Abstract
Anabolic androgenic steroids (AAS) are synthetic derivatives of testosterone used by over half a million adolescents in the United States for their tissue-building potency and performance-enhancing effects. AAS also affect behavior, including reports of heightened aggression and changes in sexual libido. The expression of sexual and aggressive behaviors is a function of complex interactions among hormones, social context, and the brain, which is extensively remodeled during adolescence. Thus, AAS may have different consequences on behavior during adolescence and adulthood. Using a rodent model, these studies directly compared the effects of AAS on the expression of male sexual and aggressive behaviors in adolescents and adults. Male Syrian hamsters were injected daily for 14 days with either vehicle or an AAS cocktail containing testosterone cypionate (2 mg/kg), nandrolone decanoate (2 mg/kg), and boldenone undecylenate (1 mg/kg), either during adolescence (27–41 days of age) or in adulthood (63–77 days of age). The day after the last injection, males were tested for either sexual behavior with a receptive female or agonistic behavior with a male intruder. Adolescent males treated with AAS showed significant increases in sexual and aggressive behaviors relative to vehicle-treated adolescents. In contrast, AAS-treated adults showed significantly lower levels of sexual behavior compared with vehicle-treated adults and did not show heightened aggression. Thus, adolescents, but not adults, displayed significantly higher behavioral responses to AAS, suggesting that the still-developing adolescent brain is more vulnerable than the adult brain to the adverse consequences of AAS on the nervous system and behavior.
Keywords: Adolescence, Anabolic androgens, Males, Steroid hormones, Aggression, Sexual behavior, Development
Introduction
Adolescence is the developmental transition from childhood to adulthood and it encompasses reproductive, neural, and behavioral maturation (Andersen, 2003; Casey et al., 2000; Lenroot et al., 2007; Sisk and Foster, 2004; Sisk and Zehr, 2005; Spear, 2000; Yurgelun-Todd, 2007). The onset of puberty marks the onset of adolescence, and gonadal steroid hormones, which become elevated during puberty, influence subsequent adolescent brain development by both organizing and activating neural circuits underlying social behaviors (Delville et al., 1998; Primus and Kellogg, 1989; Primus and Kellogg, 1990; Romeo, 2005; Schulz and Sisk, 2006; Sisk and Foster, 2004; Spear, 2004). Importantly, the organizational effects of pubertal hormones program their activational effects in adulthood. For example, studies in the Syrian hamster show that the presence of testicular hormones during adolescence results in adults that more readily display male-typical social behaviors relative to adults that matured in the absence of testosterone (T) (Schulz et al., 2006, 2004; Sisk et al., 2003). Thus, the adolescent brain is exquisitely sensitive to endogenous testicular steroids, and the action of testicular hormones during adolescence has both short-term and long-term consequences on social behaviors.
Given the significance of endogenous T in normal development of the adolescent male brain, it is important to consider how anabolic androgenic steroids (AAS) influence the adolescent brain and behavior. AAS are synthetic compounds structurally related to T that promote muscle growth (anabolic) and have masculinizing (androgenic) effects. AAS are clinically used to treat malnutrition and mood disorders (Kopera, 1993; Perry et al., 2002); however, the benefits they have for treating physiological and psychological disorders may become detriments when they are used inappropriately (Blue and Lombardo, 1999; Corcoran and Longo, 1992). Adolescents that use AAS are often described by their peers, teachers, and psychologists as defiant, aggressive, and antisocial. In addition, teenagers may display either increased or decreased sexual libido after AAS misuse (Thiblin and Petersson, 2005). In the United States, the onset of AAS use can be as early as 10 years of age, and use among high school males has been as high as 12% in the last decade (Ahima and Harlan, 1992; Bahrke and Yesalis, 2004; Bahrke et al., 1990, 1998; Buckley et al., 1988; Terney and McLain, 1990). Although there is evidence that severe consequences occur after misuse of AAS by adolescents, there has been relatively little systematic investigation of how these exogenous compounds affect the adolescent brain and behavior.
AAS affect the expression of social behaviors in most rodent models. The behavioral responses to AAS depend on the chemical structure of the steroid administered, whether steroid treatment consists of a single compound or as a cocktail, and the age and duration of treatment. Intact adult male rats treated with T and testosterone cypionate (TC) do not display altered reproductive behaviors when compared to controls (Clark et al., 1997; Farrell and McGinnis, 2003). However, T administered to intact adolescent male rats increased sexual behaviors in adulthood (Wesson and McGinnis, 2006). Also, long-term (12 weeks) treatment of intact male rats with oxymethelone, stanozolol, nandrolone, or 17α-methyltestosterone during adolescence and continuing to adulthood results in a decrease in sexual behaviors (Clark et al., 1997; Farrell and McGinnis, 2003; Feinberg et al., 1997). AAS also affect aggressive behaviors in rodents. Single compounds such as T and testosterone propionate (TP) increase aggression in intact adult male rats and mice (Albert et al., 1989; Clark and Barber, 1994; Farrell and McGinnis, 2003; Lumia et al., 1994; Martinez-Sanchis et al., 1998). However, adolescent exposure to single compounds such as nandrolone, 17α-methyltestosterone, or stanozolol has little or no effect on aggression in rats or hamsters (Breuer et al., 2001; Farrell and McGinnis, 2003; McGinnis, 2004; Wesson and McGinnis, 2006). Adolescent male Syrian hamsters exposed to a cocktail of AAS for 2 or 4 weeks show an increase in aggressive behaviors (DeLeon et al., 2002; Grimes and Melloni, 2002; Grimes et al., 2003; Melloni and Ferris, 1996; Ricci et al., 2004; Salas-Ramirez and Sisk, 2005). It is clear that a number of variables determine the overall effects of AAS on sexual and aggressive behaviors, and age has not been systematically studied.
The use of anabolic steroids by adolescent males and the growing evidence that the adolescent brain is particularly sensitive to hormones and experience call for a better understanding of how age at the time of exposure to AAS influence behavioral responses to AAS. The objective of this study was to directly compare the immediate effects of AAS on sexual and aggressive behaviors in adolescent and adult intact male Syrian hamsters. Because adolescent male hamsters are more responsive than adults to the organizational effects of endogenous testicular hormones on male social behaviors (Schulz and Sisk, 2006), we hypothesized that adolescents would also be more responsive to activational effects of exogenous AAS, leading to the prediction that AAS will elicit greater behavioral responses in adolescent compared to adult males.
Materials and methods
Animals
Eighteen-day-old male Syrian hamsters (Mesocricetus auratus) were obtained from Harlan Sprague–Dawley Laboratories (Madison, WI). Upon arrival, males were housed in groups of eight animals per cage (polycarbonate, 33 × 38 × 17 cm) with ad libitum access to food (Telkad Rodent Diet No. 8640, Harlan) and water. Animal colony temperature was maintained at 22 ± 2 °C, and animals were kept on a light–dark cycle of 14:10 L:D (lights off at 1600 h EST). At 25 days of age, animals were singly housed in polycarbonate cages (30.5 × 10 × 20 cm, for Experiment 1; 33 × 38 × 17 cm, for Experiment 2). All animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Michigan State University All-University Committee for Animal Use and Care.
Treatments
A cocktail of AAS containing 0.8 mg/ml of testosterone cypionate (TC; Sigma Aldrich, Inc., St. Louis, MO), 0.8 mg/ml of nandrolone decanoate (ND; Sigma Aldrich, Inc.), and 0.4 mg/ml of boldenone undecylenate (BU; Steraloids, New Port, Rhode Island) was dissolved in 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich, Inc.). The AAS cocktail was prepared the day before treatment began for each age group in each experiment and was stored at 4 °C.
Experimental design
Two separate experiments were conducted. The experimental designs were identical except that the final behavioral test in Experiment 1 assessed sexual behavior with a receptive female whereas in Experiment 2 it assessed agonistic behaviors with an intruder male. At 27 days of age, gonad-intact male hamsters were randomly divided into AAS-treated or vehicle-treated groups. These males received a daily subcutaneous (sc) injection of either the AAS cocktail (2 mg/kg TC, 2 mg/kg ND, 1 mg/kg BU) or vehicle (2-hydroxypropyl-β-cyclodextrin), either during adolescence (from 27 to 41 days of age) or during adulthood (from 63 to 77 days of age). Group sizes were originally n = 10/age and treatment group in Experiment 1 (assessment of sexual behavior) and n = 15/age and treatment group in Experiment 2 (assessment of aggressive behavior). Body weight for each animal was obtained daily in order to calculate the injection volume required to administer the desired dose. Volume ranged from 0.1 ml to 0.25 ml over the 2-week course of administration across age groups. Tests for either sexual or aggressive behaviors were conducted 1 day after the last injection, at 42 days of age for males treated during adolescence and at 78 days of age for males treated as adults. All behavioral interactions were videotaped with a low-light color video camera and were scored using a computer program that tags each behavioral code with an elapsed time (software generously provided by Dr. Kim Wallen, Emory University, Atlanta, GA). This system allows the calculation of counts, duration, and latency for each behavior.
Sexual behavior tests (Experiment 1)
Males were sexually inexperienced prior to a standardized behavior test with a receptive female. Tests were conducted between 1700 and 2030 h EST under red-light illumination, as described in previous studies (Heise et al., 2003; Romeo et al., 2002; Romeo and Sisk, 2001; Schulz et al., 2004). Following a 5-min acclimation to the test chamber (a 51 × 26 × 31.5 cm glass aquarium with a mirror underneath), males interacted with a stimulus female for 15 min. Ovariectomized stimulus females were hormonally primed to induce receptivity with an estradiol benzoate injection (10 μg in 0.05 ml sesame oil) 48 h before and a progesterone injection (500 μg in 0.1 ml sesame oil) 4 h before behavior testing. Before the 15-min behavior test with the subject male, stimulus females were tested for receptivity with a sexually experienced stud male. Anogenital investigation of the female, mounts, intromissions, and ejaculations was scored and quantified as previously described (Schulz et al., 2004). Data from two adolescent and two adult subjects were excluded because females did not remain receptive throughout the 15-min test. Data from three other adolescent and adult males were excluded because of technical difficulties during videotaping of the behavioral tests. Two adult AAS-treated animals died during treatment. Thus, final sample sizes for Experiment 1 were as follows: adolescent vehicle-treated, n = 7; adolescent AAS-treated, n = 8; adult vehicle-treated, n = 9; adult AAS-treated, n = 7.
Agonistic behavior tests (Experiment 2)
Animals were tested for agonistic behaviors using a resident–intruder paradigm. The socially naive males were tested between 1700 and 2030 h EST in a red-light illuminated room. A Plexiglas extension was placed in the home cage of the subject (AAS or vehicle treated) in order to make the walls of the cage taller. After a 5-min acclimation period, an age- and weight-matched intruder male was placed in the treated male’s home cage. Intruder males were purchased from the same vendor and arrived at the same time as the subject males. Ten-minute behavior tests were videotaped and scored for aggressive and submissive behaviors.
The aggressive/dominant behaviors quantified during each behavior test were defined as follows:
Flank marks: the subject rubbed its pigmented sebaceous flank gland against the wall of his home cage. In male–male social encounters, flank marking serves to communicate dominance status (Drickamer et al., 1973; Ferris et al., 1987; Johnston, 1975).
Total contact time: the length of time the subject male approached the intruder male and showed interest by sniffing or remaining in close proximity.
Attacks: the subject moved quickly toward the intruder male to bite or to attempt to bite. The pair either tumbled around or the treated male chased the intruder male.
Bites: the subject male bit the intruder.
Offensive posturing: the subject stood upright and faced the intruder with his front paws raised.
Defensive/submissive behaviors were defined as follows:
Defensive posturing: the two males approached each other and the subject male twisted sideways with his paw or paws stretched toward the other male to ward off an attack.
Tail-up walking: the subject walked around the home cage with his tail raised and back arched. This behavior was usually exhibited in response to the intruder male sniffing or investigating the hindquarters and communicated submissive status.
Escape dashes: a rapid movement away from the intruder male, which occurred only after some form of social interaction, indicating that the subject was fearful of the intruder male.
To assess overall aggressiveness of each subject, we obtained a composite aggression/dominance score by adding the number of flank marks, attacks, and bites and by subtracting tail-up walking.
Two adult males were excluded from the statistical analyses of aggressive behaviors because of extremely low levels of social interaction during the test; they were determined to be outliers by the Dixon outlier test. In addition, two males assigned to the adult vehicle-treated group were not used because of poor health prior to the treatment period. Data from six other males were excluded because of technical difficulties during videotaping of the behavioral tests. Thus, final sample sizes for Experiment 2 were as follows: adolescent vehicle-treated, n = 13; adolescent AAS-treated, n = 14; adult vehicle-treated, n = 11; adult AAS-treated, n = 12.
Physiological measures
Immediately following the behavior test, animals were administered an overdose of sodium pentobarbital (130 mg/kg i.p.). Testes and seminal vesicles were removed and weighed. At the time of sacrifice, flank gland diameters were assessed by shaving the hindquarters and measuring the gland with a caliper.
Statistics
Two-by-two ANOVAs were used to determine main effects and interactions between age at treatment (adolescent or adult) and treatment (vehicle or AAS treated) on aggressive and reproductive behaviors, testes weights, seminal vesicle weights, and flank gland diameters (Statview; JMP, Gary, NC). To investigate significant interactions, we performed simple main effects analyses to compare vehicle- and AAS-treated groups within each age. The Dixon outlier test was used to determine any behavioral outliers. Chi-square analyses were performed to determine whether there were differences in the proportion of AAS-treated adolescent males that showed defensive/submissive behaviors compared with the proportion of males in the other three treatment groups showing these behaviors. All differences were considered significant if they resulted in a p ≤ 0.05. Data in all figures are reported as group means ± SEM.
Results
Experiment 1: the effects of AAS on sexual behaviors in adolescent and adult male Syrian hamsters
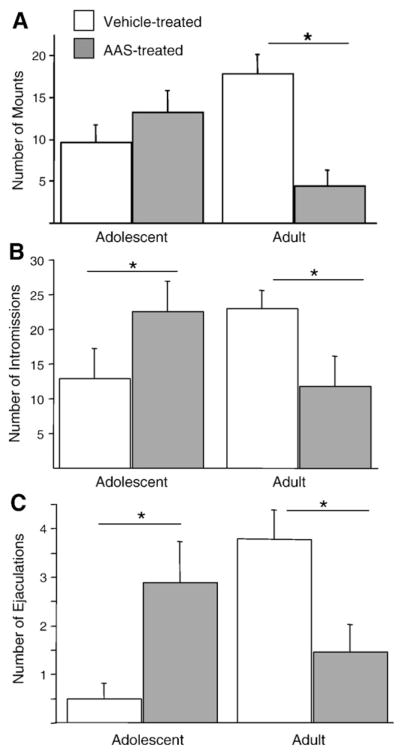
AAS did not affect the amount of time that either adolescents or adults spent investigating the receptive female (data not shown). There was a significant interaction between treatment and age on the number of mounts [F(1, 27) = 17.172; p <0.0002], intromissions [F(1, 27) = 7.449; p <0.01], and ejaculations [F(1, 27) = 15.005; p <0.0006]. In general, AAS facilitated reproductive behavior in adolescent hamsters and reduced reproductive behavior in adults (Fig. 1). Post hoc analyses showed a trend toward increased number of mounts and a significant increase in the number of intromissions [F(1, 13) = 7.482; p = 0.01] and ejaculations [F(1, 13) = 7.316; p <0.02] in AAS-treated adolescents relative to vehicle-treated adolescents. In contrast, AAS-treated adults showed significantly fewer mounts [F(1, 14) = 17.256; p <0.001], intromissions [F(1, 14) = 5.716; p <0.05], and ejaculations [F(1, 14); p <0.015] compared to vehicle-treated adults. The same pattern of results was found for latencies to show sexual behavior in that AAS treatment reduced latencies in adolescents and lengthened latencies in adults. Thus, there were significant interactions between treatment and age on latency to mount [F(1, 27) = 10.773; p <0.003], intromit [F(1, 27) = 14.332; p <0.001], and ejaculate [F(1, 27) = 8.646; p <0.007] (data not shown).
Fig. 1.
AAS affect male sexual behaviors differently in adolescent and adult hamsters. Two-way ANOVA revealed age by treatment interactions in which, relative to age-matched vehicle-treated adolescents (n = 8) and vehicle-treated adults (n = 9), AAS-treated adolescents (n = 7) displayed more (A) mounts, (B) intromissions, and (C) ejaculations, whereas these behaviors were decreased in AAS-treated adults (n = 7). Asterisk indicates significant difference (p<0.05) between AAS and vehicle treatment within an age as determined by post hoc analysis.
Experiment 2: the effects of AAS on agonistic behaviors in adolescent and adult male Syrian hamsters
Aggressive/dominant behaviors
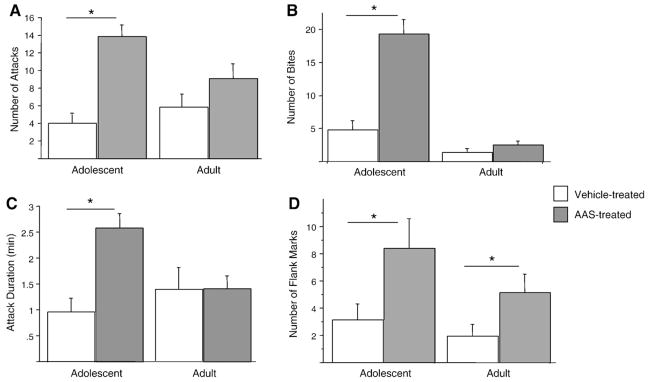
ANOVAs revealed a significant interaction between age and treatment on overall aggression scores [F(1, 46) = 7.417; p<0.009]. Post hoc analyses showed that AAS treatment significantly increased the overall aggression score in adolescents [F(1, 25) = 26.809; p<0.001], but adults only showed a trend to increase overall aggression [F(1, 21) = 2.781; p<0.1].
Significant interactions between age and treatment were also observed when individual aggressive behaviors were analyzed [number of attacks [F(1, 46) = 5.573; p<0.03], number of bites [F(1, 46); p<0.0001], and attack duration [F(1, 45) = 6.510; p <0.02]. Post hoc tests determined that AAS treatment significantly increased the number of attacks [F(1, 25) = 31.750; p<0.0001)], number of bites [F(1, 25) = 27.629; p<0.0001], and attack duration [F(1, 25) = 17.240; p<0.0003] in adolescents. In contrast, none of these measures were significantly increased by AAS treatment in adulthood (Figs. 2A–C). A two-way ANOVA showed a significant interaction between age and treatment [F(1, 46) = 7.089; p <0.01] on flank marking (Fig. 2D). Although both AAS-treated adolescents [F(1, 25) = 1.25; p<0.5] and adults [F(1, 20); p<0.05] displayed significantly more flank marks compared to vehicle-treated males, the significant interaction was due to an apparently greater response by AAS-treated adolescent males.
Fig. 2.
AAS affect male aggressive behaviors differently in adolescent and adult hamsters. Two-way ANOVA revealed age by treatment interactions on (A) attacks, (B) bites, (C) attack duration (minutes), and (D) flank marking behavior. The asterisks indicate significant difference (p<0.05) between AAS and vehicle treatment within an age as determined by post hoc analysis.
Defensive/submissive behaviors
Two-way ANOVAs revealed no effects of treatment or age nor any interactions on defensive behaviors. This was primarily because only occasionally did AAS-treated adolescent males even show these behaviors, and in addition, within-group variability of the other treatment groups was high. None of the AAS-treated adolescent males displayed any escape dashes or tail-up walking and only two AAS-treated adolescents displayed defensive posturing (Table 1). Therefore, chi-square analyses were performed to determine whether the proportion, or frequency distribution pattern, of males exhibiting defensive behaviors was different in AAS-treated adolescents compared with the other three groups. The proportion of vehicle-treated adolescents and vehicle-treated adults displaying tail-up walking and escape dashes was significantly greater than the proportion of AAS-treated adolescents (Table 1). Similarly, the proportion of AAS-treated adults exhibiting tail-up walking (p<0.05) and defensive postures (p <0.05) was greater than that of AAS-treated adolescents. Only two AAS-treated adults displayed escape dashes, and therefore this group was not significantly different from AAS-treated adolescents on this measure.
Table 1.
AAS-treated adolescent males were less likely to display submissive behaviors than vehicle controls and adult males
| Submissive behavior | Adolescent—vehicle (n = 13) | Adolescent—AAS treated (n = 14) | Adult—vehicle (n = 11) | Adult—AAS treated (n = 12) |
|---|---|---|---|---|
| Tail-up walking | 3 ± 1.5 (5/13)* | 0 (0/14) | 3.4 ± 1.9 (3/11)* | 1.8 ± 1.2 (3/12)* |
| Escape dashes | 3.5 ± 1.8 (4/13)* | 0 (0/14) | 6 ± 3.2 (4/11)* | 4.3 ± 3.1 (2/12) |
| Defensive posturing | 4.3 ± 1.6 (7/13)* | 0.3 ± 0.2 (2/14) | 7 ± 2 (5/11) | 5.2 ± 1.5 (6/12)* |
Each treatment group was compared to the adolescent AAS-treated males by using chi-square analysis to determine the frequency distribution of each behavior. Numbers in parentheses indicate the number (proportion) of subjects in each group that displayed at least one instance of tail-up walking, escape dashes, or defensive posturing.
p<0.05 in chi-square analysis of frequency of behavior in AAS-treated adolescents vs. each of the other three groups.
Physiological measures in Experiments 1 and 2
Main effects of both age and treatment were found on testes weight, seminal vesicle weight and flank gland diameter (Table 2). As expected, in both experiments, testes and seminal vesicle weights and flank gland diameters were all larger in adults compared with adolescents. In males tested for sexual behaviors (Experiment 1), testes [F(1, 29) = 22.053; p<0.0001] and seminal vesicle [F(1, 29) = 20.155; p<0.0001] weights were less in AAS-treated than in vehicle-treated males. In contrast, AAS treatment increased flank gland diameter [F(1, 29) = 5.530; p<0.03]. A similar pattern was seen in males tested for agonistic behaviors (Exp 2), where AAS treatment reduced testes [F(1, 47) = 11.147; p<0.002] and seminal vesicle [F(1, 47) = 3.805; p<0.05] weights and led to a trend towards increased flank gland diameter [F(1, 47) = 2.893; p<0.1]. Males were randomly assigned to treatment groups and there were no statistical differences in their body weights at the beginning of treatment. At the end of the treatment period in Experiment 2, however, mean body weight of AAS-treated adolescent and adults males was less than the corresponding vehicle-treated males at each age (p <0.005; Table 2).
Table 2.
Peripheral measures in vehicle- and AAS-treated adolescent and adult males
| Experiment | Adolescent males |
Adult males |
||
|---|---|---|---|---|
| Vehicle treated (mean ± SEM) | AAS treated (mean ± SEM) | Vehicle treated (mean ± SEM) | AAS treated (mean ± SEM) | |
| Testes weight (g) | ||||
| 1 | 2.31 ± 0.04 | 2.0 ± 0.04 * | 3.85 ± 0.13 | 3.29 ± 0.10 * |
| 2 | 2.06 ± 0.07 | 1.70 ± 0.07 * | 3.86 ± 0.09 | 3.67 ± 0.07 * |
| Seminal vesicle weight (g) | ||||
| 1 | 0.167 ± 0.01 | 0.114 ± 0.02 * | 0.344 ± 0.02 | 0.273 ± 0.02 * |
| 2 | 0.21 ± 0.01 | 0.16 ± 0.01 * | 0.36 ± 0.03 | 0.32 ± 0.03 * |
| Flank gland diameter (mm) | ||||
| 1 | 6.77 ± 0.33 | 8.24 ± 0.34 * | 7.6 ± 0.40 | 7.75 ± 0.19 * |
| 2 | 7 ± 0.3 | 7.9 ± 0.3 | 8.5 ± 0.26 | 8.5 ± 0.25 |
| Body weight—beginning of treatment period (g) | ||||
| 1 | 56.41 ± 1.12 | 59.49 ± 2.0 | 106.14 ± 4.17 | 107 ± 2.03 |
| 2 | 49.67 ± 1.0 | 47.23 ± 1.6 | 107.78 ± 2.6 | 108.61 ± 1.9 |
| Body weight—end of treatment period (g) | ||||
| 1 | 93.36 ± 2.33 | 90.10 ± 1.81 | 113.32 ± 4.83 | 111.39 ± 2.36 |
| 2 | 86.9 ± 1.8 | 81.8 ± 2.1 * | 120.53 ± 2.9 | 116.0 ± 2.1 * |
Main effect of treatment in two-way ANOVA, p<0.05.
Discussion
These experiments demonstrate that 2-week treatment of male hamsters with a cocktail of testosterone cypionate, boldenone undecylenate, and nandrolone decanoate at doses comparable to what a heavy or chronic AAS user would administer leads to increased levels of aggressive and reproductive behaviors in adolescent males as compared to both vehicle-treated adolescents and AAS-treated adults. This is the first direct comparison of the behavioral effects of anabolic steroid exposure at two different developmental stages, adolescence and adulthood, and in two different social contexts, sexual interactions with a receptive female or agonistic interactions with a male of similar age and weight. The different behavioral responses to anabolic steroids in adolescence and adulthood most likely reflect structural and functional differences in the neural circuits underlying social behaviors at these two stages of life.
Normally, the postnatal maturation of reproductive behaviors in male Syrian hamsters is a function of the increase in circulating testicular hormones that occurs with the onset of puberty. Previous work from this laboratory has demonstrated that male reproductive behavior cannot be activated by gonadal steroids in prepubertal animals (Meek et al., 1997; Romeo et al., 2002) and that the pubertal rise in testosterone organizes (further masculinizes and defeminizes) neural circuits to enhance or maximize the activation of reproductive behavior in adulthood (Schulz et al., 2004). In the present experiment, adolescent exposure to AAS resulted in levels of sexual behavior comparable to those typically observed in gonad-intact adult hamsters, demonstrating that behavioral responsiveness of adolescent males is greater than that of prepubertal males. Adolescent males may also be more responsive than adults to the activational effects of supraphysiological levels of androgens because in this experiment, AAS treatment increased sexual behavior in adolescents, but not adults. In fact, we found that AAS in adulthood decreases sexual behavior. Similar reductions in sexual behavior in response to exposure to supraphysiological levels of androgens have been reported in rats (Farrell and McGinnis, 2003) and hamsters (Meek et al., 1997). The decrement in sexual behavior induced by high levels of exogenous androgen in adulthood may be secondary to a general suppression of endogenous hypothalamic–pituitary–gonadal axis function. If so, then the current experiments indicate that the adolescent brain is either less responsive to these inhibitory effects, or that there are other mechanisms that override them to result in enhancement of sexual behavior by AAS in adolescence.
Aggressive behavior in Syrian hamsters is not under strong activational control by endogenous testosterone. In spite of this fact, adolescent animals treated with AAS in the present study showed higher levels of aggressive behaviors relative to controls and spent at least one-third of the 10-min test attacking their opponent, similar to previous reports of effects of AAS on aggression in adolescent hamsters (Grimes et al., 2006; Melloni et al., 1997; Melloni and Ferris, 1996). In addition, AAS-treated adolescent males in the present study did not express submissive behaviors such as defensive posturing, escape dashes, or tail-up walking. In contrast to the heightened aggression observed in AAS-treated adolescent males, AAS treatment in adulthood did not result in increased levels of overt aggression, consistent with our previous findings that aggressive behavior is not facilitated by testosterone in adulthood (Romeo et al., 2003). Testicular hormones organize flank marking behavior during adolescence in male Syrian hamsters (Schulz et al., 2006). Interestingly, both adolescent and adult males showed increased levels of flank marking behavior after AAS treatment, suggesting that this behavior may be organized and activated during early to mid adolescence. Thus, the neural mechanisms that in adults favor display of communicative agonistic behaviors (such as e.g. flank marking) over overt aggression (e.g. bites, attacks) appear to be either not engaged or overridden in adolescent males, which in the presence of AAS show heightened overt aggression.
The heightened sexual and aggressive behaviors observed with adolescent exposure to AAS may be indicative of either hyper-responsivity to steroids or to a relative lack of inhibition of the underlying neural circuits at this stage of development (Forbes and Dahl, 2005; Yurgelun-Todd, 2007). The neural circuitries underlying sexual and agonistic behaviors in the male hamster have both overlapping and distinctive components (Kollack-Walker and Newman, 1995). The medial amygdala, lateral septum, and bed nucleus of the stria terminalis (BNST) are common elements of the two circuits, while the medial preoptic area and anterior hypothalamus are areas selectively linked to sexual or agonistic behaviors, respectively (Ferris and Delville, 1994; Swann, 1997; Wood and Coolen, 1997; Wood and Swann, 2005). Most of these structures have direct or indirect connections with cognitive control and reward areas, e.g. nucleus accumbens and prefrontal cortex (Cunningham et al., 2002; Ernst et al., 2006; Yurgelun-Todd, 2007). This study suggests that adolescent males may not be able to regulate the expression of their social behaviors and/or have atypical expression of behavior after AAS exposure when compared to adult males. Our results fit with previously reported relationships between high levels of endogenous testosterone and heightened aggression and the importance of context in the display of social behaviors in human adolescents (Davey et al., 2008; Rowe et al., 2004).
Hormonal facilitation of reproductive and aggressive behaviors includes intracellular actions of both androgens and estrogens (Bodo and Rissman, 2006; Nelson and Trainor, 2007; Romeo et al., 2002; Vagell and McGinnis, 1998). Therefore, the mechanisms by which AAS influence behavior are likely to include regulation and activation of nuclear androgen (AR) and estrogen receptors (ER). All three androgens in the cocktail used in these experiments are aromatizable to estrogenic metabolites that are known to have effects on both the central nervous system and the periphery (Clark and Henderson, 2003). When given to adult male rats, this cocktail upregulates AR immunoreactivity widely throughout the brain (Menard and Harlan, 1993). Self-administration of testosterone induces AR and ER immunoreactivity in steroid-sensitive regions such as the medial amygdala and preoptic area in adult male Syrian hamsters (Dimeo and Wood, 2006). Given that these areas are receptor rich and that there are differential effects of AAS on adolescent and adult males, one can hypothesize that AR and ER expression may be differentially influenced by anabolic steroids in adolescent and adult males.
In the last decade, increased understanding that pubertal gonadal hormones organize the adolescent brain has heightened interest in discovering how AAS affect the adolescent brain and behavioral maturation. This study was the first to directly compare males treated with anabolic steroids during adolescence and adulthood, and we found significant differences in the direction and magnitude of AAS effects on sexual and aggressive behavior. The particular cocktail used in this study is comparable to what a heavy or chronic anabolic steroid user would use to enhance muscle growth and athletic performance (Menard and Harlan, 1993). An increase in body image concerns during adolescence along with a decrease in the perceived risk of taking AAS has resulted in increased use of AAS among U.S. teenagers. Importantly, through neuroimaging and behavioral studies, early adolescence has been shown to be a sensitive period of neural, cognitive, and emotional development. Our data underscore the need for further research on age-specific effects of AAS on brain circuitry and organization throughout development and whether AAS use during adolescence has enduring consequences on social behaviors.
Acknowledgments
We would like to thank Lisa Rogers, Sara Hunter, Jane Venier, and Anibal Rivera-Rivera for their technical support. We would also like to thank Kalynn Schulz, Eman Ahmed, Heather Molenda-Figueira, Margaret Bell, and Julia Zehr for their feedback during the editing of this manuscript. Lastly, we would like to thank the ULAR staff Constance Montville Crew, Bernadette Bentley, and Patrick Lee for always providing our animals with excellent care. This work was funded by NIH R01-MH068764 awarded to C.L. Sisk, the McNair-SROP Fellowship awarded to P.R. Montalto, and the American Psychological Association—Diversity in Neuroscience Program award to K. Y. Salas-Ramirez.
References
- Ahima RS, Harlan RE. Regulation of glucocorticoid receptor immunoreactivity in the rat hippocampus by androgenic–anabolic steroids. Brain Res. 1992;585 (1–2):311–314. doi: 10.1016/0006-8993(92)91226-5. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Petrovic DM, Walsh ML, Jonik RH. Medial accumbens lesions attenuate testosterone-dependent aggression in male rats. Physiol Behav. 1989;46 (4):625–631. doi: 10.1016/0031-9384(89)90342-9. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27 (1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CE. Abuse of anabolic androgenic steroids and related substances in sport and exercise. Curr Opin Pharmacol. 2004;4 (6):614–620. doi: 10.1016/j.coph.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CE, III, Wright JE. Psychological and behavioural effects of endogenous testosterone levels and anabolic–androgenic steroids among males. A review Sports Med. 1990;10 (5):303–337. doi: 10.2165/00007256-199010050-00003. [DOI] [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CE, Brower KJ. Anabolic–androgenic steroid abuse and performance-enhancing drugs among adolescents. Child Adolesc Psychiatr Clin N Am. 1998;7 (4):821–838. [PubMed] [Google Scholar]
- Blue JG, Lombardo JA. Steroids and steroid-like compounds. Clin Sports Med. 1999;18(3):667–689. ix. doi: 10.1016/s0278-5919(05)70175-7. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27 (2):217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Breuer ME, McGinnis MY, Lumia AR, Possidente BP. Aggression in male rats receiving anabolic androgenic steroids: effects of social and environmental provocation. Horm Behav. 2001;40 (3):409–418. doi: 10.1006/hbeh.2001.1706. [DOI] [PubMed] [Google Scholar]
- Buckley WE, Yesalis CE, III, Friedl KE, Anderson WA, Streit AL, Wright JE. Estimated prevalence of anabolic steroid use among male high school seniors. JAMA. 1988;260 (23):3441–3445. [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54 (1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Clark AS, Barber DM. Anabolic–androgenic steroids and aggression in castrated male rats. Physiol Behav. 1994;56 (5):1107–1113. doi: 10.1016/0031-9384(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic–androgenic steroids. Neurosci Biobehav Rev. 2003;27 (5):413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Clark AS, Harrold EV, Fast AS. Anabolic–androgenic steroid effects on the sexual behavior of intact male rats. Horm Behav. 1997;31 (1):35–46. doi: 10.1006/hbeh.1997.1355. [DOI] [PubMed] [Google Scholar]
- Corcoran JP, Longo ED. Psychological treatment of anabolic–androgenic steroid-dependent individuals. J Subst Abuse Treat. 1992;9 (3):229–235. doi: 10.1016/0740-5472(92)90065-v. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453 (2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Davey CG, et al. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- DeLeon KR, Grimes JM, Melloni RH., Jr Repeated anabolic–androgenic steroid treatment during adolescence increases vasopressin V (1A) receptor binding in Syrian hamsters: correlation with offensive aggression. Horm Behav. 2002;42 (2):182–191. doi: 10.1006/hbeh.2002.1802. [DOI] [PubMed] [Google Scholar]
- Delville Y, Melloni RH, Jr, Ferris CF. Behavioral and neurobiological consequences of social subjugation during puberty in golden hamsters. J Neurosci. 1998;18 (7):2667–2672. doi: 10.1523/JNEUROSCI.18-07-02667.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimeo AN, Wood RI. ICV testosterone induces Fos in male Syrian hamster brain. Psychoneuroendocrinology. 2006;31:237–249. doi: 10.1016/j.psyneuen.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Vandenbergh JG, Colby DR. Predictors of dominance in the male golden hamster (Mesocricetus auratus) Anim Behav. 1973;21 (3):557–563. doi: 10.1016/s0003-3472(73)80016-8. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36 (3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell SF, McGinnis MY. Effects of pubertal anabolic–androgenic steroid (AAS) administration on reproductive and aggressive behaviors in male rats. Behav Neurosci. 2003;117 (5):904–911. doi: 10.1037/0735-7044.117.5.904. [DOI] [PubMed] [Google Scholar]
- Feinberg MJ, Lumia AR, McGinnis MY. The effect of anabolic–androgenic steroids on sexual behavior and reproductive tissues in male rats. Physiol Behav. 1997;62 (1):23–30. doi: 10.1016/s0031-9384(97)00105-4. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y. Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinology. 1994;19 (5–7):593–601. doi: 10.1016/0306-4530(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Axelson JF, Shinto LH, Albers HE. Scent marking and the maintenance of dominant/subordinate status in male golden hamsters. Physiol Behav. 1987;40 (5):661–664. doi: 10.1016/0031-9384(87)90114-4. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Dev Psychopathol. 2005;17 (3):827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes JM, Melloni RH., Jr Serotonin modulates offensive attack in adolescent anabolic steroid-treated hamsters. Pharmacol Biochem Behav. 2002;73 (3):713–721. doi: 10.1016/s0091-3057(02)00880-8. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH., Jr Glutamic acid decarboxylase (GAD65) immunoreactivity in brains of aggressive, adolescent anabolic steroid-treated hamsters. Horm Behav. 2003;44 (3):271–280. doi: 10.1016/s0018-506x(03)00138-7. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH., Jr Plasticity in anterior hypothalamic vasopressin correlates with aggression during anabolic–androgenic steroid withdrawal in hamsters. Behav Neurosci. 2006;120 (1):115–124. doi: 10.1037/0735-7044.120.1.115. [DOI] [PubMed] [Google Scholar]
- Heise MT, White LJ, Simpson DA, Leonard C, Bernard KA, Meeker RB, Johnston RE. An attenuating mutation in nsP1 of the Sindbis-group virus S.A.AR86 accelerates nonstructural protein processing and up-regulates viral 26S RNA synthesis. J Virol. 2003;77 (2):1149–1156. doi: 10.1128/JVI.77.2.1149-1156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE. Scent marking by male golden hamsters (Mesocricetus auratus). II. The role of the flank gland scent in the causation of marking. Z Tierpsychol. 1975;37 (2):138–144. [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66 (3):721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kopera H. Miscellaneous uses of anabolic steroids. Wien Med Wochenschr. 1993;143 (14–15):397–398. [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36 (4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumia AR, Thorner KM, McGinnis MY. Effects of chronically high doses of the anabolic androgenic steroid, testosterone, on intermale aggression and sexual behavior in male rats. Physiol Behav. 1994;55 (2):331–335. doi: 10.1016/0031-9384(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Martinez-Sanchis S, Salvador A, Moya-Albiol L, Gonzalez-Bono E, Simon VM. Effects of chronic treatment with testosterone propionate on aggression and hormonal levels in intact male mice. Psychoneuroendocrinology. 1998;23 (3):275–293. doi: 10.1016/s0306-4530(98)00005-5. [DOI] [PubMed] [Google Scholar]
- McGinnis MY. Anabolic androgenic steroids and aggression: studies using animal models. Ann NY Acad Sci. 2004;1036:399–415. doi: 10.1196/annals.1330.024. [DOI] [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Horm Behav. 1997;31 (1):75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr, Ferris CF. Adolescent anabolic steroid use and aggressive behavior in golden hamsters. Ann NY Acad Sci. 1996;794:372–375. doi: 10.1111/j.1749-6632.1996.tb32546.x. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr, Connor DF, Hang PT, Harrison RJ, Ferris CF. Anabolic–androgenic steroid exposure during adolescence and aggressive behavior in golden hamsters. Physiol Behav. 1997;61 (3):359–364. doi: 10.1016/s0031-9384(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Menard CS, Harlan RE. Up-regulation of androgen receptor immunoreactivity in the rat brain by androgenic–anabolic steroids. Brain Res. 1993;622 (1–2):226–236. doi: 10.1016/0006-8993(93)90823-6. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev, Neurosci. 2007;8 (7):536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Yates WR, Williams RD, Andersen AE, MacIndoe JH, Lund BC, Holman TL. Testosterone therapy in late-life major depression in males. J Clin Psychiatry. 2002;63 (12):1096–1101. doi: 10.4088/jcp.v63n1202. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22 (6):633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Horm Behav. 1990;24 (3):311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Grimes JM, Melloni RH., Jr Serotonin type 3 receptors modulate the aggression-stimulating effects of adolescent cocaine exposure in Syrian hamsters (Mesocricetus auratus) Behav Neurosci. 2004;118 (5):1097–1110. doi: 10.1037/0735-7044.118.5.1097. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Neuroendocrine and behavioral development during puberty: a tale of two axes. Vitam Horm. 2005;71:1–25. doi: 10.1016/S0083-6729(05)71001-3. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Sisk CL. Pubertal and seasonal plasticity in the amygdala. Brain Res. 2001;889 (1–2):71–77. doi: 10.1016/s0006-8993(00)03111-5. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Richardson HN, Sisk CL. Puberty and the maturation of the male brain and sexual behavior: recasting a behavioral potential. Neurosci Biobehav Rev. 2002;26 (3):381–391. doi: 10.1016/s0149-7634(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Schulz KM, Nelson AL, Menard TA, Sisk CL. Testosterone, puberty, and the pattern of male aggression in Syrian hamsters. Dev Psychobiol. 2003;43 (2):102–108. doi: 10.1002/dev.10125. [DOI] [PubMed] [Google Scholar]
- Rowe R, Maughan B, Worthman CM, Costello EJ, Angold A. Testosterone, antisocial behavior, and social dominance in boys: pubertal development and biosocial interaction. Biol Psychiatry. 2004;55 (5):546–552. doi: 10.1016/j.biopsych.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Salas-Ramirez K, Sisk CL. Anabolic Androgenic Steroids Increase Aggression in Adolescent Male Syrian Hamsters. Society for Neuroscience. 2005 Abstract. [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45 (4):242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Horm Behav. 2006;50 (3):477–483. doi: 10.1016/j.yhbeh.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7 (10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Schulz KM, Zehr JL. Puberty: a finishing school for male social behavior. Ann NY Acad Sci. 2003;1007:189–198. doi: 10.1196/annals.1286.019. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26 (3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24 (4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Ann NY Acad Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Swann JM. Gonadal steroids regulate behavioral responses to pheromones by actions on a subdivision of the medial preoptic nucleus. Brain Res. 1997;750 (1–2):189–194. doi: 10.1016/s0006-8993(96)01348-0. [DOI] [PubMed] [Google Scholar]
- Terney R, McLain LG. The use of anabolic steroids in high school students. Am J Dis Child. 1990;144 (1):99–103. doi: 10.1001/archpedi.1990.02150250111046. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Petersson A. Pharmacoepidemiology of anabolic androgenic steroids: a review. Fundam Clin Pharmacol. 2005;19 (1):27–44. doi: 10.1111/j.1472-8206.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Vagell ME, McGinnis MY. The role of gonadal steroid receptor activation in the restoration of sociosexual behavior in adult male rats. Horm Behav. 1998;33 (3):163–179. doi: 10.1006/hbeh.1998.1445. [DOI] [PubMed] [Google Scholar]
- Wesson DW, McGinnis MY. Stacking anabolic androgenic steroids (AAS) during puberty in rats: a neuroendocrine and behavioral assessment. Pharmacol Biochem Behav. 2006;83 (3):410–419. doi: 10.1016/j.pbb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Wood RI, Coolen LM. Integration of chemosensory and hormonal cues is essential for sexual behaviour in the male Syrian hamster: role of the medial amygdaloid nucleus. Neuroscience. 1997;78 (4):1027–1035. doi: 10.1016/s0306-4522(96)00629-x. [DOI] [PubMed] [Google Scholar]
- Wood RI, Swann JM. The bed nucleus of the stria terminalis in the Syrian hamster: subnuclei and connections of the posterior division. Neuroscience. 2005;135:155–179. doi: 10.1016/j.neuroscience.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17 (2):251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]