Abstract
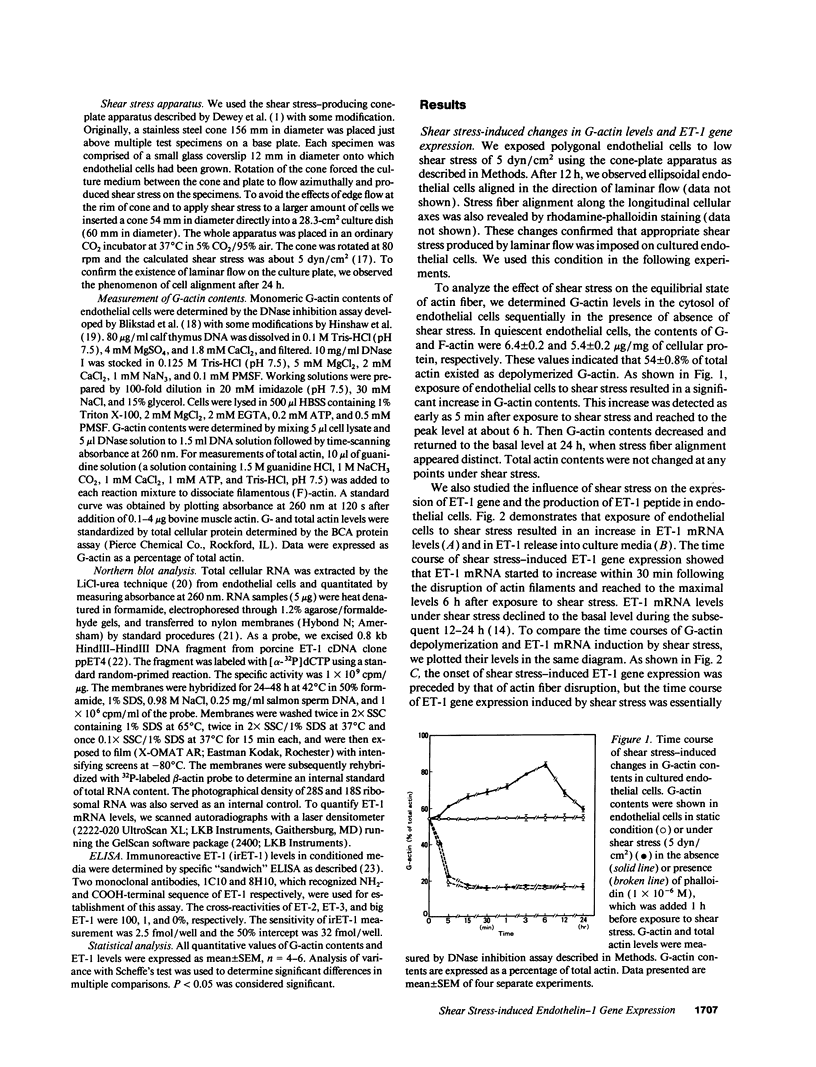
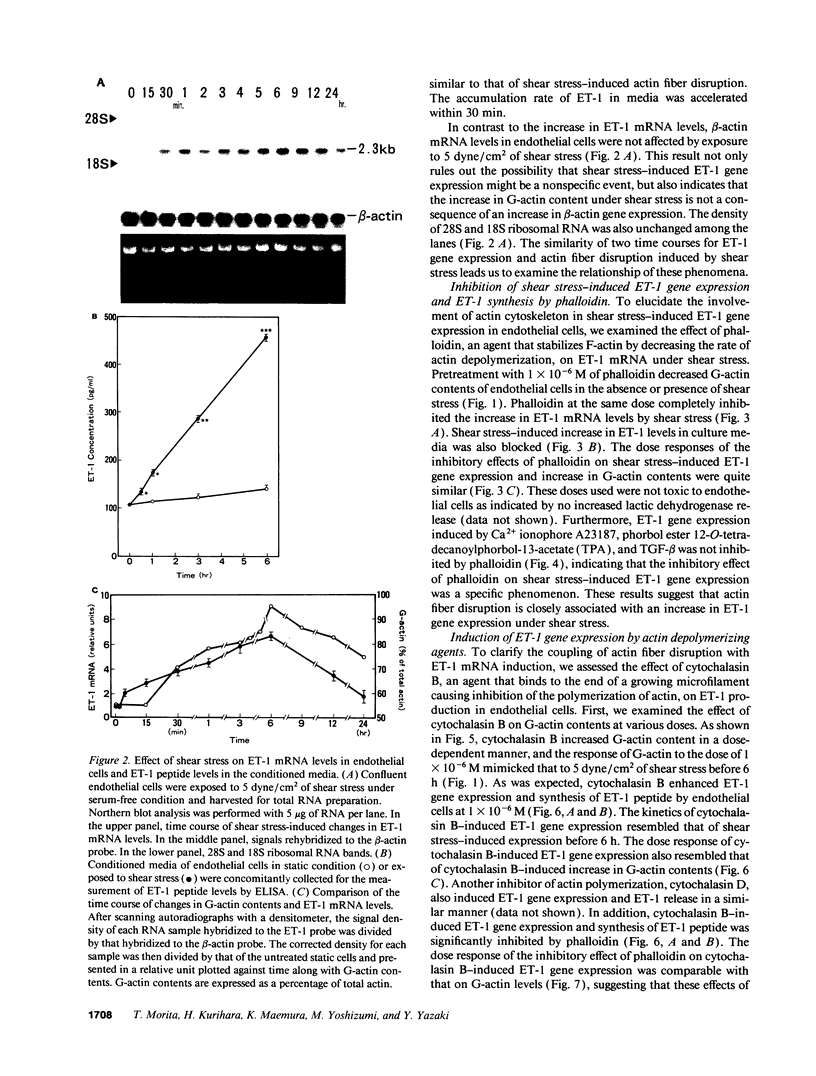
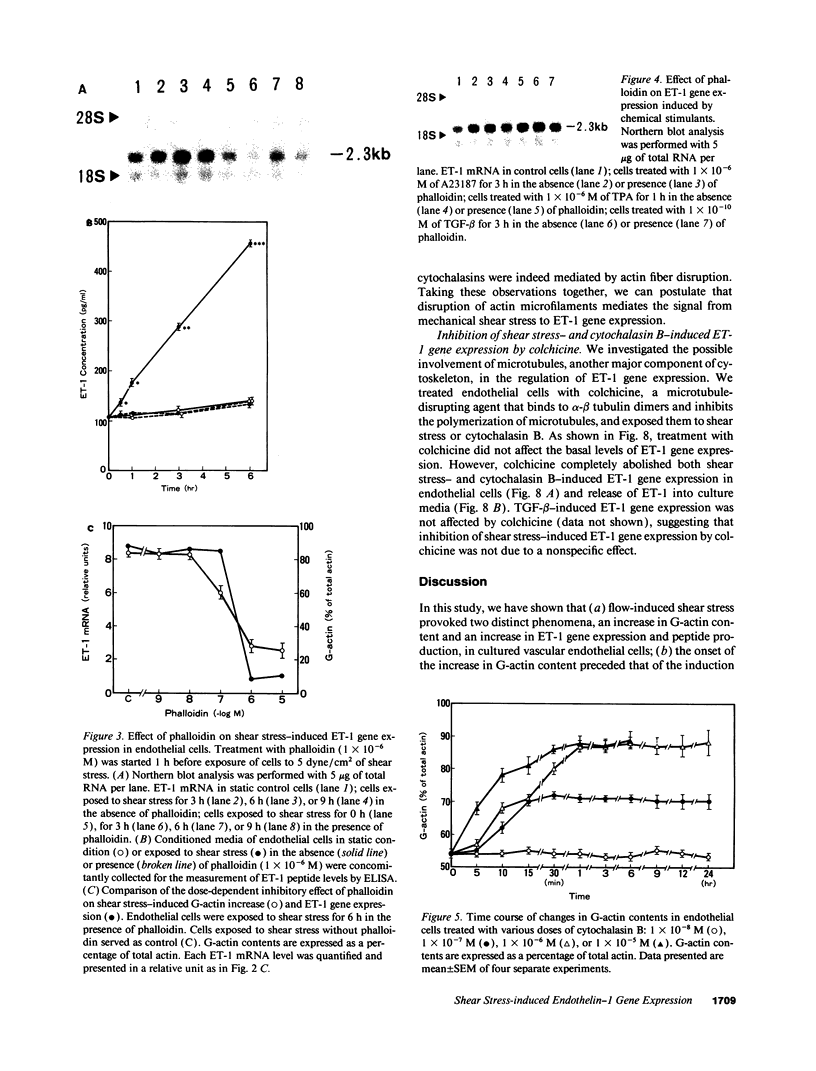
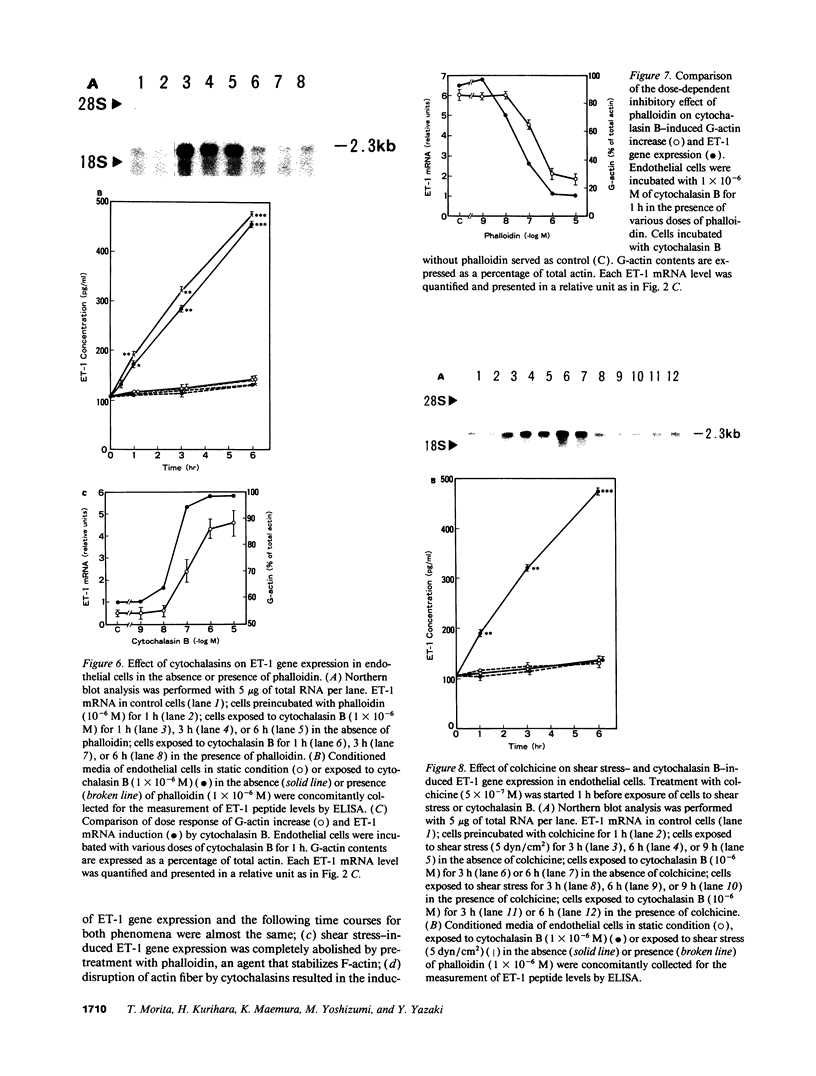
Hemodynamic shear stress alters the architecture and functions of vascular endothelial cells. We have previously shown that the synthesis of endothelin-1 (ET-1) in endothelial cells is increased by exposure to shear stress. Here we examined whether shear stress-induced alterations in cytoskeletal structures are responsible for increases in ET-1 synthesis in cultured porcine aortic endothelial cells. Exposure of endothelial cells to 5 dyn/cm2 of low shear stress rapidly increased monomeric G-actin contents within 5 min without changing total actin contents. The ratio of G- to total actin, 54 +/- 0.8% in quiescent endothelial cells, increased to 87 +/- 4.2% at 6 h and then decreased. Following the disruption of filamentous (F)-actin into G-actin, ET-1 mRNA levels in endothelial cells also increased within 30 min and reached a peak at 6 h. The F-actin stabilizer, phalloidin, abolished shear stress-induced increases in ET-1 mRNA; however, it failed to inhibit increases in ET-1 mRNA secondary to other stimulants. This indicates that shear stress-induced increases in ET-1 mRNA levels may be mediated by the disruption of actin fibers. Furthermore, increases in ET-1 gene expression can be induced by actin-disrupting agents, cytochalasin B and D. Another cytoskeleton-disrupting agent, colchicine, which inhibits dimerization of tubulin, did not affect the basal level of ET-1 mRNA. However, colchicine completely inhibited shear stress- and cytochalasin B-induced increases in ET-1 mRNA levels. These results suggest that shear stress-induced ET-1 gene expression in endothelial cells is mediated by the disruption of actin cytoskeleton and this induction is dependent on the integrity of microtubules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bhagyalakshmi A., Frangos J. A. Mechanism of shear-induced prostacyclin production in endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):31–37. doi: 10.1016/s0006-291x(89)80172-x. [DOI] [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Matrisian L. M., Gesnel M. C., Staub A., Leroy P. Sequences coding for part of oncogene-induced transin are highly conserved in a related rat gene. Nucleic Acids Res. 1987 Feb 11;15(3):1139–1151. doi: 10.1093/nar/15.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buga G. M., Gold M. E., Fukuto J. M., Ignarro L. J. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991 Feb;17(2):187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- Dewey C. F., Jr, Bussolari S. R., Gimbrone M. A., Jr, Davies P. F. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981 Aug;103(3):177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- Diamond S. L., Sharefkin J. B., Dieffenbach C., Frasier-Scott K., McIntire L. V., Eskin S. G. Tissue plasminogen activator messenger RNA levels increase in cultured human endothelial cells exposed to laminar shear stress. J Cell Physiol. 1990 May;143(2):364–371. doi: 10.1002/jcp.1041430222. [DOI] [PubMed] [Google Scholar]
- Ferrua B., Manie S., Doglio A., Shaw A., Sonthonnax S., Limouse M., Schaffar L. Stimulation of human interleukin 1 production and specific mRNA expression by microtubule-disrupting drugs. Cell Immunol. 1990 Dec;131(2):391–397. doi: 10.1016/0008-8749(90)90263-q. [DOI] [PubMed] [Google Scholar]
- Frangos J. A., Eskin S. G., McIntire L. V., Ives C. L. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985 Mar 22;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Franke R. P., Gräfe M., Schnittler H., Seiffge D., Mittermayer C., Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984 Feb 16;307(5952):648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- Glagov S., Zarins C., Giddens D. P., Ku D. N. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988 Oct;112(10):1018–1031. [PubMed] [Google Scholar]
- Hsieh H. J., Li N. Q., Frangos J. A. Shear-induced platelet-derived growth factor gene expression in human endothelial cells is mediated by protein kinase C. J Cell Physiol. 1992 Mar;150(3):552–558. doi: 10.1002/jcp.1041500316. [DOI] [PubMed] [Google Scholar]
- Kitazumi K., Mio M., Tasaka K. Involvement of the microtubular system in the endothelin-1 secretion from porcine aortic endothelial cells. Biochem Pharmacol. 1991 Aug 8;42(5):1079–1085. doi: 10.1016/0006-2952(91)90291-c. [DOI] [PubMed] [Google Scholar]
- Kurihara H., Yoshizumi M., Sugiyama T., Takaku F., Yanagisawa M., Masaki T., Hamaoki M., Kato H., Yazaki Y. Transforming growth factor-beta stimulates the expression of endothelin mRNA by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1435–1440. doi: 10.1016/0006-291x(89)92270-5. [DOI] [PubMed] [Google Scholar]
- Langille B. L., Adamson S. L. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ Res. 1981 Apr;48(4):481–488. doi: 10.1161/01.res.48.4.481. [DOI] [PubMed] [Google Scholar]
- Levesque M. J., Liepsch D., Moravec S., Nerem R. M. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis. 1986 Mar-Apr;6(2):220–229. doi: 10.1161/01.atv.6.2.220. [DOI] [PubMed] [Google Scholar]
- Masuda H., Shozawa T., Hosoda S., Kanda M., Kamiya A. Cytoplasmic microfilaments in endothelial cells of flow loaded canine carotid arteries. Heart Vessels. 1985 May;1(2):65–69. doi: 10.1007/BF02066350. [DOI] [PubMed] [Google Scholar]
- Mo M., Eskin S. G., Schilling W. P. Flow-induced changes in Ca2+ signaling of vascular endothelial cells: effect of shear stress and ATP. Am J Physiol. 1991 May;260(5 Pt 2):H1698–H1707. doi: 10.1152/ajpheart.1991.260.5.H1698. [DOI] [PubMed] [Google Scholar]
- Nishida E., Iida K., Yonezawa N., Koyasu S., Yahara I., Sakai H. Cofilin is a component of intranuclear and cytoplasmic actin rods induced in cultured cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5262–5266. doi: 10.1073/pnas.84.15.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nollert M. U., Eskin S. G., McIntire L. V. Shear stress increases inositol trisphosphate levels in human endothelial cells. Biochem Biophys Res Commun. 1990 Jul 16;170(1):281–287. doi: 10.1016/0006-291x(90)91271-s. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M. Endothelium-dependent pressure-induced contraction of isolated canine carotid arteries. Am J Physiol. 1988 Oct;255(4 Pt 2):H783–H788. doi: 10.1152/ajpheart.1988.255.4.H783. [DOI] [PubMed] [Google Scholar]
- Sharefkin J. B., Diamond S. L., Eskin S. G., McIntire L. V., Dieffenbach C. W. Fluid flow decreases preproendothelin mRNA levels and suppresses endothelin-1 peptide release in cultured human endothelial cells. J Vasc Surg. 1991 Jul;14(1):1–9. [PubMed] [Google Scholar]
- Suttorp N., Polley M., Seybold J., Schnittler H., Seeger W., Grimminger F., Aktories K. Adenosine diphosphate-ribosylation of G-actin by botulinum C2 toxin increases endothelial permeability in vitro. J Clin Invest. 1991 May;87(5):1575–1584. doi: 10.1172/JCI115171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechezak A. R., Viggers R. F., Sauvage L. R. Fibronectin and F-actin redistribution in cultured endothelial cells exposed to shear stress. Lab Invest. 1985 Dec;53(6):639–647. [PubMed] [Google Scholar]
- Wechezak A. R., Wight T. N., Viggers R. F., Sauvage L. R. Endothelial adherence under shear stress is dependent upon microfilament reorganization. J Cell Physiol. 1989 Apr;139(1):136–146. doi: 10.1002/jcp.1041390120. [DOI] [PubMed] [Google Scholar]
- Werb Z., Hembry R. M., Murphy G., Aggeler J. Commitment to expression of the metalloendopeptidases, collagenase and stromelysin: relationship of inducing events to changes in cytoskeletal architecture. J Cell Biol. 1986 Mar;102(3):697–702. doi: 10.1083/jcb.102.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. E., Fujiwara K. Expression and intracellular distribution of stress fibers in aortic endothelium. J Cell Biol. 1986 Jul;103(1):63–70. doi: 10.1083/jcb.103.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. J., Pollard T. D., Herman I. M. Actin filament stress fibers in vascular endothelial cells in vivo. Science. 1983 Feb 18;219(4586):867–869. doi: 10.1126/science.6681677. [DOI] [PubMed] [Google Scholar]
- Wong M. K., Gotlieb A. I. Endothelial monolayer integrity. Perturbation of F-actin filaments and the dense peripheral band-vinculin network. Arteriosclerosis. 1990 Jan-Feb;10(1):76–84. doi: 10.1161/01.atv.10.1.76. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Inoue A., Takuwa Y., Mitsui Y., Kobayashi M., Masaki T. The human preproendothelin-1 gene: possible regulation by endothelial phosphoinositide turnover signaling. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S13–S18. [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M., Kurihara H., Sugiyama T., Takaku F., Yanagisawa M., Masaki T., Yazaki Y. Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):859–864. doi: 10.1016/0006-291x(89)92679-x. [DOI] [PubMed] [Google Scholar]
- van Grondelle A., Worthen G. S., Ellis D., Mathias M. M., Murphy R. C., Strife R. J., Reeves J. T., Voelkel N. F. Altering hydrodynamic variables influences PGI2 production by isolated lungs and endothelial cells. J Appl Physiol Respir Environ Exerc Physiol. 1984 Aug;57(2):388–395. doi: 10.1152/jappl.1984.57.2.388. [DOI] [PubMed] [Google Scholar]









