Abstract
Background
Depending on the light dose and concentration of photosensitizer for photodynamic treatment (PDT), a multitude of dose-related events are demonstrable in PDT-treated cells. Sublethal doses may result in the alteration of cytokine release and consequently modify immune actions, rather than cause cell death.
Objective
The purpose of this study was to investigate cytokine expression in cultured HaCaT cells after intense pulse light (IPL) treatment or PDT utilizing 5-aminolevulinic acid (ALA) and IPL at sublethal doses.
Methods
Cultured HaCaT cells were treated with either IPL only (4, 8 and 12 J/cm2) or ALA-IPL PDT (100µmol/L of ALA; 0, 4, 8, and 12 J/cm2 of IPL). The expression of IL-10, TGF-β1 and TNF-α was investigated by reverse transcription-polymerase chain reaction and enzyme linked immunosorbent assay.
Results
IL-10 protein increased up to 5.95-fold after IPL treatment and up to 2.85-fold after PDT. TGF-β1 mRNA and protein showed slight increases after both IPL treatment and PDT, of which the latter induced slightly larger increases. TNF-α mRNA and protein showed no induction or reduction after PDT.
Conclusion
Increased expressions of IL-10 and TGF-β1 was observed after PDT. The induction of IL-10 may contribute to the anti-inflammatory effect, which explains the therapeutic benefit of PDT for inflammatory dermatoses, and that of TGF-β1 may be related to the therapeutic effect for psoriasis. The finding that IL-10 induction was more marked after IPL treatment than after PDT suggests that other mechanisms than IL-10 induction in keratinocytes after PDT may participate in the anti-inflammatory effect of PDT.
Keywords: IL-10, Keratinocyte, Photodynamic therapy, TGF-β1, TNF-α
INTRODUCTION
Topical photodynamic therapy (PDT) using 5-aminolevulinic acid (ALA) is a well-established treatment method for dermato-oncologic conditions, such as actinic keratoses, Bowen's disease, in situ squamous cell carcinoma and superficial basal cell carcinoma. However, a therapeutic benefit of PDT is also evident for inflammatory dermatoses, such as acne vulgaris, psoriasis, granuloma annulare, localized scleroderma and lichen sclerosus1. The light sources available for PDT include blue and red lights, incoherent lamps, and light emitting diodes. Recently, studies using intense pulse light (IPL) as a light source have shown therapeutic effects for photoaging and acne vulgaris2.
Depending on the light dose and the concentration of the photosensitizer for PDT, a multitude of dose-related events is demonstrable in PDT-treated cells from tumor destruction by cytotoxic effects when high light and drug doses are applied to immunomodulation when lower doses are applied. The mechanism of immunomodulation is not clearly identified, but alteration of cytokine expression is suspected to be involved3. Interleukin (IL)-6 expression was strongly enhanced in PDT-treated tumors, which suggest that the general inflammatory response to PDT may be mediated, at least in part, by IL-6. Additionally, IL-10 expression was markedly induced in the skin of mice exposed to PDT which implies that the enhanced IL-10 expression may participate in the inhibition of contact hypersensitivity seen following PDT4. In addition, the expression of IL-1α and tumor necrosis factor (TNF)-α increased up to 10-fold and 2.3-fold, respectively, in keratinocytes exposed to photodynamic treatment (PDT) in vitro5.
The purpose of this study was to examine, in an in vitro setting, the expression of IL-10, tumor growth factor (TGF)-β1 and TNF-α in cultured keratinocytes (HaCaT cells) after IPL treatment or PDT utilizing 5-ALA and IPL at sublethal doses.
MATERIALS AND METHODS
Materials
1) Cell cultures
HaCaT cells were seeded 3×105 cells/dish in a 35×10 mm dish (Falcon, USA) and cultivated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 50µl/ml penicillin and 50µg/ml streptomycin (GIBCO BRL, Gaithersburg, MD, USA) to subconflence. Cells were cultured in a 5% CO2 incubator at 37℃.
2) Photosensitizer and light source
5-ALA was used as a photosensitizer and a Vascular Applicator Type (VL-2; emission wavelength λem 555~950 nm) of I2PL (Ellipse FLEX®) was used as a light source.
Methods
1) Dose-response curve
A dose-response curve of PDT for HaCaT cells was evaluated to determine the appropriate sublethal doses of ALA and IPL. After application of the medium containing ALA at 0.01, 0.1, 1, 10, 100, 1,000 or 10,000µmol/L and IPL at 0, 4, 8, 12 or 16 J/cm2, cell viability was measured and the conditions resulting in <10% of cell death were determined. At 100µmol/L of ALA, cell death was less than 10% up to 16 J/cm2 of IPL.
2) Photodynamic treatment (PDT) group
(1) 5-ALA treatment
After removing medium and rinsing twice with phosphate-buffered saline (PBS), serum-free medium containing ALA (100µmol/L) was added. Cells were allowed to take up ALA for 6 hours. The medium containing ALA was removed and the cells were rinsed with PBS twice before being submerged in PBS. All the processes were performed in a dark room under a Wood's light.
(2) IPL irradiation
After removing PBS, irradiation of the ALA-treated cell cultures was performed with IPL at 0, 4, 8 or 12 J/cm2. Culture medium was added after irradiation and the cells were cultured in a 5% CO2 incubator at 37℃. The cells and the culture medium were harvested at 48 hour following irradiation.
3) IPL treatment group
Serum-free medium without ALA, was used. Otherwise, the procedures were the same as for the PDT group.
4) Control group
As with the IPL group, controls were treated with serum-free medium without ALA, but received no light irradiation.
5) Reverse transcription-polymerase chain reaction (RT-PCR)
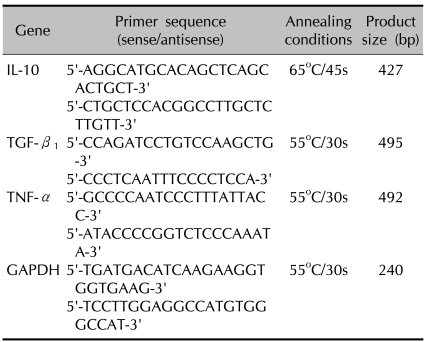
Total RNA was isolated from cells using easy-BLUE™ total RNA extraction kit (iNtRON, Korea). Total RNA was stored at -80℃ until use in RT-PCR analysis. Reverse transcription was performed using the reverse transcription system (Promega, USA). The GoTaq® polymerase system (Promega, USA) was used for amplification. Specific oligonucleotide primers for IL-10, TGF-β1, TNF-α, and GAPDH which served as an internal standard, were used in PCR amplification (Table 1).
Table 1.
Oligonucleotide primers used for PCR
Completed samples were run on an ethidium bromide/1.5% agarose gel. Bands were identified and quantified photofluorometrically by Quantity One® (Bio-Rad Laboratory, 2000, USA). They were normalized to GAPDH to correct for loading error.
6) Enzyme-linked immunosorbent assay (ELISA)
The culture medium harvested at 48 hours following irradiation was evaluated. The IL-10, TGF-β1, and TNF-α ELISA kits, and monoclonal antibodies against these molecules, were obtained from R&D systems (USA). Each ELISA was performed in triplicate.
7) Interpretation
The measured mRNA and protein levels were converted into x-fold changes compared with the untreated control group.
8) Statistics
Independent experiments were performed at least in duplicate. Data values are expressed as mean±SEM (standard error of the mean) obtained from triplicate determinations of at least two independent experiments for each case. Statistical significance was evaluated in paired analyses using Student's paired t-test of SPSS for windows (version 12.0) and defined as p<0.05.
RESULTS
Expression of IL-10, TGF-β1 and TNF-α mRNA
RT-PCR was performed a single time for each experiment.
1) IL-10
No bands were observed.
2) TGF-β1
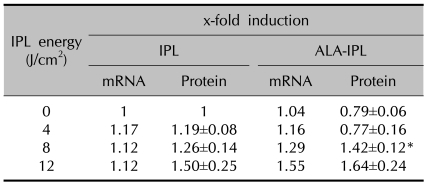
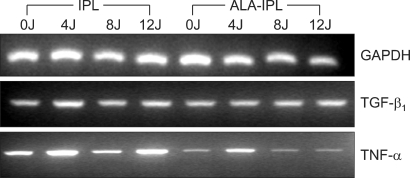
TGF-β1 mRNA was slightly increased in both the IPL treatment group (1.17-, 1.12- and 1.12-fold induction at 4, 8 and 12 J/cm2, respectively) and the PDT group (1.04-, 1.16-, 1.29- and 1.55-fold at 0, 4, 8 and 12 J/cm2, respectively), of which the PDT group showed slightly larger increases than the IPL treatment group (Table 2, Fig. 1).
Table 2.
TGF-β1 mRNA and protein expression after IPL or ALA-IPL
Data represent mean±SEM from triplicate determinations of at least two independent experiments for each case. *A significant difference compared with control that was treated with neither ALA nor IPL (p<0.05).
Fig. 1.
RT-PCR analysis of mRNA expression of TGF-β1 and TNF-α.
3) TNF-α
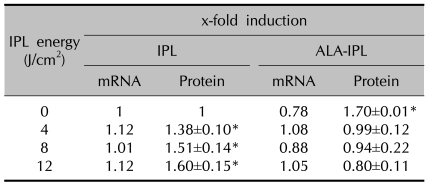
TNF-α mRNA showed no marked change in both the IPL treatment group (1.12-, 1.01- and 1.12-fold at 4, 8 and 12 J/cm2, respectively) and PDT group (0.78-, 1.08-, 0.88- and 1.05-fold at 0, 4, 8 and 12 J/cm2, respectively) (Table 3, Fig. 1).
Table 3.
TNF-α mRNA and protein expression after IPL or ALA-IPL
Data represent mean±SEM from triplicate determinations of at least two independent experiments for each case. *A significant difference compared with control that was treated with neither ALA nor IPL (p<0.05).
Expression of IL-10, TGF-β1 and TNF-α protein
1) IL-10
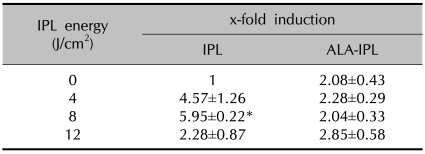
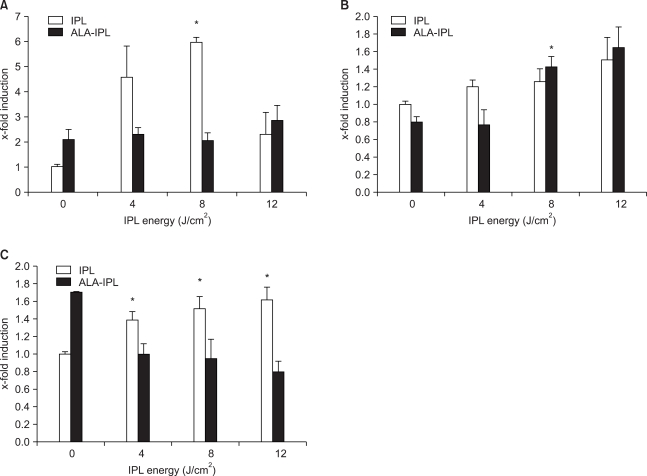
IL-10 protein was increased in both the IPL treatment group (4.57±1.26-, 5.95±0.22- and 2.28±0.87-fold inductions at 4, 8 and 12 J/cm2, respectively) and PDT group (2.08±0.43-, 2.28±0.29-, 2.04±0.33- and 2.85±0.58-fold at 0, 4, 8 and 12 J/cm2, respectively), of which the IPL group showed more marked increases (Table 4, Fig. 2).
Table 4.
IL-10 protein expression after IPL or ALA-IPL
Data represent mean±SEM from triplicate determinations of at least two independent experiments for each case. *A significant difference compared with control that was treated with neither ALA nor IPL (p<0.05).
Fig. 2.
Expression of IL-10 (A), TGF-β1 (B), and TNF-α (C) protein after IPL or ALA-IPL. Data are represented as mean±SEM from triplicate determinations of at least two independent experiments for each case. *A significant difference compared with control (that was treated with neither ALA nor IPL) (p<0.05).
2) TGF-β1
TGF-β1 protein showed slight increases in the IPL treatment group (1.19±0.08-, 1.26±0.14- and 1.50±0.25-fold at 4, 8 and 12 J/cm2, respectively). In the PDT group, the increases were slightly larger than in the IPL treatment group at 8 and 12 J/cm2 (1.42±0.12-, 1.64±0.24-fold, respectively) (Table 2, Fig. 2).
3) TNF-α
TNF-α protein was increased in the IPL treatment group (1.38±0.10-, 1.51±0.14- and 1.60±0.15-fold at 4, 8 and 12 J/cm2, respectively), whereas in PDT group no marked change of TNF-α protein was noted (0.99±0.12-, 0.94±0.22- and 0.80±0.11-fold at 4, 8 and 12 J/cm2, respectively) (Table 3, Fig. 2).
Comparison of mRNA and protein expressions of TGF-β1 and TNF-α
TGF-β1 mRNA and protein were increased in both the IPL treatment group and PDT group with larger increases in the PDT group (Table 2). TNF-α mRNA and protein were slightly increased in the IPL treatment group, but there was no marked change in the PDT group (Table 3).
DISCUSSION
PDT is a two-step therapeutic technique in which the topical or systemic delivery of photosensitizing drugs is followed by irradiation with light, and requires simultaneous presence of photosensitizer, light and oxygen inside the tissue. Although systemic photosensitizers are widely used for the treatment of a variety of internal cancers, topical photosensitizers are preferred in dermatology to avoid prolonged photosensitivity and the side effects like nausea and vomiting caused by a systemic photosensitizer.
5-ALA is the most popular topical photosensitizer. It is a small molecule (167.6 daltons) which diffuses most readily into cutaneous tissue from a topical delivery system. After penetration into the skin, ALA is selectively accumulated at a greater extent, and/or retained longer, by hyperproliferative tumoral and inflammatory cell populations and by sebaceous glands. This explains the therapeutic effects of PDT for tumors, inflammatory dermatoses and acne vulgaris. However, keratinocytes, the major cell type in skin, are also suggested to be a target of PDT5.
ALA is not a photosensitizer by itself. However, it is metabolized to photosensitive protoporphyrin IX (PpIX) through the intrinsic cellular heme biosynthetic pathway. PpIX has a high absorption peak corresponding to the Soret band at about 405 nm and other absorption maxima - the Q-bands - at approximately 510, 545, 580 and 630 nm. Although the Q-bands are 10-20-fold smaller than the peak in the Soret band, most clinical studies have used lights corresponding to Q-bands as they allow for deeper penetration into the skin, for example 630 nm red light penetrates 5~10 mm into the skin6. Recently, IPL, which emits a wide range of wavelengths, has been used to take advantage of the properties of various wavelengths. We used IPL, in the present study, with an emission spectrum (555~950 nm) that corresponds to Q bands.
Biologic effects after PDT can be modulated by different combinations of light and drug doses. At higher doses of one or both components of PDT, the disruption of cell membranes and organelles cause necrosis, which contributes to the formation of an inflammatory state. At intermediate combinations of light and drug, cells may undergo apoptosis. With low light and drug doses, cell viability may be maintained while other traits (signaling activity, cytokine formation, receptor expression) may be altered7. The apoptosis-inducing action and sublethal effects of PDT typically occur within a relatively narrow range of photosensitizer concentrations. Immunomodulatory PDT utilizes apoptotic and sublethal doses and is used to treat a variety of inflammatory dermatoses. In vivo studies have shown that PDT was effective in alleviating contact hypersensitivity (CHS) and retention of allogenic skin grafts8,9, and also was effective in reducing disease severity when applied in different models of autoimmunity10.
IL-10 is one of the regulatory cytokines that inhibit cytokine production in activated T lymphocytes and antigen-presenting cells. Epidermal keratinocytes are a major source of IL-10 in skin and its production by keratinocytes is upregulated after UV irradiation, leading to local and systemic immunosuppression. IL-10 was markedly induced in the skin of mice exposed to PDT using porfirmer and a 630 nm argon dye laser at doses that strongly inhibited the CHS. This suggests that enhanced IL-10 expression might play a role in the suppression of cell-mediated responses seen following PDT4.
In this study, IL-10 protein increased in both the IPL treatment and PDT groups, with larger increases noted in the IPL treatment group. Compared to an approximately 9-fold increase of IL-10 in the skin of mice exposed to a systemic PDT regime using porfirmer and 630 nm argon dye, we observed only a 2.85-fold increase, at maximum, after PDT. This difference in magnitude of IL-10 induction may be attributable to the fact that different photosensitizers and light sources were used, and that other cells besides keratinocytes would have participated in vivo. In addition, IL-10 was more markedly increase only after IPL treatment, which may suggest the possibility that mechanisms other than IL-10 are involved in the anti-inflammatory effect of PDT. Gollnick et al11 also suggested the implication of different mechanisms, as the ability of PDT to induce CHS suppression was sustained in IL-10 knockout mice, and after administration of anti-IL-10 antibodies.
TGF-β1 is known to be a potent stimulus for fibrosis, which stimulates fibroblast proliferation and dramatically increases the production of collagen and other extracellular matrix proteins. It also inhibits degradation of these matrix proteins by reducing metalloproteinase synthesis. In addition, TGF-β1 is a pivotal immunosuppressive cytokine which promotes inflammation resolution. It is also intimately involved in the development of immune tolerance. Being produced by regulatory T cells, it suppresses Th1 and Th2 immune responses and is able to convert CD4+ T lymphocytes to CD25+ regulatory T cells. Besides, TGF-β1 is the most potent known inhibitor of keratinocyte proliferation, and causes practical growth arrest12.
In this study, TGF-β1 slightly increased in both the IPL treatment and PDT groups, with slight larger increases in the latter group. The increase of TGF-β1 after PDT may be implicated in the beneficial effect of PDT for psoriasis and in extended engraftment of allogenic skin grafts with PDT.
TNF-α evokes two types of responses in cells: proinflammatory effects and induction of apoptotic cell death. It is an important mediator of cutaneous inflammation and is produced by keratinocytes after stimulation, such as with UV light. In previous studies, TNF-α gene transcription was found to increase in the keratinocytes of mice treated with phthalocyanine and light13, and the expression of TNF-α protein was reported to increase up to 2.3-fold in cultured keratinocytes treated with sublethal doses of ALA and red light5.
In this study, however, TNF-α mRNA and protein showed no marked change in the PDT group. The fact our findings disagree with these previous studies may be attributable to the different conditions, such as different cell types, light sources and energy, reported here.
There are some limitations of our study. Because this study was an in vitro study, the concentration of ALA and the energy of IPL used in the study cannot be applied in vivo. Moreover, normal keratinocytes and abnormal keratinocytes in diseased states may respond differently to HaCaT cells. In an in vivo condition, many other cell types, like fibroblasts, may also respond to PDT, and various cytokines other than those investigated in this study may also participate in the reaction. Thus, the actual biologic responses are difficult to predict.
In conclusion, we observed the increased expression of IL-10 and TGF-β1 in HaCaT cells after PDT. The induction of IL-10 may contribute to the anti-inflammatory effect, which explains the therapeutic benefit of PDT for inflammatory dermatoses. The induction of TGF-β1 may be related to its therapeutic effect for psoriasis. The finding that IL-10 induction was more marked after IPL treatment than after PDT suggests that some mechanism other than IL-10 induction participates in the anti-inflammatory effect of PDT. We believe that these findings may contribute to a better understanding of the immunologic effects of PDT, and that further studies, including in vivo procedures, are required.
References
- 1.Babilas P, Karrer S, Sidoroff A, Landthaler M, Szeimies RM. Photodynamic therapy in dermatology--an update. Photodermatol Photoimmunol Photomed. 2005;21:142–149. doi: 10.1111/j.1600-0781.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- 2.Gold MH. Acne and PDT: new techniques with lasers and light sources. Lasers Med Sci. 2007;22:67–72. doi: 10.1007/s10103-006-0420-z. [DOI] [PubMed] [Google Scholar]
- 3.Calzavara-Pinton PG, Venturini M, Sala R. Photodynamic therapy: update 2006. Part 1: Photochemistry and photobiology. J Eur Acad Dermatol Venereol. 2007;21:293–302. doi: 10.1111/j.1468-3083.2006.01902.x. [DOI] [PubMed] [Google Scholar]
- 4.Gollnick SO, Liu X, Owczarczak B, Musser DA, Henderson BW. Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res. 1997;57:3904–3909. [PubMed] [Google Scholar]
- 5.Karrer S, Bosserhoff AK, Weiderer P, Landthaler M, Szeimies RM. Keratinocyte-derived cytokines after photodynamic therapy and their paracrine induction of matrix metalloproteinases in fibroblasts. Br J Dermatol. 2004;151:776–783. doi: 10.1111/j.1365-2133.2004.06209.x. [DOI] [PubMed] [Google Scholar]
- 6.Nyman ES, Hynninen PH. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J Photochem Photobiol B. 2004;73:1–28. doi: 10.1016/j.jphotobiol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Hunt DW, Chan AH. Influence of photodynamic therapy on immunological aspects of disease - an update. Expert Opin Investig Drugs. 2000;9:807–817. doi: 10.1517/13543784.9.4.807. [DOI] [PubMed] [Google Scholar]
- 8.Ratkay LG, Waterfield JD, Hunt DW. Photodynamic therapy in immune (non-oncological) disorders: focus on benzoporphyrin derivatives. Bio Drugs. 2000;14:127–135. doi: 10.2165/00063030-200014020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Obochi MO, Ratkay LG, Levy JG. Prolonged skin allograft survival after photodynamic therapy associated with modification of donor skin antigenicity. Transplantation. 1997;63:810–817. doi: 10.1097/00007890-199703270-00004. [DOI] [PubMed] [Google Scholar]
- 10.Hunt DW, Levy JG. Immunomodulatory aspects of photodynamic therapy. Expert Opin Investig Drugs. 1998;7:57–64. doi: 10.1517/13543784.7.1.57. [DOI] [PubMed] [Google Scholar]
- 11.Gollnick SO, Musser DA, Oseroff AR, Vaughan L, Owczarczak B, Henderson BW. IL-10 does not play a role in cutaneous Photofrin photodynamic therapy-induced suppression of the contact hypersensitivity response. Photochem Photobiol. 2001;74:811–816. doi: 10.1562/0031-8655(2001)074<0811:idnpar>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Seaton ED, Mouser PE, Charakida A, Alam S, Seldon PM, Chu AC. Investigation of the mechanism of action of nonablative pulsed-dye laser therapy in photorejuvenation and inflammatory acne vulgaris. Br J Dermatol. 2006;155:748–755. doi: 10.1111/j.1365-2133.2006.07429.x. [DOI] [PubMed] [Google Scholar]
- 13.Anderson C, Hrabovsky S, McKinley Y, Tubesing K, Tang HP, Dunbar R, et al. Phthalocyanine photodynamic therapy: disparate effects of pharmacologic inhibitors on cutaneous photosensitivity and on tumor regression. Photochem Photobiol. 1997;65:895–901. doi: 10.1111/j.1751-1097.1997.tb01940.x. [DOI] [PubMed] [Google Scholar]