Abstract
Mycobacterium massiliense, an emerging pathogen that is increasingly reported as a causative agent in infections occurring during medical procedures, is difficult to be identified using conventional methods. Here we report the case of a cutaneous M. massiliense infection that was associated with repeated surgical procedures and that was identified via a comparative sequence analysis of rpoB and hsp65. The patient showed a substantial response to treatment with a combination of antimicrobial therapies consisting of clarithromycin, amikacin, and cefoxitin for 6 months.
Keywords: Mycobacterium massiliense
INTRODUCTION
Rapidly growing mycobacteria (RGM) are widely distributed in the environment and can contaminate reagents and medical equipment1,2. Most RGM infections in humans are caused by species belonging to the Mycobacterium (M.) fortuitum, M. chelonae, M. abscessus, or M. smegmatis groups1,3,4. Because of their low virulence, these species usually cause respiratory tract or disseminated infections only in persons with predisposing factors or in immunocompromised patients3,5,6. Recently, however, reports of RGM infections in immunocompetent people have markedly increased. These cases were soft-tissue infections associated with trauma and injection that have occurred in hospitals4,6. Ninety-five percent of soft-tissue RGM infections are caused by members of the M. chelonae complex, which includes M. chelonae, M. abscessus, and M. immunogenum1.
M. massiliense was first described as a new species, separate from the M. chelonae/M. abscessus group, by Adekambi et al.1 in 2004. M. massiliense is very closely related to M. abscessus and M. chelonae7. It has been isolated from a patient with pneumonia, a patient with pacemaker pocket infection, an outbreak associated with intramuscular injections of antimicrobial agents, and outbreaks associated with laparoscopic surgeries and cosmetic procedures2,5,7-10. Here we report the case of a cutaneous M. massiliense infection associated with a surgical procedure in an immunocompetent patient.
CASE REPORT
A 20-year-old woman presented with an erythematous-to-brownish ulcerated plaque on her left thigh that had appeared 5 months ago. She was otherwise healthy. At first, a small papule was observed on her left thigh. Although she applied antibiotic cream and squeezed it herself, the condition did not get better. At the local clinic, the doctor did repeated local incisions and pus drainage accompanied by saline irrigation in parallel with topical or systemic antibiotic treatment, but the skin lesion showed little improvement. After 3 months, she was referred to our dermatologic department because the skin lesion worsened and was accompanied by local heating and pain. On examination, the patient had a fluctuating mass and fistulization with serous secretions (Fig. 1). Sabouraud agar plates were inoculated with clinical specimens (pus and tissue debris) obtained by skin biopsy and cultured at 25℃ and 35℃ in an incubator to isolate causative agents. The patient was treated empirically with antibiotics (cefradine) which had been prescribed for common skin bacterial pathogens and surgical debridement, but she showed no response to the treatment. The biopsy specimens showed epithelial hyperplasia and a neutrophilic abscess with granulomatous inflammation in the dermis (Fig. 2), which is indicative of a mycobacterial, nontuberculous mycobacterial, or deep fungal infection. However, Gram, Ziehl-Neelsen, and periodic acid-Schiff after diastase staining gave negative results. Magnetic resonance imaging of the patient's thigh showed an area with homogeneous high signal intensity on T2-weighted images and with contrast enhancement that was 5.5×4.2×0.59 (in depth) cm in size. The signal was confined to the skin and subcutaneous tissue, with partial foci abutting fascia of the vastus lateralis anterolaterally. For the first 3 weeks, initial microbiologic examinations with staining and culturing of tissue biopsy specimens failed to demonstrate any causative microorganism. The patient received a total 4 weeks of treatment with oral cefradine and itraconazole but showed no improvement.
Fig. 1.
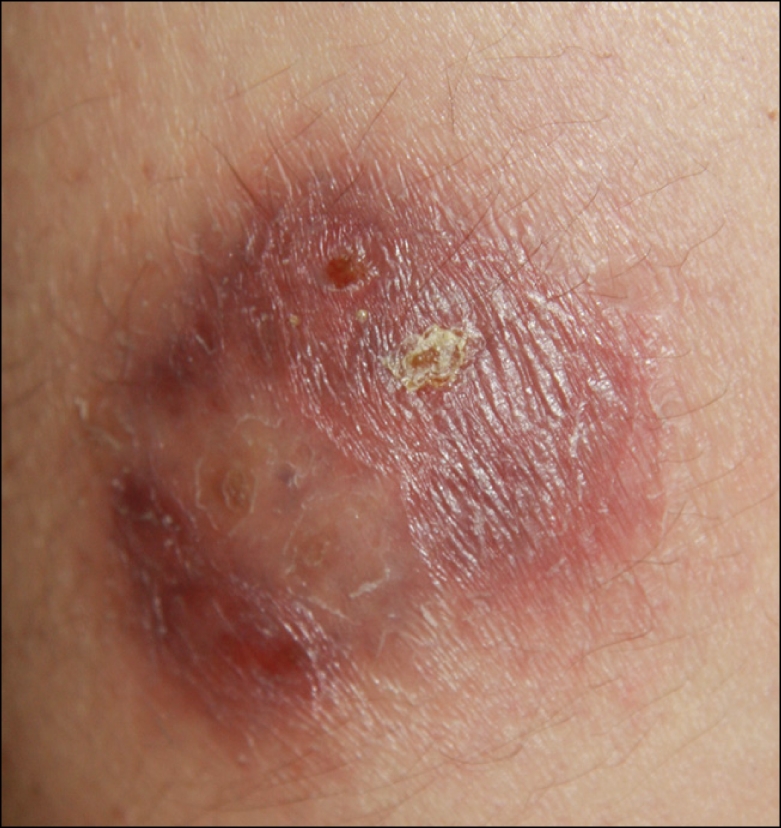
A tender, erythematous-to-brownish plaque located on the left thigh.
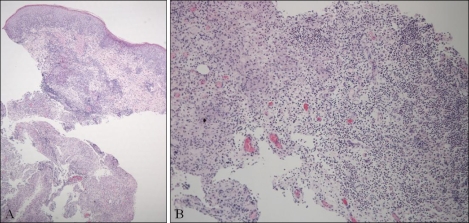
Fig. 2.
Histopathological findings of the lesion showed epithelial hyperplasia and a neutrophilic abscess with granulomatous inflammation in the dermis (H&E, A: ×40; B: ×100).
After 4 weeks of culture, small white colonies were observed on a Sabouraud agar plate (Fig. 3A). The isolates cultivated on the plate were subcultured on a blood agar plate at 37℃ and 5% CO2 in an incubator and on Ogawa medium at 37℃ in an incubator to observe colony morphology and purity. Pure cultured small colonies were observed on the blood agar plate after 5 days of culture; the colonies were ivory-colored and smooth, with elevated centers (Fig. 3B). Growth on the blood agar plate was faster than on the Sabouraud agar plate or on Ogawa medium. Microscopic examination of colonies revealed Gram-positive and acid-fast bacilli in Ziehl-Neelsen-stained smears. According to their growth and staining characteristics, the organisms were tentatively identified as members of a group of RGM species.
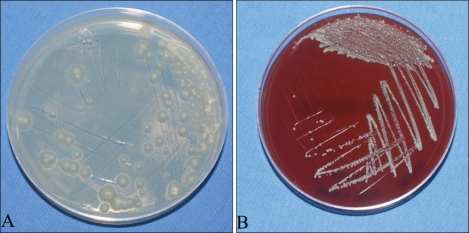
Fig. 3.
(A) Small white colonies were observed on the Sabouraud agar plate after 4 weeks of culture. (B) Ivory-coloured, smooth colonies with elevated centers were observed on the blood agar plate after 5 days of culture.
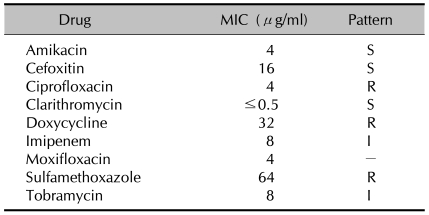
Pure isolates cultivated on the blood agar plates were used for species identification via comparative sequence analysis of the 16S rRNA genes, rpoB, and hsp65. Total DNA was extracted using the bead beater-phenol extraction method11 and used as a template for PCR. The following primer pairs were used: 285 (5'-GAG AGT TTG ATC CTG GCT CAG-3') and 244 (5'-CCC ACT GCT GCC TCC CGT AG-3') for the 16S rRNA gene (351 bp)12, RGMF (5'-GAC GAC ATC GAC CAC TTC GG-3') and RGMR (5'-GGG GTC TCG ATC GGG CAC AT-3') for rpoB (365 bp)8, and HSPF3 (5'-ATC GCC AAG GAG ATC GAG CT-3') and HSPR4 (5'-AAG GTG CCG CGG ATC TTG TT-3') for hsp65 (644 bp)13. PCR products were purified using QIAEX II gel extraction kits (Qiagen, Hilden, Germany) and sequenced directly using forward and reverse primers with an Applied Biosystems automated sequencer (model 377) and BigDye Terminator cycle sequencing kits (Perkin-Elmer Applied Biosystems, Warrington, UK). The results of the 16S rDNA PCR test were negative, but the hsp65 and rpoB sequences of the isolate had 100% homology with the corresponding sequences of Mycobacterium massiliense. Consequently, we diagnosed the patient's lesion as an M. massiliense infection and did an antimicrobial susceptibility test. Before results for testing for susceptibility to antibiotics were obtained, we started treatment empirically with intravenous amikacin (1 g/day) and intravenous cefoxitin (250 mg/day) for 1 week. After intravenous antibiotic therapy, oral doxycycline (200 mg/day) and clarithromycin (500 mg/day) were prescribed based on previous reports7,8. Susceptibility testing for antibiotics was done using the broth microdilution method according to the criteria of the Clinical and Laboratory Standards Institute. The minimal inhibitory concentrations (MICs) of nine antimicrobial agents (amikacin, cefoxitin, ciprofloxacin, clarithromycin, doxycycline, imipenem, moxifloxacin, sulfamethoxazole, and tobramycin) were estimated. Strains sensitive, intermediate, or resistant to particular antibiotics were identified from their MICs. The isolate was susceptible to clarithromycin (MIC≤0.5µg/ml), in agreement with a previous report7,8,10. The isolate was also susceptible to amikacin (MIC=4µg/ml) and cefoxitin (MIC=16µg/ml), and it showed intermediate susceptibility to imipenem (MIC=8µg/ml) and tobramycin (MIC=8µg/ml). However, the isolate was resistant to doxycycline (MIC=32µg/ml) (Table 1). Based on these results, we stopped treatment with doxycycline and treated only with clarithromycin. After 6 months of treatment, the lesion had significantly improved, with only post-inflammatory hyperpigmentation remaining.
Table 1.
Antimicrobial drug susceptibility pattern of the Mycobacterium massiliense strain isolated from the patient in Korea (broth microdilution method)
MIC: minimal inhibitory concentrations, S: susceptible, R: resistant, I: intermediate resistance.
DISCUSSION
The rate of isolation of M. massiliense from infected patients in Korea is much higher than that in recent reports6,9. An epidemic occurred in Korea associated with intramuscular injection of antimicrobial agents, and the causative microorganism was finally identified as M. massiliense8. The prevalence of this M. massiliense strain may be due to its atypical survival ability in different environments, resistance to biocides, and/or enhanced biofilm formation. Moreover, this strain may be unusually resistant to disinfectants2. The pathogenic potential of M. massiliense has been demonstrated by human infections in both immunocompetent and immunocompromised hosts, and by its intracellular survival and growth2,6-8. In the present case, repeated incision and drainage at a local clinic failed to eradicate M. massiliense. However, the worsening of the skin lesion after the patient underwent treatments at the local clinic strongly suggests that the surgical procedures performed at the clinic may have been the source of the infection.
To manage infectious diseases, the causative microorganisms must be correctly identified at the species level. Although several unique phenotypes that can differentiate M. massiliense from M. abscessus and M. chelonae have been identified6,7, these species are difficult to characterize in routine clinical laboratories. Since the early 1990s, genotype analysis, especially PCR sequencing, has greatly contributed to the precise and rapid identification of mycobacterial species8. Partial 16S rRNA gene sequence analysis is the most widely used method for identifying nontuberculous mycobacteria9,12. However, the 16S rRNA gene sequence of M. massiliense is identical to that of M. chelonae/M. abscessus2,7,8; therefore, 16S rRNA gene sequencing cannot discriminate between these species. M. massiliense can be differentiated from M. abscessus by sequencing of the rpoB, hsp65, sodA, and recA genes, or the 16S-23S rRNA internal transcribed spacer sequence6-9. Any one of these sequences can be used to identify both species. In the present case, the 16S rDNA PCR was negative. This result may have been due to insufficient amplification during PCR. However, the hsp65 and rpoB sequences of the isolate showed 100% homology with the corresponding sequences of M. massiliense. Consequently, the patient was diagnosed with M. massiliense infection.
Nontuberculous mycobacteria can grow in solid and liquid media that are frequently used in routine bacteriology5. RGM are defined as mycobacteria that grow and form visible colonies on solid agar media within 7 days. They can be grown on various microbiological media such as blood agar for isolation of bacteria, Sabouraud agar for isolation of fungi, and Lowenstein-Jensen and Ogawa media for isolation of mycobacteria. In this case, we did not consider the possibility of mycobacterial infection at first, so culture for mycobacteria was not performed. Furthermore, white colonies were observed on a Sabouraud agar plate, indicating that the patient's skin lesion might be a fungal infection. The white colonies on the Sabouraud agar plate appeared similar but not identical to yeast colonies. This similarity caused clinical confusion, but a subculture spread onto a blood agar plate revealed smooth white colonies, and the isolate was identified as an acid-fast bacillum in a Ziehl-Neelsen stained smear. Clinicians should keep in mind that Mycobacterium species can grow not only on media specific for mycobacteria, such as Ogawa media, but also on Sabouraud agar plates or blood agar plates.
Since empirical therapeutic approaches commonly used to treat cutaneous infections are not effective against nontuberculous mycobacteria, early clinical suspicion and microbiological diagnosis are key factors in reducing morbidity. Causative microorganisms should be correctly identified at the species level, and clinicians should be aware of the susceptibility of these microorganisms to antimicrobial agents because treatment regimens and management are directly affected by the results obtained with these agents. M. massiliense is very closely related to M. abscessus and M. chelonae, but these groups show differences in their antimicrobial susceptibility. Some studies previously proposed that susceptibility to doxycycline might serve as a surrogate marker to differentiate M. abscessus from M. massiliense7. M. massiliense was reported to be susceptible to doxycycline, which could distinguish it from resistant M. abscessus isolates7. Isolates with intermediate susceptibility to doxycycline have also been identified8. In another study, nine M. massiliense isolates from surgical patients were shown to be resistant to doxycycline2. The isolate in the present case was also resistant to doxycycline. These results agree with those reported by Simmon et al.10 indicating that M. massiliense does not have a unique pattern of susceptibility to doxycycline9. Moreover, the doxycycline resistance and the identity of the rpoB region V sequences of Brazilian and U.S. isolates might suggest the existence of a variant clone of M. massiliense9. This treatment method was empirically selected because no controlled studies have been carried out to establish optimal therapy against this organism.
Clarithromycin (0.5 g per os once every 12 h for adults and 7.5 mg/kg of body weight per os once every 12 h [maximum, 1 g/day] for children) was recommended to clinicians managing registered patients with definite or suspected abscesses due to M. massiliense infection8. Our isolates were found to be markedly sensitive to clarithromycin (≤0.5µg/ml) among the antimicrobial susceptibility profiles. Some of our results were concordant with previous reports in that M. massiliense strains, which were in the susceptible range of CLSI criteria, showed very low MICs (0.125~0.5µg/ml)7,8,10. The patient showed a significant response to treatment with a combination of antimicrobial therapies consisting of clarithromycin, doxycycline, amikacin, and cefoxitin for 6 months.
We had no opportunity to investigate the source of contamination. The source of infection in this case remains obscure because M. massiliense was isolated only from the patient's clinical specimens. Although no evidence exists in this case, infection caused by RGM after surgical intervention and medical injections through nonsterile contaminated needles and cannulas is well known1,2,10 and should be considered. Before visiting our hospital, the patient had undergone an incision and pus drainage with saline irrigation several times in the same local clinic. We can only speculate that inadequate aseptic techniques during the procedure were responsible for the cutaneous infection. This case underscores the absolute necessity for strict asepsis of the surgical instruments and aseptic techniques during medical and surgical procedures.
References
- 1.Adekambi T, Reynaud-Gaubert M, Greub G, Gevaudan MJ, La Scola B, Raoult D, et al. Amoebal coculture of "Mycobacterium massiliense" sp. nov. from the sputum of a patient with hemoptoic pneumonia. J Clin Microbiol. 2004;42:5493–5501. doi: 10.1128/JCM.42.12.5493-5501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown-Elliott BA, Wallace RJ., Jr Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting Rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15:716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon YH, Lee GY, Kim WS, Kim KJ. A case of skin and soft tissue infection caused by Mycobacterium abscessus. Ann Dermatol. 2009;21:84–87. doi: 10.5021/ad.2009.21.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HS, Park HJ, Lee JY, Cho BK. Mycobacterium fortuitum infection caused by a nerve block. Ann Dermatol. 2007;19:9–12. [Google Scholar]
- 5.Cardoso AM, Martins de Sousa E, Viana-Niero C, Bonfim de Bortoli F, Pereira das Neves ZC, Leao SC, et al. Emergence of nosocomial Mycobacterium massiliense infection in Goias, Brazil. Microbes Infect. 2008;10:1552–1557. doi: 10.1016/j.micinf.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Kim BJ, Lee SH, Lyu MA, Kim SJ, Bai GH, Chae GT, et al. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB) J Clin Microbiol. 1999;37:1714–1720. doi: 10.1128/jcm.37.6.1714-1720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H, Kim SH, Shim TS, Kim MN, Bai GH, Park YG, et al. PCR restriction fragment length polymorphism analysis (PRA)-algorithm targeting 644 bp Heat Shock Protein 65 (hsp65) gene for differentiation of Mycobacterium spp. J Microbiol Methods. 2005;62:199–209. doi: 10.1016/j.mimet.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Kim HY, Yun YJ, Park CG, Lee DH, Cho YK, Park BJ, et al. Outbreak of Mycobacterium massiliense infection associated with intramuscular injections. J Clin Microbiol. 2007;45:3127–3130. doi: 10.1128/JCM.00608-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HY, Kook Y, Yun YJ, Park CG, Lee NY, Shim TS, et al. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J Clin Microbiol. 2008;46:3384–3390. doi: 10.1128/JCM.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmon KE, Pounder JI, Greene JN, Walsh F, Anderson CM, Cohen S, et al. Identification of an emerging pathogen, Mycobacterium massiliense, by rpoB sequencing of clinical isolates collected in the United States. J Clin Microbiol. 2007;45:1978–1980. doi: 10.1128/JCM.00563-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Springer B, Stockman L, Teschner K, Roberts GD, Bottger EC. Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. J Clin Microbiol. 1996;34:296–303. doi: 10.1128/jcm.34.2.296-303.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tortoli E, Gabini R, Galanti I, Mariottini A. Lethal Mycobacterium massiliense sepsis, Italy. Emerg Infect Dis. 2008;14:984–985. doi: 10.3201/eid1406.080194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viana-Niero C, Lima KV, Lopes ML, Rabello MC, Marsola LR, Brilhante VC, et al. Molecular characterization of Mycobacterium massiliense and Mycobacterium bolletii in isolates collected from outbreaks of infections after laparoscopic surgeries and cosmetic procedures. J Clin Microbiol. 2008;46:850–855. doi: 10.1128/JCM.02052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]