Abstract
Background
Psoriasis is a chronic, inflammatory, immune-mediated skin disease. Recently, several psoriasis-linked genetic loci have been reported; PSORS4 contains S100A8 (calgranulin A), and PSOR6 (19p13) locus harbors JunB (19p13.2). S100A8 is considered to be a marker of inflammation in a variety of diseases. The expression of JunB and c-Jun have been reported to be reduced in psoriatic lesions.
Objective
We attempted to assess the role and correlation of S100A8, JunB, and c-Jun in the pathogenesis of guttate psoriasis and psoriasis vulgaris by studying whether any difference of immunohistochemical expression existed.
Methods
Skin biopsy specimens from patients with psoriasis vulgaris (n=37) and guttate psoriasis (n=17), and a normal skin controls (n=9) were utilized in the study. Formalin-fixed and paraffin-embedded tissue sections were prepared and JunB, c-Jun, and calgranulin A were immunohistochemically stained in order to compare the expression of those three proteins in each group.
Results
Reduced JunB expression was observed in patients with psoriasis vulgaris and guttate psoriasis, as compared to patients in the control group; however, c-Jun expression was reduced only in the psoriasis vulgaris group. The expression of S100A8 increased in the psoriasis groups as compared to the control group. In addition, the expression of S100A8 was different between the psoriasis vulgaris and guttate psoriasis groups; S100A8 was expressed more profoundly in the guttate psoriasis group (p<0.05).
Conclusion
Our results indicate that S100A8 contributes to the pathogenesis of guttate psoriasis, and it may be a good target for therapy for guttate psoriasis provoked by microorganisms.
Keywords: c-Jun, Guttate psoriasis, JunB, Psoriasis vulgaris, S100A8 (calgranulin A)
INTRODUCTION
Psoriasis is a chronic inflammatory skin disease characterized by thickened, scaly skin lesions. Histologically, the affected lesions have dermal infiltrations of inflammatory cells, including lymphocytes, neutrophils, dendritic cells, and macrophages. The causes of psoriasis are not apparent, but are thought to be involved in the cellular, molecular, and genetic predispositions to psoriasis. Psoriasis is considered to be a T-cell-mediated inflammatory disease; specifically, psoriasis is a consequence of deregulation of the inflammatory process. Recently, the relationship between innate immunity and the pathogenesis of psoriasis has focused on cytokines, acute phase proteins, and peptides, including pentraxin and the defensins. A genetic predisposition to psoriasis has also been reported, and psoriasis-linked genetic loci have been identified and named, including PSOR1 (MHC region) and PSOR2 through PSOR91. PSORS4 contains S100A8 (calgranulin A), and PSOR6 (19p13) contains JunB (19p13.2). S100A8 is an intracellular calcium-binding protein, and it is induced by myeloid cells that enhance the recruitment of neutrophils/monocytes. Such S100 proteins are considered to be markers of inflammation in a variety of diseases2. According to Zenz et al3, the expression of JunB and c-Jun in psoriatic lesions is reduced.
Psoriasis has been shown to affect approximately 1% of the Korean population. Psoriasis vulgaris is the most common form of psoriasis. Guttate psoriasis, another form of psoriasis, affects approximately 10% of psoriasis patients in Korea4. Guttate psoriasis does, however, represent a common form of the disease in children. Guttate psoriasis can be provoked or exacerbated, particularly by infections (e.g., Streptococcus pyogenes) or drugs (e.g., lithium or beta-blockers). The progression rate of the disease into the chronic plaque form is 33% in 10 years5.
In the current study, we compared the difference in expression of JunB, c-Jun, and S100A8 in the skin of patients with psoriasis vulgaris and guttate psoriasis and normal skin in order to understand better the pathogenesis of these two forms of psoriasis.
MATERIALS AND METHODS
Patients
Thirty-seven patients with psoriasis vulgaris, 17 patients with guttate psoriasis, and 9 volunteers in the control group participated in this study. The diagnoses in the patients were histologically confirmed and the control group was chosen in healthy subjects with pigmentary disorders.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue sections were prepared from skin biopsies for diagnosis during outpatient visits. Sections were mounted on glass slides, deparaffinized via two incubations in xylene and sequential rehydration steps with graded ethanol, and then washed in water. The tissue sections were then incubated with blocking solution (DakoCytomation, Glostrup, Denmark) for 30 min at room temperature in order to terminate endogenous peroxidase activity, followed by 15 min of protein blocking (DakoCytomation). The primary antibodies used in these experiments were anti-JunB (C-11, mouse IgG1) and anti-calgranulin A (C-19, goat polyclonal) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-c-Jun Ab (60A8, rabbit IgG; Cell Signaling Technology, Danvers, MA, USA). Antibody diluents (1:50 in phosphate-buffered saline) were added to the sections and incubated overnight at 4℃. After washing the slides, the sections were incubated for 30 min with secondary antibody solution (Dako Cytomation), washed, and added to horseradish peroxidase-conjugated streptavidin solutions. After extensive washing, the sections were incubated with 3,3'-diaminobenzidine (DAB) substrate (DakoCytomation) for color development. The negative control sections were prepared by omitting the incubation step with a primary antibody solution. The sections were counterstained with hematoxylin. The slides were imaged using microscopes equipped with analySIS (Soft Imaging System GmBH, Lakewood, CO, USA) for image analysis.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism (version 5; GraphPad Software, Inc., La Jolla, CA, USA). The values are expressed as the means±standard deviation (SD). Differences between groups were evaluated by Student's t-test and a p<0.05 was considered to be significant.
RESULTS
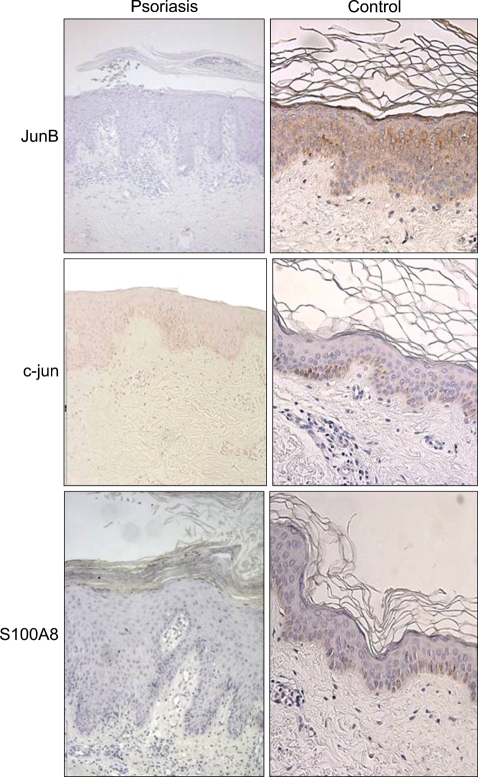
JunB was stained throughout the epidermal layer in the control group, but was stained less prominently in the psoriasis groups. The staining pattern of c-Jun in the control group was mostly confined to the basal layer; in the psoriatic groups, c-Jun-positive cells were confined to the basal layer and part of the Malpighian layer. S100A8 was stained in the basal and keratin layers, and part of the granular layers (Fig. 1).
Fig. 1.
Expression of JunB, c-Jun, and S100A8 on the epidermal layers from psoriasis and control patients.
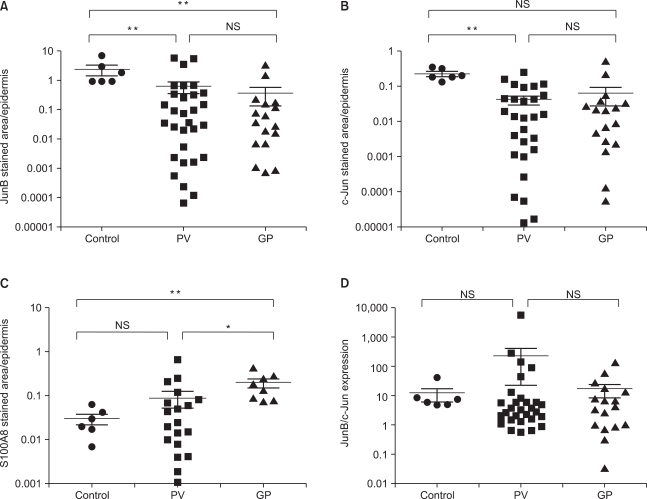
Reduced expression of JunB (Fig. 2A) was observed in patients with psoriasis vulgaris and guttate psoriasis as compared to the subjects in the control group (p<0.01), but the expression of c-Jun (Fig. 2B) was reduced only in of the patients with psoriasis vulgaris (p<0.01). Increased expression of S100A8 was noted in the psoriasis groups as compared to the control group (p<0.01). In addition, the expression of S100A8 differed between the psoriasis vulgaris and guttate psoriasis groups, with greater expression in the guttate psoriasis group (Fig. 2C; p<0.05). The ratio of JunB/c-Jun among the three groups did not differ significantly (Fig. 2D).
Fig. 2.
Quantification of cells expressing JunB, c-Jun, and S100A8 in the epidermis. The pixel area that is stained brown was divided from the hematoxylin-stained area, which was designated as the epidermal layer, in order to compare the level of expression of the three proteins in different tissues from the patients and controls. The dots represent the ratio of each of the tissue sections (*p<0.05, **p<0.01).
DISCUSSION
In this study, we compared the expression of JunB, c-Jun, and S100A8 in the epidermal layers of skin biopsies obtained from psoriasis vulgaris, guttate psoriasis, and unaffected patients. We determined that the expression of JunB and c-Jun was less, but expression of S100A8 was more in psoriatic lesions than in unaffected skin.
One of the damage-associated molecular pattern (DAMP) molecules, S100 has been reported to mediate inflammatory processes, including the recruitment of inflammatory cells to the site of tissue damage6. S100A8 and S100A9 have been mapped to the psoriasis susceptibility region, PSORS4. In particular, S100A8/S100A9 complex proteins exerted proinflammatory roles by upregulating integrin (e.g., CD11b/CD18) expression and by recruiting inflammatory cells via IL-8 and inducing the expression of ICAM-16. These two endogenous DAMP molecules are secreted from activated phagocytes, such as macrophages, while interacting with activated endothelial cells. DAMP molecules can be recognized by the multi-ligand receptor for advanced glycation end products (RAGE) or Toll-like receptors (TLRs). The latter species, TLRs, detect the pathogen-associated molecular pattern (PAMP), as well as DAMP.
The pathophysiology of guttate psoriasis, which is involved in infections or drugs7, may explain the different patterns of S100A8 between psoriasis vulgaris and guttate psoriasis. Several microorganisms have been reported to provoke or exacerbate psoriasis skin lesions via the activation of interferon γ-producing Th1 cells, and these T cells have been proposed to bind to streptococcal antigen or skin-specific antigen. In addition to T cell activation, such microorganisms harbor PAMP molecules which can activate inflammatory cells, and this could result in release of DAMP molecules, as we observed with S100A8 (Fig. 2C).
JunB was mapped in the PSORS6 locus and the down-regulation of JunB and c-Jun in the epidermis has been reported to be crucial to the pathogenesis of psoriasis3; additionally, mice lacking JunB and c-Jun exhibit upregulated expression of S100A8 and S100A93, as depicted in Fig. 2. As there were no significant differences in the level of expression of JunB and c-Jun between the patients with psoriasis vulgaris and guttate psoriasis, our results revealed that the function of Jun proteins in the pathogenesis of these two kinds of psoriasis might not be different.
In our experiments, we determined that the levels of S100A8 differ between patients with psoriasis vulgaris and guttate psoriasis, in contrast to the level of Jun proteins. This suggests that S100A8 contributes to the pathogenesis of guttate psoriasis, and thus S100A8 might represent a good target for therapy to treat guttate psoriasis induced by microorganisms.
Footnotes
This work was financially supported by The 2004 Alumni Association of the Department of Dermatology, Medical College, The Catholic University of Korea.
References
- 1.Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- 2.Pouliot P, Plante I, Raquil MA, Tessier PA, Olivier M. Myeloid-related proteins rapidly modulate macrophage nitric oxide production during innate immune response. J Immunol. 2008;181:3595–3601. doi: 10.4049/jimmunol.181.5.3595. [DOI] [PubMed] [Google Scholar]
- 3.Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, Mehic D, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–375. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- 4.Youn JI, Jo SJ. Clinical study on 3,123 psoriatic patients: observation of the patients registered for the past 20 years (1982~2002) at Seoul National University Hospital Psoriasis Clinic. Korean J Dermatol. 2004;42:1536–1542. [Google Scholar]
- 5.Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717–718. doi: 10.1001/archderm.1996.03890300147032. [DOI] [PubMed] [Google Scholar]
- 6.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 7.Christophers E. Explaining phenotype heterogeneity in patients with psoriasis. Br J Dermatol. 2008;158:437–441. doi: 10.1111/j.1365-2133.2007.08307.x. [DOI] [PubMed] [Google Scholar]