Abstract
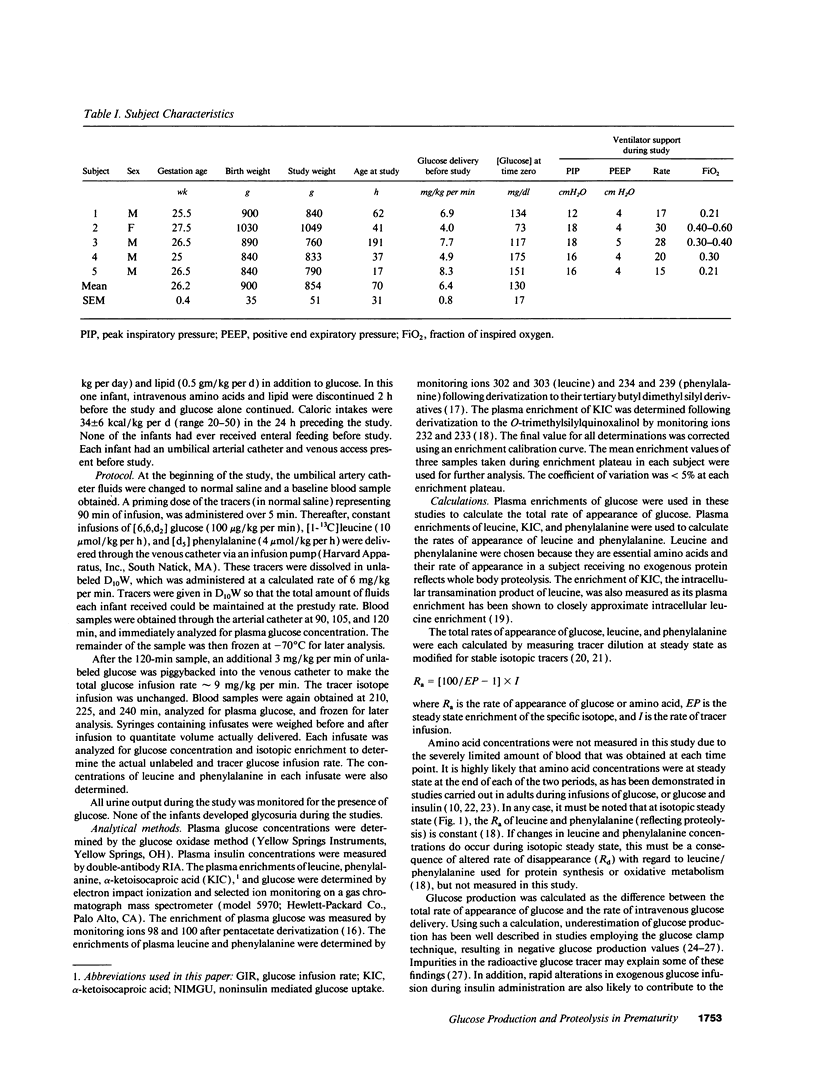
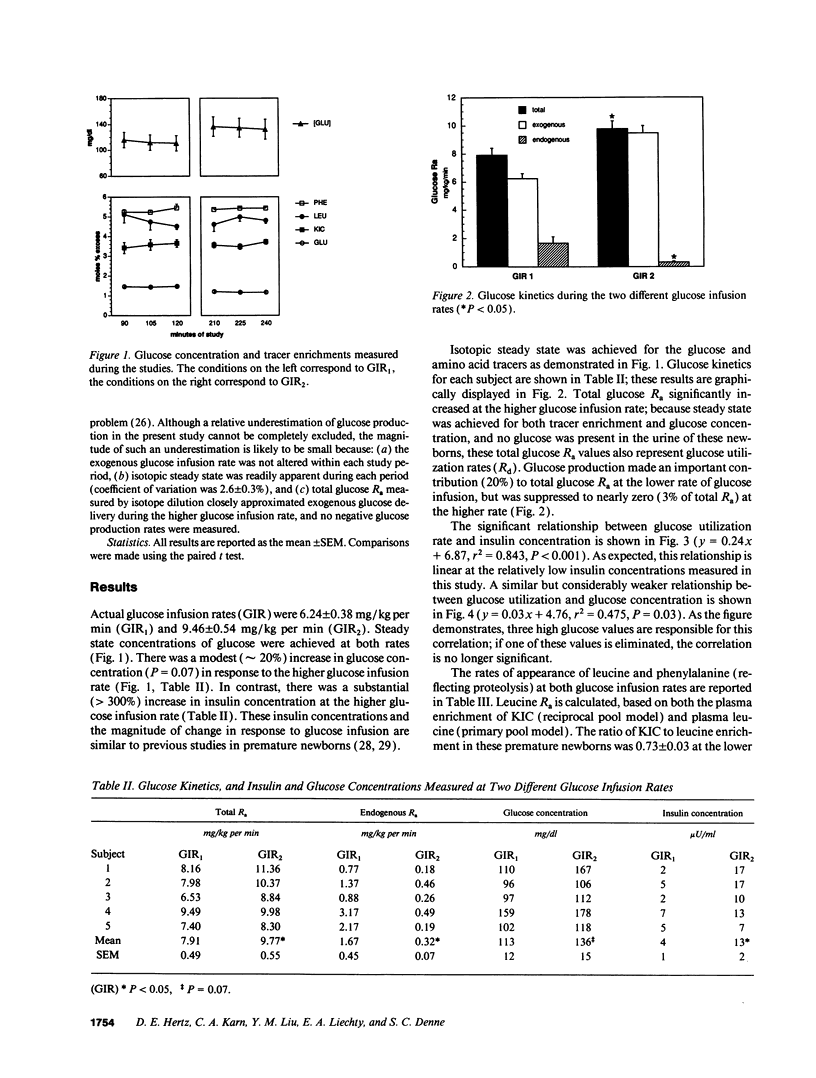
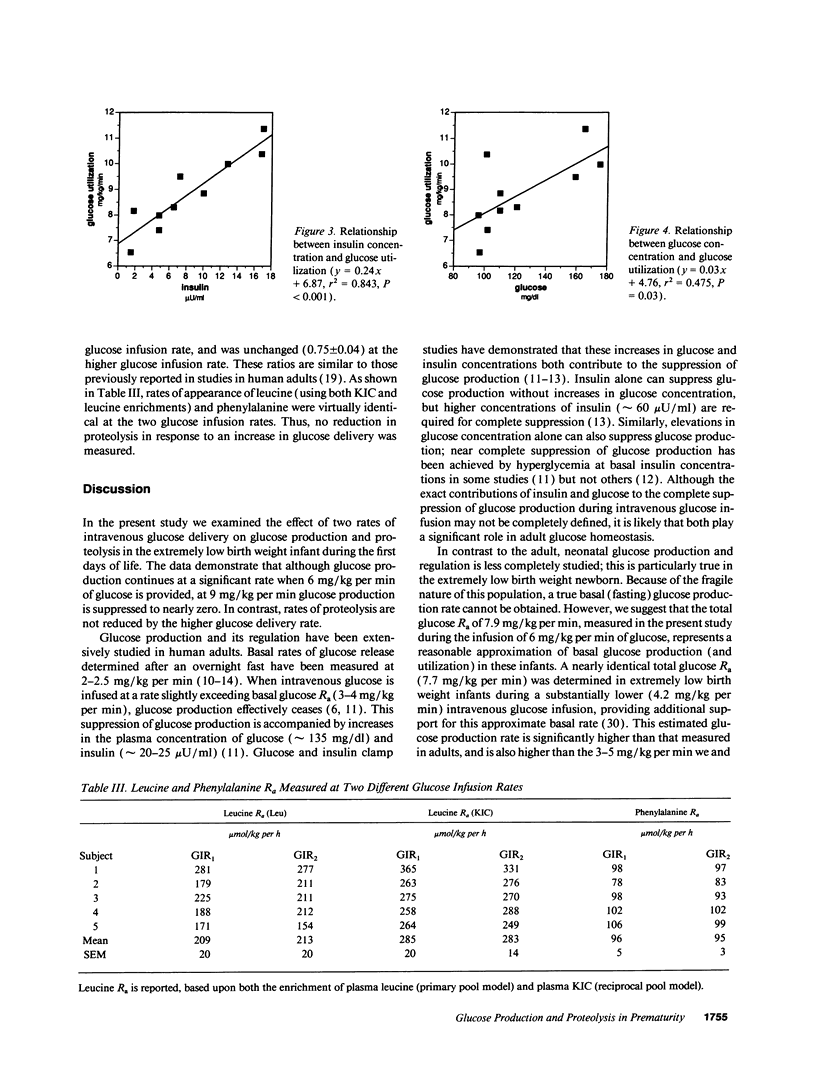
To ascertain whether the inability to suppress glucose production and increase glucose utilization in response to glucose infusion is an inherent characteristic of immature individuals, we determined glucose rate of appearance (R(a)) in minimally stressed, clinically stable, extremely premature infants (approximately 26-wk gestation) at two glucose infusion rates (6.2 +/- 0.4 and 9.5 +/- 0.5 mg/kg per min). We also assessed whether an increase in glucose delivery suppresses proteolysis by measuring the R(a) of phenylalanine and leucine. Glucose R(a) (and utilization) increased significantly at the higher glucose infusion rate (7.9 +/- 0.5 vs. 9.8 +/- 0.6 mg/kg per min). Glucose production persisted at the lower glucose infusion rate but was suppressed to nearly zero at the higher rate (1.7 +/- 0.5 vs. 0.3 +/- 0.1 mg/kg per min). Proteolysis was unaffected by the higher glucose infusion rate as reflected by no change in the rates of appearance of either phenylalanine (96 +/- 5 vs. 95 +/- 3 mumol/kg per h) or leucine (285 +/- 20 vs. 283 +/- 14 mumol/kg per h). Thus, clinically stable, extremely premature infants suppress glucose production and increase glucose utilization in response to increased glucose infusion, demonstrating no inherent immaturity of these processes. In contrast, increasing the rate of glucose delivery results in no change in whole body proteolysis in these infants. The regulation of proteolysis in this population remains to be defined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argoud G. M., Schade D. S., Eaton R. P. Underestimation of hepatic glucose production by radioactive and stable tracers. Am J Physiol. 1987 May;252(5 Pt 1):E606–E615. doi: 10.1152/ajpendo.1987.252.5.E606. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Kolterman O. G., Bell J., Mandarino L. J., Olefsky J. M. Rates of noninsulin-mediated glucose uptake are elevated in type II diabetic subjects. J Clin Invest. 1985 Nov;76(5):1782–1788. doi: 10.1172/JCI112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier D. M. Intrinsically difficult problems: the kinetics of body proteins and amino acids in man. Diabetes Metab Rev. 1989 Mar;5(2):111–132. doi: 10.1002/dmr.5610050203. [DOI] [PubMed] [Google Scholar]
- Caballero B., Wurtman R. J. Differential effects of insulin resistance on leucine and glucose kinetics in obesity. Metabolism. 1991 Jan;40(1):51–58. doi: 10.1016/0026-0495(91)90192-y. [DOI] [PubMed] [Google Scholar]
- Catzeflis C., Schutz Y., Micheli J. L., Welsch C., Arnaud M. J., Jéquier E. Whole body protein synthesis and energy expenditure in very low birth weight infants. Pediatr Res. 1985 Jul;19(7):679–687. doi: 10.1203/00006450-198507000-00009. [DOI] [PubMed] [Google Scholar]
- Cowett R. M., Andersen G. E., Maguire C. A., Oh W. Ontogeny of glucose homeostasis in low birth weight infants. J Pediatr. 1988 Mar;112(3):462–465. doi: 10.1016/s0022-3476(88)80337-8. [DOI] [PubMed] [Google Scholar]
- Cowett R. M., Oh W., Schwartz R. Persistent glucose production during glucose infusion in the neonate. J Clin Invest. 1983 Mar;71(3):467–475. doi: 10.1172/JCI110791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowett R. M., Susa J. B., Oh W., Schwartz R. Endogenous glucose production during constant glucose infusion in the newborn lamb. Pediatr Res. 1978 Aug;12(8):853–857. doi: 10.1203/00006450-197808000-00010. [DOI] [PubMed] [Google Scholar]
- Denne S. C., Kalhan S. C. Leucine metabolism in human newborns. Am J Physiol. 1987 Dec;253(6 Pt 1):E608–E615. doi: 10.1152/ajpendo.1987.253.6.E608. [DOI] [PubMed] [Google Scholar]
- Denne S. C., Liechty E. A., Liu Y. M., Brechtel G., Baron A. D. Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. Am J Physiol. 1991 Dec;261(6 Pt 1):E809–E814. doi: 10.1152/ajpendo.1991.261.6.E809. [DOI] [PubMed] [Google Scholar]
- Dobbing J., Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973 Oct;48(10):757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi D., Meneilly G. S., Minaker K. L., Andersen D. K., Rowe J. W. Escape of hepatic glucose production during hyperglycemic clamp. Am J Physiol. 1989 Nov;257(5 Pt 1):E704–E711. doi: 10.1152/ajpendo.1989.257.5.E704. [DOI] [PubMed] [Google Scholar]
- Finegood D. T., Bergman R. N., Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987 Aug;36(8):914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- Flakoll P. J., Kulaylat M., Frexes-Steed M., Hourani H., Brown L. L., Hill J. O., Abumrad N. N. Amino acids augment insulin's suppression of whole body proteolysis. Am J Physiol. 1989 Dec;257(6 Pt 1):E839–E847. doi: 10.1152/ajpendo.1989.257.6.E839. [DOI] [PubMed] [Google Scholar]
- Fryburg D. A., Louard R. J., Gerow K. E., Gelfand R. A., Barrett E. J. Growth hormone stimulates skeletal muscle protein synthesis and antagonizes insulin's antiproteolytic action in humans. Diabetes. 1992 Apr;41(4):424–429. doi: 10.2337/diab.41.4.424. [DOI] [PubMed] [Google Scholar]
- Fukagawa N. K., Minaker K. L., Rowe J. W., Goodman M. N., Matthews D. E., Bier D. M., Young V. R. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985 Dec;76(6):2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. L., Hirata T. Attenuated response to insulin in very low birthweight infants. Pediatr Res. 1980 Jan;14(1):50–53. doi: 10.1203/00006450-198001000-00012. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Kelly F. J. Protein turnover and growth in the whole body, liver and kidney of the rat from the foetus to senility. Biochem J. 1984 Jan 15;217(2):507–516. doi: 10.1042/bj2170507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso S., Fallucca F., Romeo M. G., Distefano G., Sciullo E., Reitano G. Glucagon and insulin secretion in low birthweight preterm infants. The effect of glucose infusion. Acta Paediatr Scand. 1990 Mar;79(3):280–285. doi: 10.1111/j.1651-2227.1990.tb11457.x. [DOI] [PubMed] [Google Scholar]
- Grasso S., Messina A., Distefano G., Vigo R., Reitano G. Insulin secretion in the premature infant. Response to glucose and amino acids. Diabetes. 1973 May;22(5):349–353. doi: 10.2337/diab.22.5.349. [DOI] [PubMed] [Google Scholar]
- Heiling V. J., Campbell P. J., Gottesman I. S., Tsalikian E., Beaufrere B., Gerich J. E., Haymond M. W. Differential effects of hyperglycemia and hyperinsulinemia on leucine rate of appearance in normal humans. J Clin Endocrinol Metab. 1993 Jan;76(1):203–206. doi: 10.1210/jcem.76.1.8093620. [DOI] [PubMed] [Google Scholar]
- Jensen M. D., Haymond M. W. Protein metabolism in obesity: effects of body fat distribution and hyperinsulinemia on leucine turnover. Am J Clin Nutr. 1991 Jan;53(1):172–176. doi: 10.1093/ajcn/53.1.172. [DOI] [PubMed] [Google Scholar]
- Jensen M. D., Miles J. M., Gerich J. E., Cryer P. E., Haymond M. W. Preservation of insulin effects on glucose production and proteolysis during fasting. Am J Physiol. 1988 Jun;254(6 Pt 1):E700–E707. doi: 10.1152/ajpendo.1988.254.6.E700. [DOI] [PubMed] [Google Scholar]
- Kalhan S. C., Oliven A., King K. C., Lucero C. Role of glucose in the regulation of endogenous glucose production in the human newborn. Pediatr Res. 1986 Jan;20(1):49–52. doi: 10.1203/00006450-198601000-00013. [DOI] [PubMed] [Google Scholar]
- Kalhan S. C., Savin S. M., Adam P. A. Measurement of glucose turnover in the human newborn with glucose-1-13C. J Clin Endocrinol Metab. 1976 Sep;43(3):704–707. doi: 10.1210/jcem-43-3-704. [DOI] [PubMed] [Google Scholar]
- Kalhan S. C., Tserng K. Y., Gilfillan C., Dierker L. J. Metabolism of urea and glucose in normal and diabetic pregnancy. Metabolism. 1982 Aug;31(8):824–833. doi: 10.1016/0026-0495(82)90082-8. [DOI] [PubMed] [Google Scholar]
- Liechty E. A., Denne S. C., Lemons J. A., Kien C. L. Effects of glucose infusion on leucine transamination and oxidation in the ovine fetus. Pediatr Res. 1991 Nov;30(5):423–429. doi: 10.1203/00006450-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Schwarz H. P., Yang R. D., Motil K. J., Young V. R., Bier D. M. Relationship of plasma leucine and alpha-ketoisocaproate during a L-[1-13C]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism. 1982 Nov;31(11):1105–1112. doi: 10.1016/0026-0495(82)90160-3. [DOI] [PubMed] [Google Scholar]
- Pildes R. S. Neonatal hyperglycemia. J Pediatr. 1986 Nov;109(5):905–907. doi: 10.1016/s0022-3476(86)80725-9. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- Robert J. J., Bier D. M., Zhao X. H., Matthews D. E., Young V. R. Glucose and insulin effects on the novo amino acid synthesis in young men: studies with stable isotope labeled alanine, glycine, leucine, and lysine. Metabolism. 1982 Dec;31(12):1210–1218. doi: 10.1016/0026-0495(82)90006-3. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Saccà L., Vitale D., Cicala M., Trimarco B., Ungaro B. The glucoregulatory response to intravenous glucose infusion in normal man: roles of insulin and glucose. Metabolism. 1981 May;30(5):457–461. doi: 10.1016/0026-0495(81)90180-3. [DOI] [PubMed] [Google Scholar]
- Schwenk W. F., Berg P. J., Beaufrere B., Miles J. M., Haymond M. W. Use of t-butyldimethylsilylation in the gas chromatographic/mass spectrometric analysis of physiologic compounds found in plasma using electron-impact ionization. Anal Biochem. 1984 Aug 15;141(1):101–109. doi: 10.1016/0003-2697(84)90431-7. [DOI] [PubMed] [Google Scholar]
- Schwenk W. F., Butler P. C., Haymond M. W., Rizza R. A. Underestimation of glucose turnover corrected with high-performance liquid chromatography purification of [6-3H]glucose. Am J Physiol. 1990 Jan;258(1 Pt 1):E228–E233. doi: 10.1152/ajpendo.1990.258.1.E228. [DOI] [PubMed] [Google Scholar]
- Shangraw R. E., Stuart C. A., Prince M. J., Peters E. J., Wolfe R. R. Insulin responsiveness of protein metabolism in vivo following bedrest in humans. Am J Physiol. 1988 Oct;255(4 Pt 1):E548–E558. doi: 10.1152/ajpendo.1988.255.4.E548. [DOI] [PubMed] [Google Scholar]
- Sherwood W. G., Hill D. E., Chance G. W. Glucose homeostasis in preterm rhesus monkey neonates. Pediatr Res. 1977 Aug;11(8):874–877. doi: 10.1203/00006450-197708000-00002. [DOI] [PubMed] [Google Scholar]
- Staten M. A., Matthews D. E., Bier D. M. Leucine metabolism in type II diabetes mellitus. Diabetes. 1986 Nov;35(11):1249–1253. doi: 10.2337/diab.35.11.1249. [DOI] [PubMed] [Google Scholar]
- Tessari P., Trevisan R., Inchiostro S., Biolo G., Nosadini R., De Kreutzenberg S. V., Duner E., Tiengo A., Crepaldi G. Dose-response curves of effects of insulin on leucine kinetics in humans. Am J Physiol. 1986 Sep;251(3 Pt 1):E334–E342. doi: 10.1152/ajpendo.1986.251.3.E334. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., Kalhan S. C. Calculation of substrate turnover rate in stable isotope tracer studies. Am J Physiol. 1983 Sep;245(3):E308–E311. doi: 10.1152/ajpendo.1983.245.3.E308. [DOI] [PubMed] [Google Scholar]
- Varma S., Nickerson H., Cowan J. S., Hetenyi G., Jr Homeostatic responses to glucose loading in newborn and young dogs. Metabolism. 1973 Nov;23(2):1367–1375. doi: 10.1016/0026-0495(73)90252-7. [DOI] [PubMed] [Google Scholar]
- Virkamäki A., Puhakainen I., Koivisto V. A., Vuorinen-Markkola H., Yki-Järvinen H. Mechanisms of hepatic and peripheral insulin resistance during acute infections in humans. J Clin Endocrinol Metab. 1992 Mar;74(3):673–679. doi: 10.1210/jcem.74.3.1740504. [DOI] [PubMed] [Google Scholar]
- Welle S., Nair K. S. Failure of glyburide and insulin treatment to decrease leucine flux in obese type II diabetic patients. Int J Obes. 1990 Aug;14(8):701–710. [PubMed] [Google Scholar]
- Wolfe R. R., Shaw J. H., Jahoor F., Herndon D. N., Wolfe M. H. Response to glucose infusion in humans: role of changes in insulin concentration. Am J Physiol. 1986 Mar;250(3 Pt 1):E306–E311. doi: 10.1152/ajpendo.1986.250.3.E306. [DOI] [PubMed] [Google Scholar]



