zhang Y, McElrea A, Gomez A, Klumpp DA. J. Org. Chem. 2004;69:2340. doi: 10.1021/jo030327t. [DOI] [PubMed] [Google Scholar]
- 3.Neumann R, de la Vera F, Bar-On A. J. Org. Chem. 1995;60:1315. [Google Scholar]
- 4.Kojima M, Ishada A, Kuriyama Y, Wada Y, Takeya H. Bull. Chem. Soc. Jpn. 1999;72:1049. [Google Scholar]
- 5.Beller M, Seayad J, Tillack A, Jiao H. Angew. Chem., Int. Ed. 2004;43:3368. doi: 10.1002/anie.200300616. and references cited therewithin. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, McElrea A, Sanchez GV, Jr., Klumpp DA, Do D, Gomez A, Aguirre SL, Rendy R, Klumpp DA. J. Org. Chem. 2003;68:5119. doi: 10.1021/jo030024z. [DOI] [PubMed] [Google Scholar]
- 7.Assignment of the regiochemistry of deuteration was based on 1H NMR, 13C NMR, HETCOR, and mass spectral data, see Supporting Information. Further comparisons were made with published NMR data of an alkylpyrazine, see: Cox RH, Bothner-By AA. J. Phys. Chem. 1968;72:1646.
- 8.Chia AS-C, Trimble RF., Jr. J. Phys. Chem. 1961;65:863. [Google Scholar]
- 9.Olah GA, Prakash GKS, Sommer J. Superacids. Wiley; New York, NY: 1985. [Google Scholar]
- 10.(a) Fahey RC, Monahan MW. J. Am. Chem. Soc. 1970;92:2816. [Google Scholar]; (b) Emery SL, Fies CH, Hester EJ, McClusky JV. J. Org. Chem. 1999;64:3788. [Google Scholar]
- 11.Koltunov KY, Prakash GKS, Rasul G, Olah GA. J. Org. Chem. 2002;67:8943. doi: 10.1021/jo0204855. [DOI] [PubMed] [Google Scholar]
- 12.With benzene as a substrate, two phases separate: the acidic-ionic phase and the nonpolar benzene phase.
- 13.(a) Masui M, Ueda C, Moriguchi T, Michida T, Kataoka M, Ohmori H. Chem. Pharm. Bull. 1984;32(4):1392. [Google Scholar]; (b) Park JW, Choi NH, Kim JH. J. Phys. Chem. 1996;100:769. [Google Scholar]; (c) Apperloo JJ, Groenendaal LB, Verheyen H, Jayakannan M, Jayakannan M, Janssen RAJ, Dkhissi A, Beljonne D, Lazzaroni R, Bredas J-L. Chem. Eur. J. 2002;8:2384. doi: 10.1002/1521-3765(20020517)8:10<2384::AID-CHEM2384>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Although no mechnaism was specifically proposed for the reaction of acrylic acid with benzene in CF3SO3H (ref. 2a), the O,O-diprotonated intermediate is perhaps the most likely dicationic species based on earlier studies involving carboxylic acids in superacidic media. See: Prakash GKS, Rasul G, Burrichter A, Laali KK, Olah GA. J. Org. Chem. 1996;61:9253.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


