Abstract
Glaucoma is one of the leading causes of bilateral blindness affecting nearly 8 million people worldwide. Glaucoma is characterized by a progressive loss of retinal ganglion cells (RGCs) and is often associated with elevated intraocular pressure (IOP). However, patients with normal tension glaucoma (NTG), a subtype of primary open-angle glaucoma (POAG), develop the disease without IOP elevation. The molecular pathways leading to the pathology of NTG and POAG are still unclear. Here, we describe the phenotypic characteristics of transgenic mice overexpressing wild-type (Wt) or mutated optineurin (Optn). Mutations E50K, H486R and Optn with a deletion of the first (amino acids 153–174) or second (amino acids 426–461) leucine zipper were used for overexpression. After 16 months, histological abnormalities were exclusively observed in the retina of E50K mutant mice with loss of RGCs and connecting synapses in the peripheral retina leading to a thinning of the nerve fiber layer at the optic nerve head at normal IOP. E50K mice also showed massive apoptosis and degeneration of entire retina, leading to approximately a 28% reduction of the retina thickness. At the molecular level, introduction of the E50K mutation disrupts the interaction between Optn and Rab8 GTPase, a protein involved in the regulation of vesicle transport from Golgi to plasma membrane. Wt Optn and an active GTP-bound form of Rab8 complex were localized at the Golgi complex. These data suggest that alternation of the Optn sequence can initiate significant retinal degeneration in mice.
INTRODUCTION
Glaucoma is characterized by progressive loss of retinal ganglion cells (RGCs), degeneration of axons in the optic nerve and visual field defects. Primary open-angle glaucoma (POAG) is one of the major causes of irreversible blindness leading to vision loss in about 4.5 million people and accounting for 12% of global blindness (1,2). POAG is often associated with elevated intraocular pressure (IOP), which is one of main risk factors in glaucoma. However, degenerative changes in the RGC and the optic nerve head leading to progressive visual field loss may occur even in the absence of elevated IOP in a subtype of POAG called normal tension glaucoma (NTG). A recent epidemiological study in Tajimi, Japan, demonstrated that >90% of POAG cases were diagnosed as NTG (3).
At least 24 different genetic loci have been linked to various forms of glaucoma, and four glaucoma-associated genes, myocilin, cytochrome P4501B1, OPTN and WD repeat domain 36 (WDR36) have been previously identified (4–7). A significantly higher frequency of OPTN sequence alternations in glaucoma subjects compared with controls supports the contribution of this gene to the development of glaucoma (8–10). In one original report, >16.7% of NTG families had mutations in the OPTN gene (6), and a number of disease-causing amino acid substitutions including E50K, H486R and R545Q have been confirmed by others (8–10). The substitution of glutamic acid by lysine at amino acid 50 (E50K) is exclusively associated with familial and sporadic forms of NTG (6,8,11). We also identified E50K mutation in an NTG family in Japan (Supplementary Material 1). Several lines of evidence support E50K mutation could play a critical role for the severity of phenotype and pathology of glaucoma. Clinical study revealed that an NTG phenotype is more severe in subjects with the E50K mutation than in a control group of subjects with NTG but without this mutation, supporting a critical role for this mutation (8,12). In vitro cell biological study demonstrated that transfection of E50K-mutated optineurin (Optn) caused cell death of rat RGC cell line, RGC5 (13).
OPTN corresponds to the GLC1E locus for adult-onset POAG and is located in the 10p14 region. The OPTN gene contains 3 non-coding exons followed by 13 exons encoding a 577 amino acid protein. Almost all of the reported disease-causing mutations correspond to positions that are evolutionarily conserved between the mouse, monkey and human. OPTN is ubiquitously expressed in all tissues and interacts with number of proteins including huntingtin (14), transcription factor IIIA (15), Rab8 (16,17), myosin VI (18), FOS (19), ring finger protein 11 (20) and metabotrophic glutamate receptor 1-a (21), suggesting multiple cellular functions. Recent studies have shown that the OPTN promoter is induced by TNF-α (22). OPTN may function as an adaptor which regulates the assembly of TAX1BP1 and the post-translationally modified form of Tax1, leading to a sustained NF-κB activation (23).
The molecular pathways leading to glaucoma from a single gene mutation still remain unclear mainly due to (i) insufficiency of clinical and genetic information from glaucoma patients, (ii) difficulty in obtaining clinical material, such as optic nerve tissues, from patients and (iii) lack of animal models with particular gene mutations. Recently, it has been reported that glutamate transporter-deficient mice exhibit an NTG-like phenotype (24). However, to this date, no animal models have been produced based on the gene mutation found in NTG patients.
In this paper, we developed five variants of Optn overexpressing mice including the wild-type (Wt), E50K and H486R point mutants, and mutants with a deletion of the first or second leucine zipper. We used histopathology to investigate changes in the optic nerve and retina of each mutant. Using a modified protein fragment complementation method, we also investigated the effects of the E50K mutation on the interaction with OPTN-interacting protein Rab8, which controls the vesicle transport.
RESULTS
Construction of mouse Optn mutants and characterization of expression
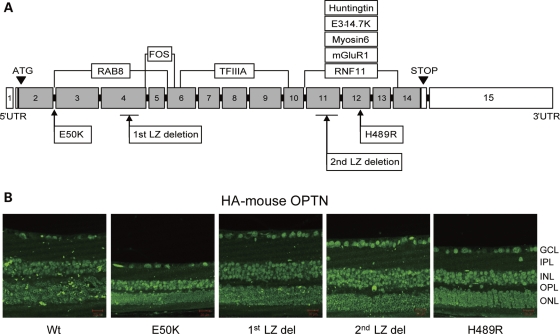
Five mouse Optn variants were overexpressed under the CMV early enhancer/chicken beta-actin (CAG) promoter in transgenic mice. These variants included Wt Optn, the E50K and H489R mutants which are mouse equivalents of the human glaucoma-causing mutations E50K and H486R, respectively, and mutants with deletion of the first (1st LZ del) or second (2nd LZ del) Optn leucine zipper domain. All transgenic mice were born at normal Mendelian ratios, weighed the same as non-transgenic littermates and appeared normal up to 16 months of age. The mutant HA-tagged proteins were ubiquitously expressed in the entire retina (Fig. 1B). The copy numbers for each mutant cDNA construct were approximately 12 to 14 per mouse as determined by TaqMan real-time PCR assay (data not shown).
Figure 1.
(A) Schematic diagram of the mouse Optn constructs used in this study. Positions of mutations and deletions are shown in lower boxes. Predicted binding sites of Optn-interacting proteins are shown in upper boxes. (B) Expression of Optn mutants in the retina of transgenic mice. Sections were immunostained with anti-HA antibody. Scale bar: 20 μm.
Comparison of histological changes in the eye of Wt and mutant Optn transgenic mice
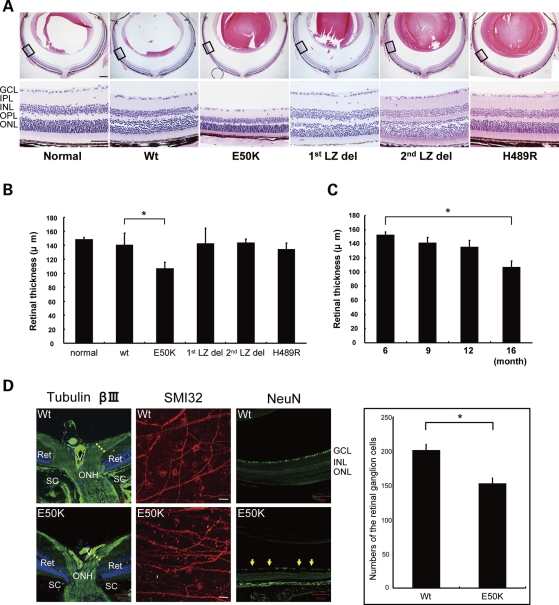
Loss of RGCs and cupping of the optic disc are the defining histological features of the retina of patients with POAG and NTG. Therefore, we examined the eyes of aged Wt, E50K, H489R, 1st LZ del and 2nd LZ del mice using histology and immunohistochemistry. Cornea, lens and anterior segment of Wt and transgenic mice were histologically normal even in 16-month-old mice. For statistical analysis, measurements of retinal thickness were made at the peripheral retina ∼1.0–1.2 mm from the optic nerve head. Remarkably, we found significant phenotypical changes in five independent transgenic mouse lines expressing the E50K mutant (Fig. 2A). The retinal thickness of E50K mice was significantly reduced compared with Wt mice at 16 months of age (*P < 0.05) (Fig. 2B), but a reduction of the retinal thickness was observed as early as 12 months of age (Fig. 2C). Owing to the loss of RGCs and their axons in the peripheral retina, β-III tubulin-stained nerve fiber layer was relatively thinner at the optic nerve head of E50K mice compared with Wt mice (Fig. 2D). The anti-SMI32 immunostaining of the whole-mounted retina demonstrated loss of large RGCs in the peripheral retina (Fig. 2D). Progressive, non-specific loss of RGCs in E50K mice was shown by counting NeuN-stained cells in the entire retina sections (Fig. 2D).
Figure 2.
Comparison of retina morphology of normal, Wt and mutant transgenic mice. (A) HE staining of retina sections of 16-month-old normal and transgenic mice. Scale bar: 200 μm (upper panel), 50 μm (lower panel). (B) Quantification of the retina thickness measurements of different transgenic lines at 16 months of age. Six retina samples were measured in each group. Significant thinning of the retina was observed only for E50K mice (*P < 0.05). (C) Quantification of the retina thickness measurements of E50K mice of different ages (6 to 16 months; n = 6 for each time point). E50K mice showed statistically significant retinal thinning at 16 months of age. (D) Tubulin β-III immunostaining of the Wt and E50K mice at the optic nerve head (scale bar: 50 μm). Reduction of the RGC number at the peripheral retina is shown by NeuN immunostaining of paraffin section and SMI32 immunostaining of flat mount retina for Wt and E50K mice (scale bar: 100 μm). RGCs were counted over entire paraffin sections for NeuN immunostaining. Right panel represents quantification of these results. Significant loss of the RGCs was observed in the E50K mice compared with Wt (*P < 0.05).
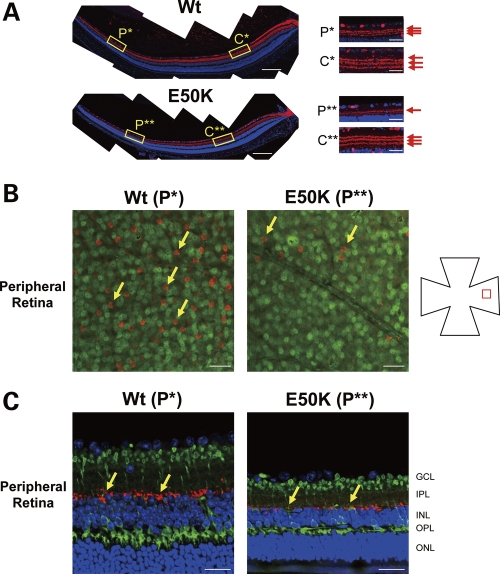
To determine which retinal cell types are vulnerable to the E50K mutation, we performed immunohistochemical analysis using retinal cell-specific markers. Immunostaining with calretinin antibodies was used to visualize synapses of RGCs and amacrine cells in the inner plexiform layer of 16-month-old Wt and E50K mice (Fig. 3A). Although there was no difference in the immunolabeling pattern of synapses in the central retina of Wt and E50K mice (Box C**), a significant degeneration of synapses was observed in the peripheral retina of E50K mice versus Wt mice (Box P**). Immunolabeling of the flat mount retina using antibodies against choline acetyltransferase (ChAT, cholinergic amacrine) revealed areas of amacrine cell loss in the peripheral retina (Box P**) (Fig. 3B, arrow). Loss and/or changes of another type of amacrine cells and rod bipolar cells in the peripheral retina of E50K mice versus Wt were detected by staining with antibodies against tyrosine hydroxylase (red, doperminergic amacrine cell) and PKC α (green, rod bipolar cell) (Fig. 3C, Box P**). The outer plexiform layer (OPL) and outer nuclear layer (ONL) were also affected in E50K mice. In the OPL, expression of synaptophysin, synaptic vesicle marker at the photoreceptor synaptic terminal, was reduced in E50K mice compared with Wt mice (Fig. 4), whereas rhodopsin-labeled outer segments were shorter in E50K mutant than in Wt mice.
Figure 3.
Changes in the retina of E50K mice. (A) Immunostaining of the retina sections with anti-calretinin antibody, a specific marker for RGCs and amacrine cells. Disruption of synapses between RGCs and amacrine cells was observed in the peripheral retina of E50K but not control mice (yellow box, P**). Scale bar: 20 μm. (B) Immunostaining of the flat mount retina with ChAT (red) and NeuN (green). A significant number of starburst amacrine cells were lost in the RGC layer in the peripheral retina of E50K mutant mice (P**) compared with Wt mice (P*). Scale bar: 50 μm. (C) Immunostaining of the retina sections with tyrosine hydroxylase (red) and PKC α (green), specific markers for dopaminergic amacrine cells and rod bipolar cells, respectively. Amacrine cell loss and size reduction of bipolar cells were observed. Scale bar: 20 μm.
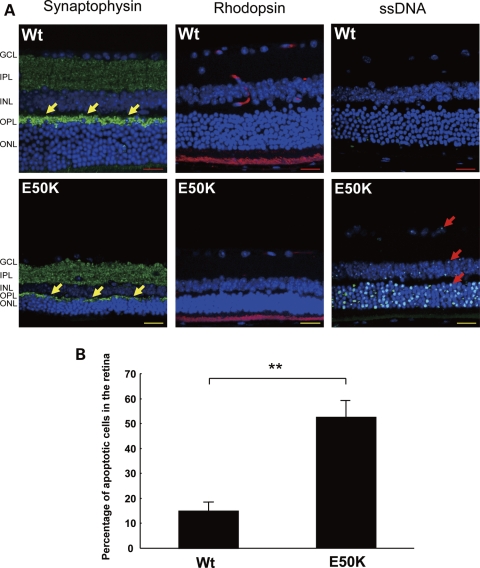
Figure 4.
Degeneration of the OPL and ONL in the peripheral retina of E50K mice. (A) Immunostaining of the retina sections with synaptophysin, rhodopsin and ssDNA, specific markers for neuronal presynaptic vesicles, rod photoreceptors and apoptosis cells, respectively. Synapse disruption (arrow), rod photoreceptor cells degeneration and apoptosis cells were observed in the OPL and/or ONL in the peripheral retina of E50K mice. Scale bar: 20 μm. (B) Percentage of apoptotic cells in the ONL (n = 6, **P < 0.01).
Apoptosis assay by single-stranded DNA immunohistochemistry
RGC death by apoptosis is one of the typical features of glaucoma pathogenesis. Immunostaining with antibodies against single-stranded DNA (ssDNA), a marker of apoptosis-associated DNA damage, was used to detect apoptotic changes in the retina of E50K mice. ssDNA-positive (apoptotic) cells were detected not only in the RGCL (Fig. 4A, Arrow) but also in the INL and ONL. At the peripheral retina, significant increase of apoptotic cell number in all retinal layers was observed in E50K mice at 16 months of age (Fig. 4B) compared with age-matched Wt mice (**P < 0.01).
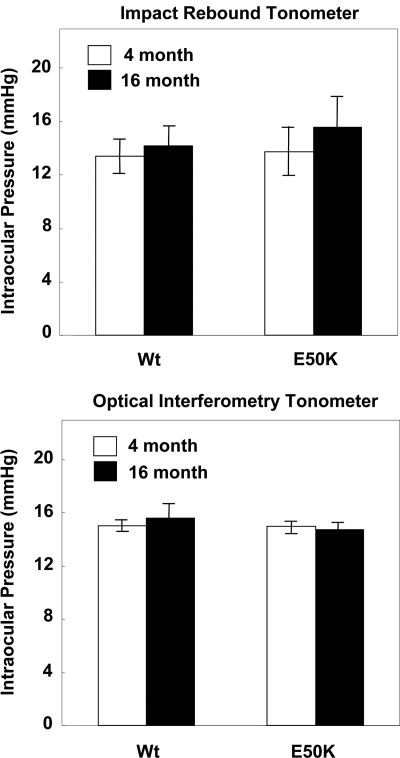
IOP of Wt and mutant Optn transgenic mice
IOP measurement is a necessary and important step to determine whether retinal degeneration in our transgenic mice is associated with the elevation of IOP or it is IOP independent. IOP was measured using an impact-rebound tonometer and an optical interferometry tonometer. The average IOP reading from both devices gave similar IOP for mutant and Wt mice in the normal range of 15 ± 1 mmHg for all examined ages (Fig. 5). These results demonstrated that pathological features of E50K mice are not due to changes in IOP.
Figure 5.
IOP measurement of Wt and E50K mice. IOP was measured using an impact-rebound tonometer and an optical interferometry tonometer between 9 AM and noon in 4-month and 16-month-old mice. Both methods gave similar results (IOP of 15 ± 1 mmHg) for Wt and transgenic mice at both ages examined (n = 6).
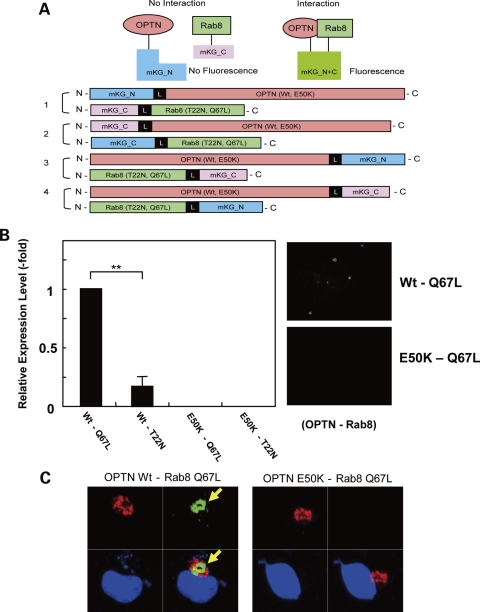
E50k mutation disrupts OPTN–Rab8 direct protein interaction
Protein–protein interaction of OPTN and Rab8 was analyzed after transfection of RGC5 or COS1 cells with constructs encoding OPTN (Wt, E50K), Rab8 [Wt, T22N (inactive GDP form) and Q67L (active GTP form)] tagged with fluorescent labels (Fig. 6A). We observed >4-fold decrease in the interaction between OPTN Wt and the active form (Q67L) versus the inactive form (T22N) of Rab8. However, E50K did not interact with either form of Rab8 in RGC5 cells (Fig. 6B). Interaction of Wt OPTN and the active form Rab8 was further supported by co-localization of the complex with a specific Golgi marker, GM130, in COS1 cells (Fig. 6C).
Figure 6.
Disruption of OPTN–Rab8 interaction by the E50K mutation. (A) A diagram of cDNA constructs used in experiments to study protein–protein interaction. (B) The protein–protein interaction of OPTN Wt and E50K with Rab8 T22N (GDP inactive form), and Q67L (GTP active form) was measured in RGC-5 cells as described in Materials and Methods. Interaction of OPTN Wt and Q67L-active form of Rab8 was increased five times over Rab8 T22N-inactive form of Rab8 protein (**P < 0.01). E50K did not show any interaction with any construct including the active form of Rab8 (n = 6). (C) Co-localization of the OPTN–Rab8 complex (green) and Golgi marker GM130 (red). COS1 cells were transfected with constructs encoding indicated constructs and stained with antibodies against GM130 48 h after transfection as described in Materials and Methods. Nuclei (blue) were stained with DAPI.
DISCUSSION
In the present study, we produced and characterized the phenotype of five different transgenic Optn mice lines including lines with overexpression of two OPTN mutations identified in glaucoma patients. Among the 15 OPTN mutations previously identified (P16A, H26D, E50K, K66R, E92V, E103D, 2 bp insertion between amino acids 127–128, V161M, H228Y, A336G, A377T, I407T, A466S, H486R and R545Q), we selected two mutations E50K and H486R, which has been confirmed by several groups to be associated with severe NTG and/or juvenile open-angle glaucoma (JOAG) (6,8–11,25–27). E50K, a substitution of glutamic acid by lysine at amino acid 50, is exclusively associated with the familial and sporadic forms of NTG (6,8,11), and that phenotype is, on an average, more severe compared with NTG without the E50K mutation (Supplementary Material) (6). A study by Hauser et al. (12) also reported a more severe glaucomatous phenotype in a patient with E50K mutation than that in the other NTG patient. The H486R mutation is reportedly associated with both NTG and JOAG (4,26). Histidine 486 is an evolutionarily conserved residue located at the C-terminus, where five other proteins, adenovirus E3-14.K, huntingtin, metabotrophic glutamate receptor 1-a, myosin VI, ring finger protein 11, can interact with Optn (Fig. 1A). On the other hand, we chose 1st and 2nd LZ del as the transgenic construct design. As shown in Figure 1A, both LZ regions are binding sites for various functional molecules—1st LZ: Rab8 and FOS; 2nd LZ: Huntingtin, E3-14.7K, Myosin6, mGluR1, RNF11. To elucidate the functional defect which may occur by deleting these regions, we generated 1st and 2nd LZ del transgenic mice.
Taken together, we hypothesized that each OPTN transgenic line would show distinct phenotype because of different locations of mutations, influencing different OPTN-interacting proteins. Surprisingly, only E50K mutant showed severe histopathological changes in mice. The E50K mice showed not only loss of RGCs, but also progressive retinal degeneration exclusively in the peripheral region (Figs. 2–4). Immunolabeling of ssDNA demonstrated that apoptotic changes occurred in all retinal cell layers. The number of cells in different retinal layers, including amacrine, bipolar and photoreceptor cells, and thickness of all retinal cell layers were reduced in the peripheral retina of E50K mice.
Herein, a question may rise from these findings in E50K mice: why is neuronal degeneration eminent at the peripheral retina, not at the central retina? Previous reports have indicated that mouse models of glaucoma follow similar natural courses of peripheral retinal degeneration. These include the well-known glaucoma mouse model, the DBA/2J mouse, and recently reported GLAST-deficient mouse, where all layers of the peripheral retina were shown to be affected, leading to a significant reduction of retinal thickness (24,28). The myocilin Tyr437His transgenic mouse, a POAG mouse model, also develops RGC loss at the peripheral retina and retinal degeneration (29,30). These three mouse models all share a pattern of peripheral degeneration with Optn E50K mice. In general, glaucoma most often affects peripheral visual field at early stages of the disease, whereas deterioration of the central retina can be seen only at later stages of the disease (31). We can speculate that the increased sensitivity of peripheral RGCs is associated with longer non-myelinated axons compared with the central RGCs, but this would not explain the degeneration of other neuronal cells. Further investigation is required to explain the difference of E50K susceptibility between mouse and human at the peripheral retina. The fact that E50K mice develop a phenotype of peripheral RGC degeneration which is similar to the previous glaucoma mouse models suggests that later stages of cellular and molecular mechanisms for neuronal degeneration are shared between NTG and POAG. Therefore, the use of E50K mice in exploring the mechanisms of NTG pathogenesis, as well as the development of new therapeutic interventions, holds great promise.
Rab8 belongs to a family of small GTP-binding proteins which act as regulators of multiple cellular processes. Rab GTPases regulate all stages of membrane trafficking, including vesicle transport, cargo sorting, transport, tethering and fusion (32). Rab8 has been shown to be involved in polarized membrane transport and regulation of vesicular transport from the trans-Golgi network (33). Recently, OPTN was demonstrated to protect survival of NIH3T3 cells under oxidative stress by relocating to the nucleus in an Rab8-dependent manner, whereas E50K lost the ability to translocate to the nucleus (34). These previous functional analyses of the protein–protein interaction reveal that OPTN–Rab8 complex is involved in multiple functions that are essential for retinal and optic nerve health. Thus, alterations of this complex by E50K mutation may influence the entire cellular function, leading to glaucomatous-like pathology.
Our study demonstrated for the first time a direct protein–protein interaction of OPTN and Rab8 at the Golgi-complex and spreading vesicles (Fig. 6C), which was completely abolished by E50K mutation. The downstream effect of this disruption can be predicted by two studies by Buss and colleagues and Canals and colleagues, who demonstrated the importance of OPTN–Rab8 complex with myosin VI or huntingtin for post-Golgi trafficking, respectively (18,35). Sahlendaer et al. (18) demonstrated that OPTN and active Rab8 interact with myosin VI and this is essential for the formation of Golgi ribbon and exocytosis. del Toro et al. (35) demonstrated that a mutation in huntingtin reduces interaction with OPTN–Rab8 complex, resulting in delocalization of the complex in Golgi and impairment of post-Golgi trafficking. In both studies, OPTN–Rab8 was considered essential component of post-Golgi trafficking system, whereas OPTN served as an effecter protein of Rab8 and a binding partner of the actin-based motor protein myosin VI. Disruption of this complex may result in a significant reduction of selected protein transport within the cell and to the cell surface for secretion. It would be interesting to investigate what type of cargo is affected by the E50K mutation and if cells be rescued by supplementing this cargo molecule. If these proteins can be identified, it may serve as potential therapeutic approach to treat glaucoma patients with E50K mutation.
In the photoreceptor, Rab8 has a pivotal role of docking and fusion in rhodopsin trafficking (36) and cooperating with Bardet–Biedl syndrome proteins in ciliary membrane biogenesis (37,38). One possible explanation about the significant apoptotic changes in the ONL suggests that disruption of OPTN–Rab8 complex may affect the trafficking not only in the RGCs but also in the photoreceptors. Until now, there are no reports about the correlation between OPTN and Rab8 in the photoreceptors, or OPTN-mutated glaucoma and photoreceptor function. Considering trafficking malfunction via OPTN mutation as the etiology of glaucoma, E50K mice provides a good animal model to explore the pathogenesis of RGC and photoreceptor.
MATERIALS AND METHODS
Cloning of mouse Optn and site-directed mutagenesis
Total RNA was extracted from a fresh C57BL/6N mouse brain tissue using TRIzol (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed into first-strand cDNA using oligo-dT adaptor primer and SuperScript First-Strand Synthesis System for RT–PCR (Invitrogen). OPTN cDNA was amplified by PCR using oligonucleotides 5′-cggaattccgatgtcccatcaacctctgag-3′ and 5′-cggaattccgtcaaatgatgcagtccatca-3′ as primers. The amplified DNA fragment was purified using a MinElute gel extraction kit (Qiagen, Hilden, Germany), ligated into pBluescript II (KS-) (Agilent Technologies, Santa Clara, CA, USA) and sequenced using the M13 primers and ABI PRISM 3130 (Applied Biosystems, Foster City, CA, USA). Site-directed mutagenesis was carried out to produce cDNA corresponding to the deletion of the E50K mutation, the first leucine zipper (1st LZ del), deletion of the second leucine zipper (2nd LZ del) and the H489R mutation. The following primers were used: 5′-cagctcaaactcaactccgg-3′ and 5′-atgctccacttcctgctcca-3′ for 1st LZ del, 5′-aaatgaaggaactcctggttaagaaccaccagctgaaagaa-3′ and 5′-ttctttcagctggtggttcttaaccaggagttccttcattt-3′ for E50K, 5′-gagaccatggccgtcctc-3′ and 5′-caacatcttgtccaccttttctg-3′ for 2nd LZ del and 5′-gcagcaagagagaagattcgtgaagaaaaggagcagc-3′ and 5′-gctgctccttttcttcacgaatcttctctcttgctgc-3′ for H489R. Plasmids were digested with EcoRI, purified by agarose gel electrophoresis and recovered using the MinElute gel extraction kit according to the manufacturer's protocol. The cDNA inserts were ligated into EcoRI-digested pCMVHA vector (Takara Bio USA, Madison, WI, USA). HA-tagged Optns were amplified by PCR using oligonucleotides 5′-ccgctcgagcgccaccatgatgtacccatacgatgttcc-3′ and 5′-ccgctcgagcggtcaaatgatgcagtccatca-3′ as primers. HA-tag was inserted at the N-terminus of Optn constructs for the detection of proteins expressed by the transgene. The amplified DNA fragments were purified using a MinElute gel extraction kit (Qiagen), ligated into the expression vector and sequenced as described earlier.
Development of transgenic mice overexpressing mutant Optn
The expression vector pCAGGS containing chicken beta-actin promoter with CMV enhancer kindly provided by Dr Junichi Miyazaki of Osaka University was used for strong ubiquitous expression of the transgene in mice. cDNA inserts were released from the pCAGGS vector using SalI and BamHI. These restriction fragments were injected into pronuclear-stage BDF1/C57BL6N embryos, and transgenic mice were generated at PhoenixBio Co., Ltd (Tochigi, Japan). Offspring were screened for the transgene by isolating genomic DNA from tail biopsies followed by PCR. Primers used for PCR were 5′-ctctagagcctctgctaaccatgt-3′ and 5′-ccatggccataagagcgtaa-3′. To determine copy number of transgenes, real-time PCR was performed using TaqMan MGB probe (Applied Biosystems), according to manufacturer's standard protocols. Primers and probe for the mouse beta-actin were 5′-AGGCCAACCGTGAAAAGATG-3′ (forward), 5′-TGAGAAGCTGGCCAAAGAGAA-3′ (reverse) and 5′-CCCAGGTCAGTATCC-3′ (probe); for the CAG promoter were 5′-CCGCAGCCATTGCCTTT-3′ (forward), 5′-TTCGGCTCCGCACAGATT-3′ (reverse) and 5′-CGCAGGGACTTCC-3′ (probe). To determine the absolute amount of the copy number of beta-actin, PCR products of mouse beta-actin amplified from genomic DNA were cloned into the pCAGGS vector. The copy number of beta-actin gene in mouse genome was measured with real-time PCR analyses, the purified plasmid DNAs were used as standards. To determine the transgene copy number, multiplex quantitative PCR was performed for both CAG promoter as a target and beta-actin gene as a reference. All experiments with mice were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Vision Research.
Histology and immunohistochemistry
Mice were sacrificed with Nembutal (150 mg/kg) i.p., and the eyes were removed quickly. For histology, mouse eyes were dissected and immersed in Davidson's solution fixative overnight at 4°C. The eyes were embedded in paraffin and sectioned at 5 μm thickness along the vertical meridian through the optic nerve head. After deparaffinization and rehydration, sections were stained with hematoxylin and eosin (HE) staining. Images of HE staining were collected with Nikon Eclipse light microscope (Nikon, Tokyo, Japan).
For immunohistochemistry, after deparaffinization and rehydration, eye sections were treated with Target Retrieval Solution (DakoCytomation, Denmark). These sections were incubated with blocking solution for 1 h followed by overnight incubation with primary antibody against HA tag (1:500 dilution; Sigma-Aldrich, St Louis, MO, USA), OPTN (1:500 dilution; kind gift from Dr Mansoor Sarfarazi, University of Connecticut), tubulin β-III isoform (1:100 dilution; Millipore, Billerica, MA, USA), NeuN (1:100 dilution; Millipore), calretinin (1:500 dilution; Sigma), tyrosine hydroxylase (1:100 dilution; Millipore), PKC α (1:500 dilution; Millipore), rhodopsin (1:200 dilution; Santa Cruz, CA, USA), synaptophysin (1:500 dilution; Abcam, Cambridge, MA, USA) or ssDNA (1:500 dilution; Immuno-Biological Laboratories, Gunma, Japan) in phosphate-buffered saline (PBS) containing 1% BSA at 4°C. Slides were washed in PBS and then incubated with Alexa 488 or Alexa 568 (1:500 dilution; Invitrogen)-conjugated anti-mouse or rabbit IgG and 4′,6′-diamidino-2-phenylindole (DAPI) for nuclear staining for 1 h at room temperature. The stained tissues were examined using confocal fluorescence laser microscope (Radiance 2000, Bio-Rad Laboratories, Hercules, CA, USA). Control slides were processed similarly, except for the omission of primary antibodies (data not shown).
Whole-mount immunostaining
The whole-mount immunostaining was performed essentially as described (39,40). Briefly, neural retinas were separated from the posterior eyes, fixed in 4% PFA/PBS for 2 h on ice and incubated with the anti-SMI32 (1:200 dilution; Sternberger Monoclonals, Baltimore, MD, USA), anti-ChAT (1:100 dilution; Millipore) or anti-active NeuN (1:250 dilution; Millipore) antibody for 7 days at 4°C. Slides were washed in PBS containing 0.1% Triton X-100 and then incubated with Alexa 488 or Alexa 568 (1:500 dilution; Invitrogen)-conjugated anti-mouse or rabbit IgG/DAPI for 2 days at 4°C. Whole-mounted retinal samples were placed on slides, with the vitreous facing up. Radial cuts were made in the peripheral retina, and the retinal tissue was flattened with a fine brush. The retinas were then mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA) and evaluated with a confocal microscope.
Measurement of IOP
The average IOP for each genotype was recorded. IOP was measured using an impact-rebound tonometer (Colonal Medical Supply, Franconia, NH, USA) and optical interferometry tonometer (FISO Technologies, Quebec, Canada) for mice of each genotype as described (29). Using the rebound tonometer, we were able to measure IOP in awake and non-sedated mice of various ages, whereas optical interferometry tonometry was performed on anesthetized animals. Measurement of IOP was always performed in the morning between 10 and 12 AM. The mice successfully assessed for each genotype and age were 18 weeks and 16 months.
Measurement of OPTN–Rab8 interaction
OPTN–Rab8 interaction analysis was performed using CoralHue® Fluo-chase Kit (MBL, Tokyo, Japan). Based on the instruction manual, we constructed OPTN Wt, E50K, Rab8 Q67L (GTP-bound active form), Rab8 T22N (GDP-bound inactive form), with fluorescence tag protein (mKG_N or mKG_C) on either N-terminal or C-terminal (Fig. 6A). RGC-5 and COS1 cells were transfected by each pair of the plasmid mixtures using Fugene HD (Roche Diagnostics, Mannheim, Germany). Forty-eight hours after transfection, the medium was replaced to PBS and the cells were observed with inverted microscope (Eclipse TE300, Nikon). To observe localization of OPTN–Rab8 complex, cells were fixed 48 h after transfection with 4% paraformaldehyde in PBS on ice for 20 min. Cells were incubated in blocking buffer (3% bovine serum albumin, 0.1% Triton X-100 in PBS) and then with anti-GM130 antibody (BD Bioscience, San Jose, CA, USA) at room temperature for 1 h each. Cells were washed three times with PBS-T (0.1% Triton X-100 in PBS) and incubated with Alexa-568-conjugated secondary antibody for 1 h at room temperature. Slides were washed, mounted and analyzed by confocal microscopy.
Statistical analysis
All data were expressed as the mean ± standard deviation. Statistical differences were analyzed by the ANOVA or Student's t-test. *P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL
FUNDING
This research was supported in part by the grants to T.I. by the Japan Ministry of Health, Labor, and Welfare, to T.I. and M.A. by the Japan Ministry of Education, Culture, Sports, Science and Technology and to T.I. for Japan Society for the Promotion Science Fellowship for Z.C. Funding to pay the Open Access publication charges for this article was provided by the Japan Ministry of Health, Labor, and Welfare.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank T.V. Johnson, S. Zigler, Jr, for critical reading of the manuscript and K. Fujinami for helpful comments.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Quigley H.A. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. doi:10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley H.A., Broman A.T. The number of people with glaucoma world wide in 2010 and 2020. Br. J. Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. doi:10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwase A., Suzuki Y., Araie M., Yamamoto T., Abe H., Shirato S., Kuwayama Y., Mishima H.K., Shimizu H., Tomita G., et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111:1641–1648. doi: 10.1016/j.ophtha.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Stone E.M., Fingert J.H., Alward W.L., Nguyen T.D., Polansky J.R., Sunden S.L., Nishimura D., Clark A.F., Nystuen A., Nichols B.E., et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. doi:10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 5.Stoilov I., Akarsu A.N., Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum. Mol. Genet. 1997;6:641–647. doi: 10.1093/hmg/6.4.641. doi:10.1093/hmg/6.4.641. [DOI] [PubMed] [Google Scholar]
- 6.Rezaie T., Child A., Hitchings R., Brice G., Miller L., Coca-Prados M., Heon E., Krupin T., Ritch R., Kreutzer D., et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. doi:10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 7.Monemi S., Spaeth G., DaSilva A., Popinchalk S., Ilitchev E., Liebmann J., Ritch R., Heon E., Crick R.P., Child A., et al. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum. Mol. Genet. 2005;14:725–733. doi: 10.1093/hmg/ddi068. doi:10.1093/hmg/ddi068. [DOI] [PubMed] [Google Scholar]
- 8.Aung T., Rezaie T., Okada K., Viswanathan A.C., Child A.H., Brice G., Bhattacharya S.S., Lehmann O.J., Sarfarazi M., Hitchings R.A. Clinical features and course of patients with glaucoma with the E50K mutation in the optineurin gene. Invest. Ophthalmol. Vis. Sci. 2005;46:2816–2822. doi: 10.1167/iovs.04-1133. doi:10.1167/iovs.04-1133. [DOI] [PubMed] [Google Scholar]
- 9.Leung Y.F., Fan B.J., Lam D.S., Lee W.S., Tam P.O., Chua J.K., Tham C.C., Lai J.S., Fan D.S., Pang C.P. Different optineurin mutation pattern in primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 2003;44:3880–3884. doi: 10.1167/iovs.02-0693. doi:10.1167/iovs.02-0693. [DOI] [PubMed] [Google Scholar]
- 10.Fuse N., Takahashi K., Akiyama H., Nakazawa T., Seimiya M., Kuwahara S., Tamai M. Molecular genetic analysis of optineurin gene for primary open-angle and normal tension glaucoma in the Japanese population. J. Glaucoma. 2004;13:299–303. doi: 10.1097/00061198-200408000-00007. doi:10.1097/00061198-200408000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Alward W.L., Kwon Y.H., Kawase K., Craig J.E., Hayreh S.S., Johnson A.T., Khanna C.L., Yamamoto T., Mackey D.A., Roos B.R., et al. Evaluation of optineurin sequence variations in 1,048 patients with open-angle glaucoma. Am. J. Ophthalmol. 2003;136:904–910. doi: 10.1016/s0002-9394(03)00577-4. doi:10.1016/S0002-9394(03)00577-4. [DOI] [PubMed] [Google Scholar]
- 12.Hauser M.A., Sena D.F., Flor J., Walter J., Auguste J., Larocque-Abramson K., Graham F., Delbono E., Haines J.L., Pericak-Vance M.A., et al. Distribution of optineurin sequence variations in an ethnically diverse population of low-tension glaucoma patients from the United States. J. Glaucoma. 2006;15:358–363. doi: 10.1097/01.ijg.0000212255.17950.42. doi:10.1097/01.ijg.0000212255.17950.42. [DOI] [PubMed] [Google Scholar]
- 13.Chalasani M.L., Radha V., Gupta V., Agarwal N., Balasubramanian D., Swarup G. A glaucoma-associated mutant of optineurin selectively induces death of retinal ganglion cells which is inhibited by antioxidants. Invest. Ophthal. Vis. Sci. 2007;48:1607–1614. doi: 10.1167/iovs.06-0834. doi:10.1167/iovs.06-0834. [DOI] [PubMed] [Google Scholar]
- 14.Faber P.W., Barnes G.T., Srinidhi J., Chen J., Gusella J.F., MacDonald M.E. Huntingtin interacts with a family of WW domain proteins. Hum. Mol. Genet. 1998;7:1463–1474. doi: 10.1093/hmg/7.9.1463. doi:10.1093/hmg/7.9.1463. [DOI] [PubMed] [Google Scholar]
- 15.Moreland R.J., Dresser M.E., Rodgers J.S., Roe B.A., Conaway J.W., Conaway R.C., Hanas J.S. Identification of a transcription factor IIIA-interacting protein. Nucleic Acids Res. 2000;28:1986–1993. doi: 10.1093/nar/28.9.1986. doi:10.1093/nar/28.9.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hattula K., Peranen J. FIP-2, a coiled-coil protein, links Huntingtin to Rab8 and modulates cellular morphogenesis. Curr. Biol. 2000;10:1603–1606. doi: 10.1016/s0960-9822(00)00864-2. doi:10.1016/S0960-9822(00)00864-2. [DOI] [PubMed] [Google Scholar]
- 17.Park B.C., Shen X., Samaraweera M., Yue B.Y. Studies of optineurin, a glaucoma gene: Golgi fragmentation and cell death from overexpression of wild-type and mutant optineurin in two ocular cell types. Am. J. Pathol. 2006;169:1976–1989. doi: 10.2353/ajpath.2006.060400. doi:10.2353/ajpath.2006.060400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahlender D.A., Roberts R.C., Arden S.D., Spudich G., Taylor M.J., Luzio J.P., Kendrick-Jones J., Buss F. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J. Cell. Biol. 2005;169:285–295. doi: 10.1083/jcb.200501162. doi:10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto-Sato E., Ishizaka M., Horisawa K., Tateyama S., Takashima H., Fuse S., Sue K., Hirai N., Masuoka K., Yanagawa H. Cell-free cotranslation and selection using in vitro virus for high-throughput analysis of protein–protein interactions and complexes. Genome Res. 2005;15:710–717. doi: 10.1101/gr.3510505. doi:10.1101/gr.3510505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colland F., Jacq X., Trouplin V., Mousin C., Groizeleau C., Hamburger A., Meil A., Wojcik J., Legrain P., Gauthier J.M. Functional proteomics mapping of a human signaling pathway. Genome Res. 2004;14:1324–1332. doi: 10.1101/gr.2334104. doi:10.1101/gr.2334104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anborgh P.H., Godin C., Pampillo M., Dhami G.K., Dale L.B., Cregan S.P., Truant R., Ferguson S.S. Inhibition of metabotropic glutamate receptor signalling by the huntingtin binding protein optineurin. J. Biol. Chem. 2005;280:34840–34848. doi: 10.1074/jbc.M504508200. doi:10.1074/jbc.M504508200. [DOI] [PubMed] [Google Scholar]
- 22.Sudhakar C., Nagabhushana A., Jain N., Swarup G. NF-kappaB mediates tumor necrosis factor alpha-induced expression of optineurin, a negative regulator of NF-kappaB. PLoS One. 2009;4:e5114. doi: 10.1371/journal.pone.0005114. doi:10.1371/journal.pone.0005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Journo C., Filipe J., About F., Chevalier S.A., Afonso P.V., Brady J.N., Flynn D., Tangy F., Israël A., Vidalain P.O. NRP/optineurin cooperates with TAX1BP1 to potentiate the activation of NF-kappaB by human T-lymphotropic virus type 1 tax protein. PLoS Pathog. 2009;5:e1000521. doi: 10.1371/journal.ppat.1000521. doi:10.1371/journal.ppat.1000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada T., Harada C., Nakamura K., Quah H.M., Okumura A., Namekata K., Saeki T., Aihara M., Yoshida H., Mitani A., et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J. Clin. Invest. 2007;117:1763–1770. doi: 10.1172/JCI30178. doi:10.1172/JCI30178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Funayama T., Ishikawa K., Ohtake Y., Tanito T., Kurosaka D., Kimura I., Suzuki K., Ideta H., Nakamoto K., Yasuda N., et al. Variants in optineurin gene and their association with tumor necrosis factor-alpha polymorphisms in Japanese patients with glaucoma. Invest. Ophthalmol. Vis. Sci. 2004;45:4359–4367. doi: 10.1167/iovs.03-1403. doi:10.1167/iovs.03-1403. [DOI] [PubMed] [Google Scholar]
- 26.Weisschuh N., Neumann D., Wolf C., Wissinger B., Gramer E. Prevalence of myocilin and optineurin sequence variants in German normal tension glaucoma patients. Mol. Vis. 2005;11:284–287. [PubMed] [Google Scholar]
- 27.Yao H.Y., Cheng C.Y., Fan B.J., Tam O.S., Tham C.Y., Wang D.Y., Lam S.C., Pang C.P. Polymorphisms of myocilin and optineurin I primary open-angle glaucoma patients. Zhonghua Yi Xue Za Zhi. 2006;86:554–559. [PubMed] [Google Scholar]
- 28.John S.W., Smith R.S., Savinova O.V., Hawes N.L., Chang B., Turnbull D., Davisson M., Roderick T.H., Heckenlively J.R. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest. Ophthalmol. Vis. Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- 29.Senatorov V., Malyukova I., Fariss R., Wawrousek E.F., Swaminathan S., Sharan S.K., Tomarev S. Expression of mutated mouse myocilin induces open-angle glaucoma in transgenic mice. J. Neurosci. 2006;26:11903–11914. doi: 10.1523/JNEUROSCI.3020-06.2006. doi:10.1523/JNEUROSCI.3020-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y., Grinchuk O., Tomarev S.I. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Invest. Ophthalmol. Vis. Sci. 2008;49:1932–1939. doi: 10.1167/iovs.07-1339. doi:10.1167/iovs.07-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster P.J., Buhrmann R., Quigley H.A., Johnson G.J. The definition and classification of glaucoma in prevalence surveys. Br. J. Ophthalmol. 2002;86:238–242. doi: 10.1136/bjo.86.2.238. doi:10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell. Biol. 2001;2:107–117. doi: 10.1038/35052055. doi:10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 33.Huber L.A., Pimplikar S., Parton R.G., Virta H., Zerial M., Simons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J. Cell. Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. doi:10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Marco N., Buono M., Troise F., Diez-Roux G. Optineurin increases cell survival and translocates to the nucleus in a Rab8-dependent manner upon an apoptotic stimulus. J. Biol. Chem. 2006;281:16147–16156. doi: 10.1074/jbc.M601467200. doi:10.1074/jbc.M601467200. [DOI] [PubMed] [Google Scholar]
- 35.del Toro D., Alberch J., Lázaro-Diéguez F., Martín-Ibáñez R., Xifró X., Egea G., Canals J.M. Mutant huntingtin impairs post-Golgi trafficking to lysosomes by delocalizing optineurin/Rab8 complex from the Golgi apparatus. Mol. Biol. Cell. 2009;20:1478–1492. doi: 10.1091/mbc.E08-07-0726. doi:10.1091/mbc.E08-07-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moritz O.L., Tam B.M., Hurd L.L., Peranen J., Deretic D., Papermaster D.S. Mutant rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol. Biol. Cell. 2001;12:2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato T., Mushiake S., Kato Y., Sato K., Sato M., Takeda N., Ozono K., Miki K., Kubo Y., Tsuji A., et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature. 2007;448:366–369. doi: 10.1038/nature05929. doi:10.1038/nature05929. [DOI] [PubMed] [Google Scholar]
- 38.Nachury M.V., Loktev A.V., Zhang Q., Westlake C.J., Peränen J., Merdes A., Slusarski D.C., Scheller R.H., Bazan J.F., Sheffield V.C., et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. doi:10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 39.Jakobs T.C., Libby R.T., Ben Y., John S.W., Masland R.H. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J. Cell Biol. 2005;171:313–325. doi: 10.1083/jcb.200506099. doi:10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howell G.R., Libby R.T., Jakobs T.C., Smith R.S., Phalan F.C., Barter J.W., Barbay J.M., Marchant J.K., Mahesh N., Porciatti V., et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. doi:10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.