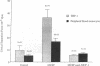Abstract
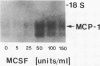
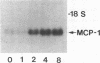
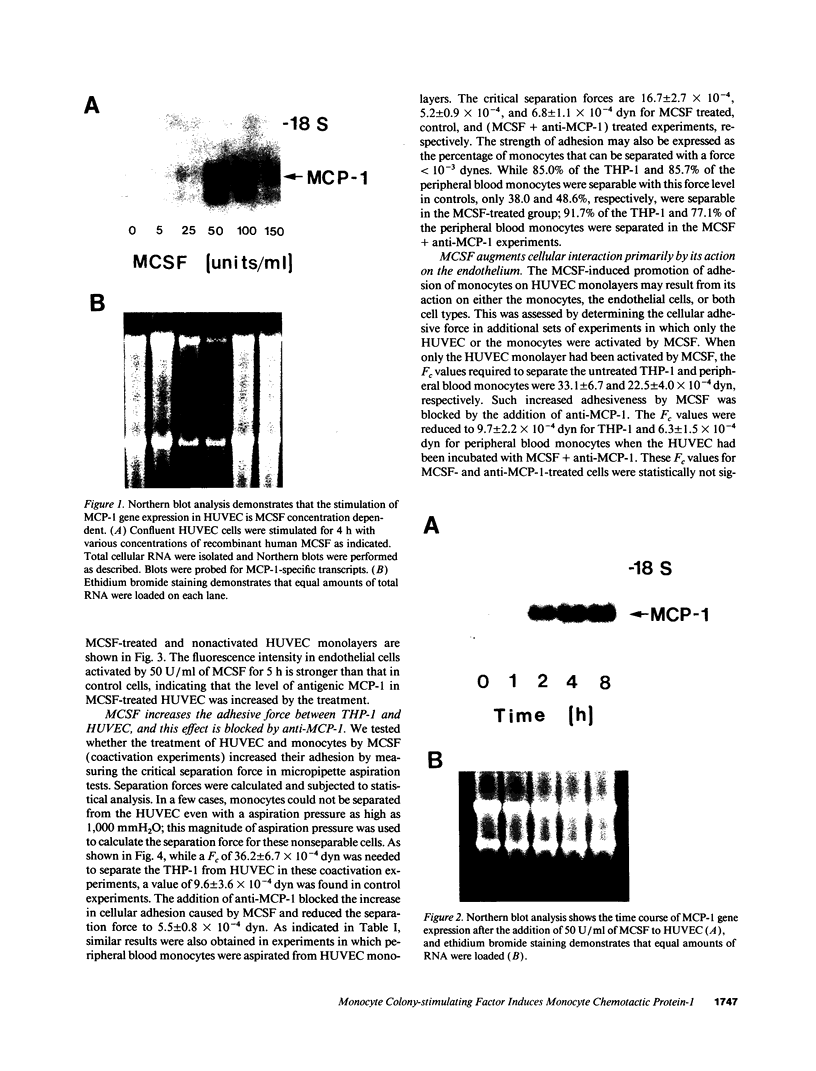
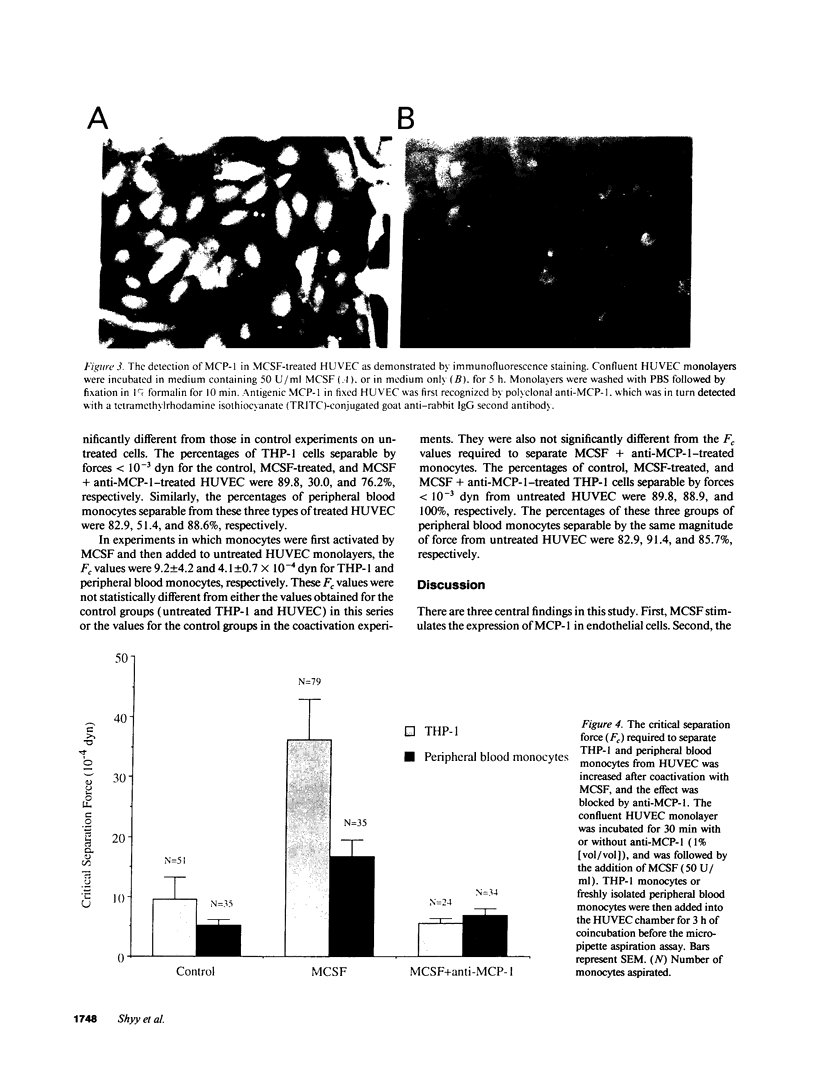
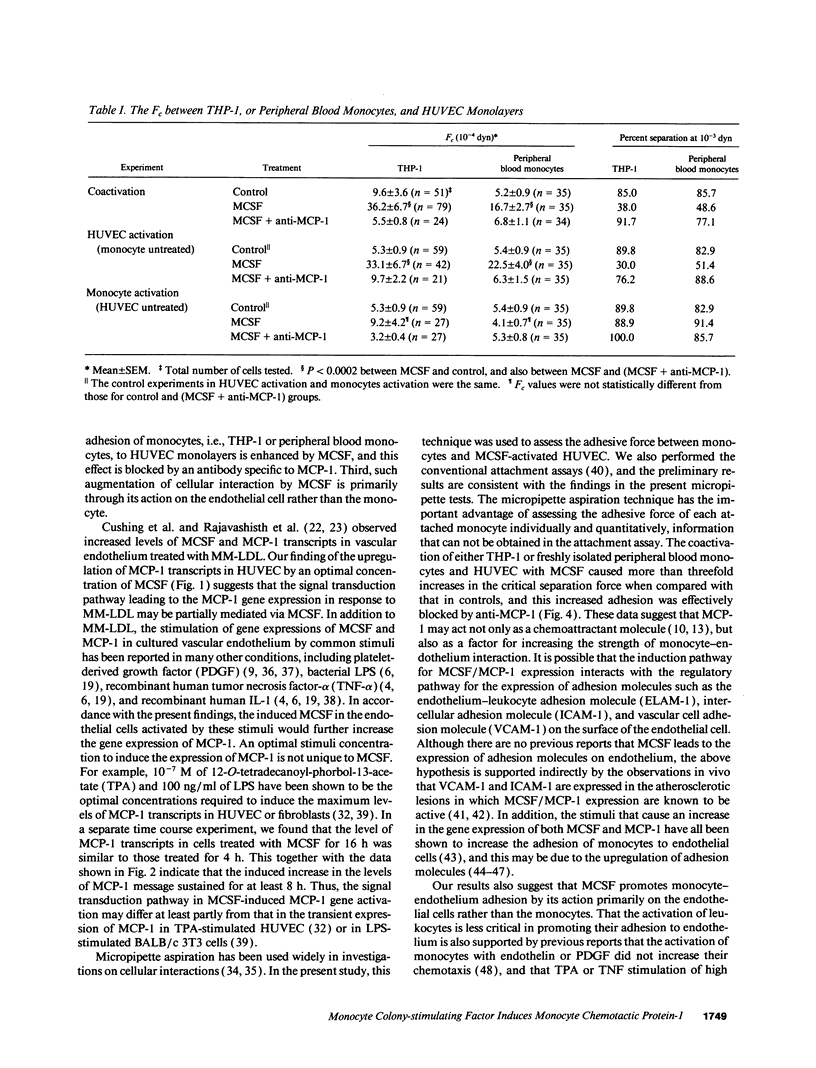
The stimulation of the human umbilical vein endothelial cell (HUVEC) with recombinant human monocyte-derived colony-stimulating factor (MCSF) increased the gene expression of monocyte chemotactic protein (MCP-1). Northern blot analysis indicated that 50 U/ml of MCSF is the optimal concentration for this effect. The elevation of MCP-1 mRNA started as early as 1 h after stimulation and was maintained for at least 8 h. An increased MCP-1 level in MCSF-treated HUVEC was also demonstrated at the protein level by immunocytochemical staining using a polyclonal MCP-1-specific antibody. HUVEC activated by 50 U/ml of MCSF for 5 h showed a stronger immunofluorescence staining than control cells. Micropipette separation of THP-1 monocytes from HUVEC showed that the activation of both THP-1 and endothelium by MCSF led to an increase in the separation force by more than three times (36.2 +/- 6.7 x 10(-4) vs. 9.6 +/- 3.6 x 10(-4) dyn). An increased adhesiveness was also observed after MCSF activation of peripheral blood monocytes and HUVEC (16.7 +/- 2.7 x 10(-4) vs. 5.2 +/- 0.9 x 10(-4) dyn). The increased adhesive force in both systems was blocked by the use of anti-MCP-1 (5.5 +/- 0.8 x 10(-4) and 6.8 +/- 1.1 x 10(-4) dyn). Similar results were obtained in experiments in which only HUVEC, but not monocytes, were activated by MCSF. This increased adhesion of untreated monocytes to MCSF-activated HUVEC was also blocked by the addition of anti-MCP-1. In contrast, experiments in which only THP-1 or peripheral blood monocytes, but not HUVEC, were treated with MCSF did not show a significant increase of adhesion between these cells. These results indicate that MCSF augments monocyte-endothelium interaction primarily by its action on the endothelial cell and that this function is probably mediated through an increased expression of MCP-1. The MCSF/MCP-1-dependent adhesive mechanism might be operative in the arterial wall in vivo to lead to the trapping of the infiltrated monocyte-macrophage in the subendothelial space during atherogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud S. L., Pinzani M. Peptide growth factors stimulate macrophage colony-stimulating factor in murine stromal cells. Blood. 1991 Jul 1;78(1):103–109. [PubMed] [Google Scholar]
- Bath P. M., Mayston S. A., Martin J. F. Endothelin and PDGF do not stimulate peripheral blood monocyte chemotaxis, adhesion to endothelium, and superoxide production. Exp Cell Res. 1990 Apr;187(2):339–342. doi: 10.1016/0014-4827(90)90102-g. [DOI] [PubMed] [Google Scholar]
- Becker S., Warren M. K., Haskill S. Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures. J Immunol. 1987 Dec 1;139(11):3703–3709. [PubMed] [Google Scholar]
- Bochner B. S., Luscinskas F. W., Gimbrone M. A., Jr, Newman W., Sterbinsky S. A., Derse-Anthony C. P., Klunk D., Schleimer R. P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991 Jun 1;173(6):1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., De Rossi M., Sica A., Colotta F., Wang J. M., Bocchietto E., Padura I. M., Bosia A., DeJana E., Mantovani A. Murine endothelioma cell lines transformed by polyoma middle T oncogene as target for and producers of cytokines. J Immunol. 1991 Oct 1;147(7):2122–2129. [PubMed] [Google Scholar]
- Chin Y. H., Cai J. P., Johnson K. Lymphocyte adhesion to cultured Peyer's patch high endothelial venule cells is mediated by organ-specific homing receptors and can be regulated by cytokines. J Immunol. 1990 Dec 1;145(11):3669–3677. [PubMed] [Google Scholar]
- Clinton S. K., Underwood R., Hayes L., Sherman M. L., Kufe D. W., Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992 Feb;140(2):301–316. [PMC free article] [PubMed] [Google Scholar]
- Colotta F., Borré A., Wang J. M., Tattanelli M., Maddalena F., Polentarutti N., Peri G., Mantovani A. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J Immunol. 1992 Feb 1;148(3):760–765. [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Das S. K., Stanley E. R. Structure-function studies of a colony stimulating factor (CSF-1). J Biol Chem. 1982 Nov 25;257(22):13679–13684. [PubMed] [Google Scholar]
- Fibbe W. E., Daha M. R., Hiemstra P. S., Duinkerken N., Lurvink E., Ralph P., Altrock B. W., Kaushansky K., Willemze R., Falkenburg J. H. Interleukin 1 and poly(rI).poly(rC) induce production of granulocyte CSF, macrophage CSF, and granulocyte-macrophage CSF by human endothelial cells. Exp Hematol. 1989 Mar;17(3):229–234. [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Bevilacqua M. P., Cybulsky M. I. Endothelial-dependent mechanisms of leukocyte adhesion in inflammation and atherosclerosis. Ann N Y Acad Sci. 1990;598:77–85. doi: 10.1111/j.1749-6632.1990.tb42279.x. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Jiang Y. L., Williamson M. J., Valente A. J. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989 Sep 29;245(4925):1490–1493. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- Hall D. J., Brownlee C., Stiles C. D. Interleukin-1 is a potent regulator of JE and KC gene expression in quiescent BALB/c fibroblasts. J Cell Physiol. 1989 Oct;141(1):154–159. doi: 10.1002/jcp.1041410123. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Seifert P. S., Stemme S. Immune mechanisms in atherosclerosis. Arteriosclerosis. 1989 Sep-Oct;9(5):567–578. doi: 10.1161/01.atv.9.5.567. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Inaba T., Shimano H., Harada K., Inoue I., Mokuno H., Mori N., Gotoda T., Takaku F., Yamada N. Monocyte colony-stimulating factor enhances uptake and degradation of acetylated low density lipoproteins and cholesterol esterification in human monocyte-derived macrophages. J Biol Chem. 1990 Aug 25;265(24):14109–14117. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Valente A. J., Williamson M. J., Zhang L., Graves D. T. Post-translational modification of a monocyte-specific chemoattractant synthesized by glioma, osteosarcoma, and vascular smooth muscle cells. J Biol Chem. 1990 Oct 25;265(30):18318–18321. [PubMed] [Google Scholar]
- Kawahara R. S., Deuel T. F. Platelet-derived growth factor-inducible gene JE is a member of a family of small inducible genes related to platelet factor 4. J Biol Chem. 1989 Jan 15;264(2):679–682. [PubMed] [Google Scholar]
- Lane T. A., Lamkin G. E., Wancewicz E. Modulation of endothelial cell expression of intercellular adhesion molecule 1 by protein kinase C activation. Biochem Biophys Res Commun. 1989 Jun 30;161(3):945–952. doi: 10.1016/0006-291x(89)91334-x. [DOI] [PubMed] [Google Scholar]
- Liao F., Berliner J. A., Mehrabian M., Navab M., Demer L. L., Lusis A. J., Fogelman A. M. Minimally modified low density lipoprotein is biologically active in vivo in mice. J Clin Invest. 1991 Jun;87(6):2253–2257. doi: 10.1172/JCI115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Cybulsky M. I., Kiely J. M., Peckins C. S., Davis V. M., Gimbrone M. A., Jr Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol. 1991 Mar 1;146(5):1617–1625. [PubMed] [Google Scholar]
- Montgomery K. F., Osborn L., Hession C., Tizard R., Goff D., Vassallo C., Tarr P. I., Bomsztyk K., Lobb R., Harlan J. M. Activation of endothelial-leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6523–6527. doi: 10.1073/pnas.88.15.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston R. N., Haskard D. O., Coucher J. R., Gall N. P., Johnson-Tidey R. R. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am J Pathol. 1992 Mar;140(3):665–673. [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Stiles C. D. A cell-cycle constraint on the regulation of gene expression by platelet-derived growth factor. Science. 1987 Nov 27;238(4831):1269–1271. doi: 10.1126/science.3685976. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Pober J. S. Interleukin-4 induces the synthesis and secretion of MCP-1/JE by human endothelial cells. Am J Pathol. 1991 Jun;138(6):1315–1319. [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Stier P., Ernst T., Wong G. G. The human homolog of the JE gene encodes a monocyte secretory protein. Mol Cell Biol. 1989 Nov;9(11):4687–4695. doi: 10.1128/mcb.9.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Walz A., Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991 Aug 15;78(4):1112–1116. [PubMed] [Google Scholar]
- Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Ylä-Herttuala S., Lipton B. A., Ord V. A., Witztum J. L., Steinberg D. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992 Feb;140(2):291–300. [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Cerami A. Small cytokine superfamily. Curr Opin Immunol. 1991 Feb;3(1):56–60. doi: 10.1016/0952-7915(91)90077-e. [DOI] [PubMed] [Google Scholar]
- Shimano H., Yamada N., Ishibashi S., Harada K., Matsumoto A., Mori N., Inaba T., Motoyoshi K., Itakura H., Takaku F. Human monocyte colony-stimulating factor enhances the clearance of lipoproteins containing apolipoprotein B-100 via both low density lipoprotein receptor-dependent and -independent pathways in rabbits. J Biol Chem. 1990 Aug 5;265(22):12869–12875. [PubMed] [Google Scholar]
- Shimano H., Yamada N., Motoyoshi K., Matsumoto A., Ishibashi S., Mori N., Takaku F. Plasma cholesterol-lowering activity of monocyte colony-stimulating factor (M-CSF). Ann N Y Acad Sci. 1990;587:362–370. doi: 10.1111/j.1749-6632.1990.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Shyy Y. J., Li Y. S., Kolattukudy P. E. Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem Biophys Res Commun. 1990 Jun 15;169(2):346–351. doi: 10.1016/0006-291x(90)90338-n. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Lipoproteins and atherosclerosis. A look back and a look ahead. Arteriosclerosis. 1983 Jul-Aug;3(4):283–301. doi: 10.1161/01.atv.3.4.283. [DOI] [PubMed] [Google Scholar]
- Sung K. L., Sung L. A., Crimmins M., Burakoff S. J., Chien S. Determination of junction avidity of cytolytic T cell and target cell. Science. 1986 Dec 12;234(4782):1405–1408. doi: 10.1126/science.3491426. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C. S., Major J. A., Poptic E. J., DiCorleto P. E., Hamilton T. A. Lipopolysaccharide induces competence genes JE and KC in Balb/C 3T3 cells. J Cell Physiol. 1990 Jul;144(1):77–83. doi: 10.1002/jcp.1041440111. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Stanley E. R. The regulation of mononuclear phagocyte entry into S phase by the colony stimulating factor CSF-1. J Cell Physiol. 1985 Feb;122(2):221–228. doi: 10.1002/jcp.1041220210. [DOI] [PubMed] [Google Scholar]
- Tözeren A., Sung K. L., Sung L. A., Dustin M. L., Chan P. Y., Springer T. A., Chien S. Micromanipulation of adhesion of a Jurkat cell to a planar bilayer membrane containing lymphocyte function-associated antigen 3 molecules. J Cell Biol. 1992 Feb;116(4):997–1006. doi: 10.1083/jcb.116.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente A. J., Graves D. T., Vialle-Valentin C. E., Delgado R., Schwartz C. J. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988 May 31;27(11):4162–4168. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989 Dec;3(14):2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Lipton B. A., Rosenfeld M. E., Särkioja T., Yoshimura T., Leonard E. J., Witztum J. L., Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Moore S. K., Appella E., Lerman M. I., Leonard E. J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989 Feb 27;244(2):487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]