Abstract
Background
Steroids are used in conventional treatment of atopic dermatitis (AD) and they are very effective for improving the symptoms, but they also have several complications. Many studies have reported that short-term use of cyclosporine (CsA) is effective for severe AD as a substitute for steroid. However, there are very few studies on the long-term use of CsA for AD in the Korean population.
Objective
The purpose of this study was to investigate whether long-term CsA therapy is effective and safe for treating AD.
Methods
We performed a retrospective study of the patients with AD and who were treated with CsA at Kyung Hee Medical Center between January 2001 and February 2008. Among 147 patients, 61 received CsA treatment for more than 6 months. To evaluate the efficacy of CsA treatment, the objective SCORAD was checked for all 61 patients at every visit. Extensive laboratory tests were performed every two months to assess the safety of treatment.
Results
The mean duration of CsA treatment was 13.5±8.4 months and the mean initial dose of CsA was 2.7±0.9 mg/kg/day. The mean objective SCORAD values significantly decreased from 34.1±11.2 at baseline to 11.4±10.7 after 6-month of CsA treatment (p<0.05). A significant decline of the SCORAD score was observed starting from 1-month of CsA treatment. The mean duration of remission was 4.5±2.9 months. A total of 13 adverse events in 10 patients were recorded during the study period. One patient dropped out due to renal dysfunction. Elevation of peripheral blood pressure was noted in 8 patients. Three patients complained of gastrointestinal troubles, and one patient had hypertrichosis, but the problems of these 4 patients were mild and easily treated.
Conclusion
We suggest that long-term, low-dose CsA treatment is safe and effective for patients who suffer from AD.
Keywords: Atopic dermatitis, Cyclosporine, Long-term treatment
INTRODUCTION
Atopic dermatitis (AD) is a chronically relapsing inflammatory skin disease that's caused by complex interactions between multiple genetic and environmental factors1. Patients with moderate to severe AD require long-term treatment with topical or systemic steroids, and this can lead to a variety of complications associated with the treatment. Significant progress has been made in steroid replacement therapy to minimize the long-term exposure to steroids. In 1970, Borel et al.2 in Switzerland, discovered and isolated cyclosporine (CsA) from the soil fungus Tolypocladium inflatumgams during a search for antifungal agents. The immunosuppressive effects of CsA were identified in 1976, and its therapeutic effects in patients with psoriasis were recognized in 1979 by Mueller and Herrmann3. Subsequently, the indications for CsA in dermatology are gradually expanded to AD, pyoderma gangrenosum, Behçet's disease, alopecia areata and vesicobullous disease4. However, the use of CsA has been limited because of its complications such as nephrotoxicity, hypertension, hyperlipidemia, and hypertrichosis5.
As the use of CsA has increased, the various reports on its safety for long-term treatment have also increased6-9. In Korea, there have been a few published studies on the efficacy of CsA treatment for patients with AD10-12. Although the drug's clinical efficacy has been proven, Korean studies on the efficacy and safety of long-term (>6 month) CsA treatment for AD are still limited. We performed a retrospective study on the clinical efficacy and adverse events of long-term CsA treatment for AD patients.
MATERIALS AND METHODS
Patients
This retrospective study focused on patients with AD and who were treated with CsA for more than 6 months, and these patients were treated at the Kyung Hee Medical Center from January 2001 to February 2008. A total of 147 patients who had moderate to severe AD were treated with CsA during the study period. Among the 147 patients, 61 patients received CsA treatment for more than 6 months. The exclusion criteria included: abnormal renal function, abnormal liver function, hyperkalemia or hyperuricemia, a history of malignancy or recent infections, uncontrolled hypertension (considered when the systolic blood pressure was greater than 140 mmHg and/or the diastolic blood pressure was over 90 mmHg on two consecutive measurements), clinically significant impairment of the hematopoietic, cardiovascular and/or cerebral function, and concomitant therapy with nephrotoxic medications13.
To determine whether the treatment results of CsA changed depending on the subtype, the patients with AD was divided into two major groups, 1) those with intrinsic AD and 2) those with extrinsic AD. The intrinsic subtype was defined by the following criteria: 1. the clinical phenotype of AD, according to Hanifin and Rajka14, 2. the absence of other atopic diseases, 3. normal total serum IgE levels and 4. no IgE sensitization detected on a skin test or in the serum15. The patients who had elevated total serum IgE and IgE sensitization against foods and inhalants, and other atopic disease were defined as having the extrinsic type AD.
Methods
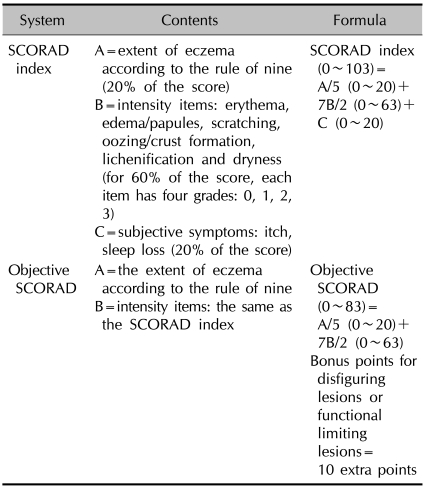
The clinical and laboratory data was obtained by a chart review. The age and gender, the changes in the objective SCORAD scores, the dose and duration of CsA treatment, the laboratory findings and the blood pressure were reviewed. The severity of disease was evaluated with the objective SCORAD score16,17. The difference between the classic SCORAD and the objective SCORAD is described in Table 1.
Table 1.
The content and formula of the SCORAD index and the objective SCORAD16
1) Treatment dosage of CsA
All the patients were initially treated with oral CsA, antihistamines and topical steroids. The mean initial dose of CsA was 2.7±0.9 mg/kg/day. The dosage of CsA was gradually reduced by 50 mg per every 4 weeks according to the therapeutic response. CsA treatment was discontinued with tapering when the objective SCORAD score was lower than 518. If AD flared up during the course of tapering, then the CsA was increased to the previous dosage.
2) Laboratory findings
To assess the safety of long-term CsA treatment, the severity and persistence of all complications were reviewed and evaluated. Prior to the initial treatment, laboratory tests that included complete blood counts with the differential, tests for the serum IgE, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, serum creatinine, triglycerides levels and urinalysis were performed in all the patients. All the same tests were repeated every two months and the 24 hour urine creatinine and creatinine clearance were evaluated every 6 months. Hypertension was defined by the criteria reported by Chobanian et al.19, stage 1 hypertension was defined as a systolic blood pressure from 140 mmHg to 160 mmHg and/or a diastolic blood pressure from 90 mmHg to 100 mmHg, and stage 2 hypertension was defined as a systolic blood pressure greater than 160 mmHg and/or a diastolic blood pressure over 100 mmHg.
3) Evaluation of the efficacy of CsA treatment
To evaluate the efficacy of CsA treatment, the objective SCORAD score was measured at every visit. In the acute stage, the follow up intervals of the patients were from 1 to 2 weeks. After the skin lesions subsided, the patients visited our clinic every month. The severity of symptoms was divided into 3 groups according to the objective SCORAD score: mild was lower than 15, moderate was from 15 to 40 and severe was more than 4016. The objective SCORAD scoring was measured by dermatologists who had been trained for the SCORAD scoring system to minimize the inter-evaluator variance.
4) Evaluation for the recurrence of AD
When itching interfered with the patients' daily living and/or the reappearance of the typical skin lesions of AD was observed, then this was considered to be recurrence of AD. If the lesion could not be controlled by oral antihistamine and topical steroid treatment only, or if the objective SCORAD score was over 15, then CsA treatment was restarted at the same dosage used in the initial treatment.
Statistical analysis
All the analyses were performed using the statistics program SPSS package ver. 13.0. Analysis of the objective SCORAD scores was performed using one-way ANOVA. The differences between the laboratory tests at baseline and those at the end of the treatment were analyzed with the paired samples t-test. For all cases, p-values<0.05 were considered to be statistically significant.
RESULTS
Age and gender distribution
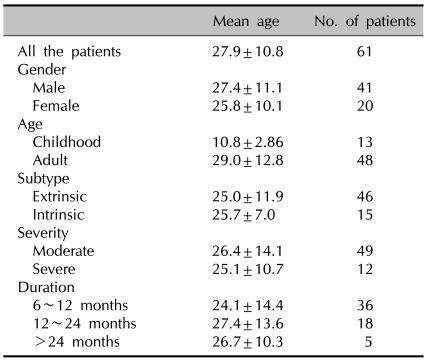
A total of 61 patients were enrolled in this retrospective study. The patient groups consisted of 41 males and 20 females (2.05:1). The age distribution of the enrolled patients ranged from 9 to 68 years of age with a mean age of 27.9±10.8 years. Among the 61 patients, 13 patients were children. This child group consisted of 8 boys and 5 girls, and they ranged from 5 to 14 years of age (mean age: 10.8±2.9). The demographic characteristics of the subjects are summarized in Table 2.
Table 2.
The demographic data of the atopic dermatitis patients who underwent cyclosporine treatment for more than 6 months
Analysis of CsA treatment
The mean duration of CsA treatment was 13.5±8.4 months. The mean treatment durations for the males and females were 14.5±8.6 and 12.2±8.7 months, respectively. There was no significant difference between the two groups.
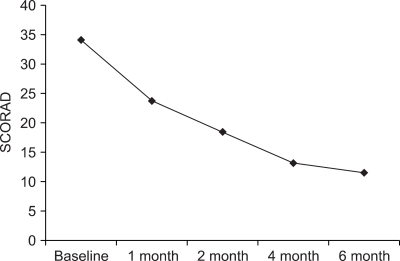
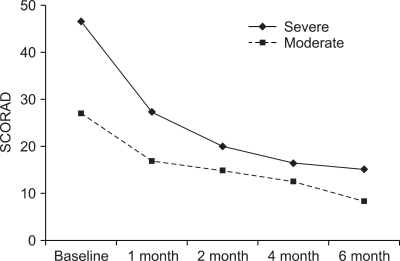
The mean scores of SCORAD were 34.1±11.2 at baseline, 23.7±14.3 after 1 month, 18.4±9.8 after 2 months, 13.1±7.9 after 4 months and 11.4±10.7 after 6 months of CsA treatment (Fig. 1). Each level of the objective SCORAD score in all the patients was significantly reduced, compared with the baseline score (p<0.05). The mean objective SCORAD score for the severe AD group was 46.6±20.3 at baseline, 27.4±15.6 after 1 month, 20.1± 14.7 after 2 months, 16.5±11.0 after 4 months and 15.2± 15.3 after 6 months. The mean objective SCORAD score for moderate AD was 27.0±7.7 at baseline, 16.9±10.1 after 1 month, 14.9±7.8 after 2 months, 12.6±8.8 after 4 months and 8.4±9.6 after 6 months (Fig. 2). Each SCORAD score in the moderate and severe AD groups was also significantly decreased, compared with the baseline score (p<0.05). Because the SCORAD scores of two patients were not decreased during the treatment, they were regarded as treatment failure.
Fig. 1.
The changes of the SCORAD score according to cyclosporine treatment. Each level of the SCORAD score after 1 month, 2 months, 4 months and 6 months of cyclosporine treatment was significantly different from the baseline score (p<0.05).
Fig. 2.
The changes of the SCORAD score according to the severity of AD. Each level of the SCORAD score in both the moderate (n=12) and severe groups (n=49) after 1 month, 2 months, 4 months and 6 months of cyclosporine treatment was significantly different from the baseline score (p<0.05).
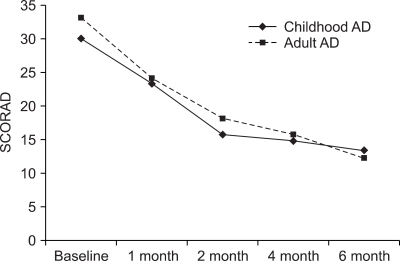
For the childhood AD group, the mean SCORAD were 30.0±14.1 at baseline, 23.3±10.1 after 1 month, 15.8±11.4 after 2 months, 14.8±9.3 after 4 months and 13.4±11.2 after 6 months of CsA treatment (Fig. 3). The mean objective SCORAD score in the adult group was 33.1± 10.49 at baseline, 24.1±10.9 after 1 month, 18.1±9.6 after 2 months, 15.7±6.2 after 4 months and 12.2±10.5 after 6 months. Both the childhood and adult AD groups showed a significant decrease of the mean SCORAD after CsA treatment (p<0.05).
Fig. 3.
The changes of the SCORAD score according to the age groups. Each level of the SCORAD score for both childhood (n=13) and adult AD (n=48) after 1 month, 2 months, 4 months, and 6 months of cyclosporine treatment was significantly different from the baseline score (p<0.05).
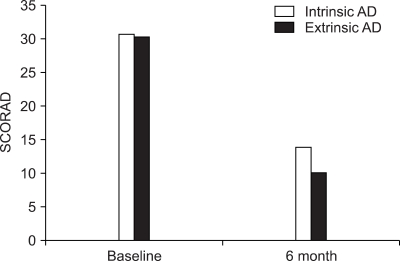
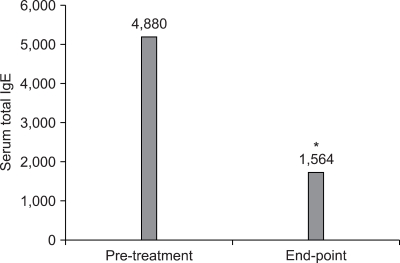
For the intrinsic AD group, the SCORAD score at baseline and 6 months after treatment was 30.7±13.6 and 13.9± 8.0, respectively. For the extrinsic AD group, the objective SCORAD score at baseline and 6 months after the treatment was 30.3±10.7 and 10.1±10.5, respectively (Fig. 4). There was no inter-group difference between the intrinsic and extrinsic AD groups. However, there was a significant difference in the objective SCORAD scores between baseline and 6-months treatment in each group (p<0.05). Of the 46 patients with extrinsic AD, 35 patients (76.1%) showed decreased levels of serum total IgE. The mean level of serum IgE was 4,880 IU/ml at baseline and 1,564 IU/ml at the end of treatment (Fig. 5). The average rate of decreased total serum IgE was 47.8% in the extrinsic AD group.
Fig. 4.
Comparison of the SCORAD score between the intrinsic (n=12) and extrinsic AD (n=41). There was no significant difference in the efficacy of cyclosporine treatment between the intrinsic and extrinsic groups.
Fig. 5.
The changes of the total serum IgE levels in the extrinsic atopic dermatitis patients according to the cyclosporine treatment. The serum IgE was significantly decreased after treatment (*p<0.05).
Recurrence and retreatment
Among 61 patients, 58 patients (95.1%) discontinued CsA treatment after prolonged remission. All the patients had recurrence of AD after discontinuation of CsA treatment and they needed additional sessions of the CsA treatment. The mean duration from the discontinuation of the CsA treatment to retreatment was 4.5±2.9 months. Two patients abruptly stopped their CsA medication on their own, but they did not have an immediate flare-up.
Assessment of adverse events
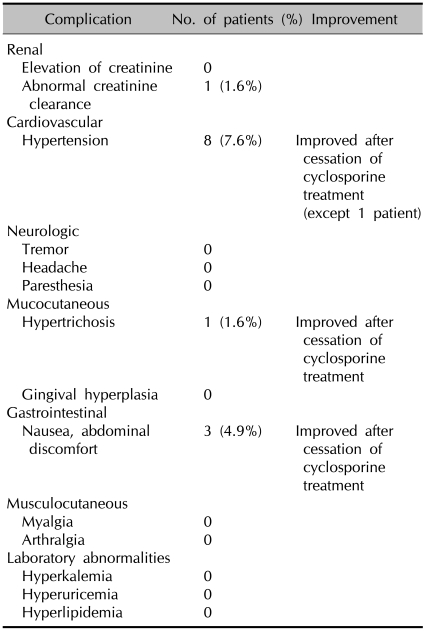
The adverse events of CsA treatment in this study are summarized in Table 3. There were 13 adverse events in 10 patients during the CsA treatment. Two patients had hypertension and abdominal discomfort, and one patient had concomitant abdominal discomfort and hypertrichosis. Most adverse events were mild and reversible after discontinuation of the CsA. Eight patients had an elevated peripheral blood pressure. Six of them had stage 1 hypertension and 2 had stage 2 hypertension. The mean interval from the start of CsA treatment to clinically detected hypertension was 5.69±1.91 months. One patient developed hypertension earlier than the other patients (6 weeks after CsA treatment). His hypertension was stage 2 and this persisted after the discontinuation of CsA treatment, yet he had a previous history of hypertension. The patients who had stage 2 hypertension were treated with antihypertensive medication. Except for one patient who developed hypertension earlier, the other patients with increased blood pressure due to CsA returned to normal after the discontinuation of CsA treatment and this occurred without taking antihypertensive medication.
Table 3.
The common complications of cyclosporine treatment in this study
The mean level of serum creatinine was 0.85±0.16 mg/L at baseline and 0.79±0.26 mg/L at the end of the treatment. There was no significant difference in the serum creatinine level between before and after treatment. One patient stopped CsA treatment due to severe adverse events of the CsA. In this patient, a serum creatinine level that rose to more than 30% above the baseline level was detected 26 months after CsA treatment. After confirming the elevation of the serum creatinine level, the dose of CsA was reduced to 100 mg/day. However, the serum creatinine level did not return to the normal range. Moreover, the glomerular filtration clearance rate was decreased on the 24-hour creatinine clearance test. Therefore, the CsA treatment was discontinued. Four months after the CsA was discontinued, the serum creatinin level returned to normal.
Three patients complained of abdominal discomfort. The abdominal discomfort of these patients occurred after 1-year of CsA treatment. The symptom was treated with cimetidine or almagate. There was one case of hypertrichosis that occurred on both arms of a female patient. This symptom was improved six months after the discontinuation of CsA treatment.
DISCUSSION
The subjects of our investigation showed a male predominance (male 2.05:female 1). This is different from the 1.0:1.3 previously reported male to female ratio for the general AD population20. In the previous reports on CsA treatment for moderate to severe AD patients, there was no significant gender predominance9,18. The frequency of AD patients presenting with intrinsic AD is variable, ranging from 16 to over 25 percent, and up to 45 percent according to different regions15. In our study, the frequency of intrinsic AD among all the AD subjects was 24.6%.
In this study, the mean initial dose of CsA was 2.7 mg/kg/day. The mean initial dosage used in this study was relatively lower than the 5.0 mg/kg/day used in the study of Harper et al.9 and the 4.2 mg/kg/day used in the study of Lee et al.18. The mean duration of AD was longer than that reported in the other prior studies (13.5±8.4 months)7,18. There were statistically significant differences in the SCORAD score between the values obtained at baseline (27.26±10.85) and at 6-months of treatment (13.93±8.10). These findings are consistent with the results of other reports showing that CsA was effective for the treatment of AD4,21-23. CsA treatment was effective for both intrinsic and extrinsic AD, and even for childhood AD.
Whether or not the total serum IgE levels change after CsA treatment remains a matter of debate. According to the investigation reported by Okudaira et al.24, the level of serum IgE didn't change after treatment with CsA. However, another report showed that CsA treatment was associated with down-regulation of IgE production via suppressing the secretion of such Th2 cytokines as IL-4 and IL-525. In our study, the serum level of total IgE was significantly decreased after the CsA treatment. In the extrinsic group, the serum levels of total IgE decreased, whereas a decrease of total serum IgE levels was not observed in the intrinsic group. It is likely that there is another mechanism of CsA for the treatment of AD and that mechanism is probably not associated with decreased serum IgE levels. This mechanism may affect the treatment of intrinsic AD.
Ninety-five percent of patients in our study showed a prolonged remission and they could discontinue the CsA treatment. After the discontinuation of CsA treatment, all the patients were followed up for at least 6 months. Once CsA treatment was stopped, the mean time to restart CsA treatment (4.5±2.9 months) was relatively longer than that of other reports. In another study, for the short-term CsA treatment (4 weeks) of Korean childhood AD, 80% of the patients experienced relapse of their disease within 6 weeks11. According to the report of Lee et al.18, recurrence of AD was observed in all patients 3 months after cessation of CsA treatment. The remission period seems to be prolonged after the long-term period of CsA treatment for AD. In the report by Hijnen et al.6, the remission periods in 55% of the patients who underwent CsA treatment for more than 6 months were at least 3 months. These results suggest there is a relationship between long-term CsA treatment and a prolonged remission period for AD.
Overall, the adverse events in this study were relatively milder and smaller in number than those in other studies by Hijnen et al.6 and Garcia-Bustinduy et al.13. In the report of Hijnen et al.6, the initially used doses of CsA varied up to 5 mg/kg. An increased serum creatinine level more than 30% above the baseline value was observed in 7 patients (9.6%). Hypertension was reported in 11 patients (15.1%). Other adverse events such as paresthesia, hypertrichosis, headache and fatigue were also observed. According to an investigation by Garcia-Bustinduy et al.13, the mean initial dose of CsA in their study was 3 mg/kg. Arterial hypertension was found in 45.3% of their patients, and a transient increase in plasma creatinine (>30% more than the baseline level) developed in 11.3% of the patients. As compared with our report, the adverse events of CsA were more commonly observed in these two reports. According to the results of our study, we suggest that the development of adverse events might be related to a higher mean initial dose of CsA. Using a low initial dose of CsA can probably decrease the complications of CsA.
In conclusion, the results of this study showed that CsA is safe and effective for the long-term treatment of AD in adults and children if close follow-up and routine laboratory monitoring are performed.
References
- 1.James WD, Berger TG, Elston DM, Odom RB. Andrews' diseases of the skin: clinical dermatology. 10th ed. Philadelphia: Saunders Elsevier; 2006. pp. 69–77. [Google Scholar]
- 2.Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6:468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 3.Mueller W, Herrmann B. Cyclosporin A for psoriasis. N Engl J Med. 1979;301:555. doi: 10.1056/NEJM197909063011016. [DOI] [PubMed] [Google Scholar]
- 4.Nousari CH, Anhalt GJ. Immunosuppressive and immunomodulatory drugs. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th ed. New York: McGraw-Hill; 2008. pp. 2217–2223. [Google Scholar]
- 5.Lee CS, Koo JYM. Cyclosporine. In: Wolverton SE, editor. Comprehensive dermatologic drug therapy. 2nd ed. Indiana: Saunders Elsevier; 2007. pp. 219–238. [Google Scholar]
- 6.Hijnen DJ, ten Berge O, Timmer-de Mik L, Bruijnzeel-Koomen CA, de Bruin-Weller MS. Efficacy and safety of long-term treatment with cyclosporin A for atopic dermatitis. J Eur Acad Dermatol Venereol. 2007;21:85–89. doi: 10.1111/j.1468-3083.2006.01877.x. [DOI] [PubMed] [Google Scholar]
- 7.Harper JI, Berth-Jones J, Camp RD, Dillon MJ, Finlay AY, Holden CA, et al. Cyclosporin for atopic dermatitis in children. Dermatology. 2001;203:3–6. doi: 10.1159/000051694. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DE, Kemp AS. Cyclosporine restores cytokine imbalance in childhood atopic dermatitis. J Allergy Clin Immunol. 1997;99:857–859. doi: 10.1016/s0091-6749(97)80025-5. [DOI] [PubMed] [Google Scholar]
- 9.Harper JI, Ahmed I, Barclay G, Lacour M, Hoeger P, Cork MJ, et al. Cyclosporin for severe childhood atopic dermatitis: short course versus continuous therapy. Br J Dermatol. 2000;142:52–58. doi: 10.1046/j.1365-2133.2000.03241.x. [DOI] [PubMed] [Google Scholar]
- 10.Park C, Kang YS, Lee CH. Immunologic changes after treatment with cyclosporine A in atopic dermatitis. Korean J Dermatol. 1993;31:210–216. [Google Scholar]
- 11.Lee SW, Lee YS, Lee SC. The effect of cyclosporin in the treatment of childhood atopic dermatitis. Korean J Dermatol. 2000;38:466–471. [Google Scholar]
- 12.Lee JH, Kim KH, Park KC, Chung JH, Suh DH. The efficacy of cyclosporine in patients with severe atopic dermatitis. Ann Dermatol. 2001;13:12–15. [Google Scholar]
- 13.Garcia-Bustinduy M, Escoda M, Guimera FJ, Saez M, Dorta S, Fagundo E, et al. Safety of long-term treatment with cyclosporin A in resistant chronic plaque psoriasis: a retrospective case series. J Eur Acad Dermatol Venereol. 2004;18:169–172. doi: 10.1111/j.1468-3083.2004.00877.x. [DOI] [PubMed] [Google Scholar]
- 14.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44–47. [Google Scholar]
- 15.Schmid-Grendelmeier P, Simon D, Simon HU, Akdis CA, Wuthrich B. Epidemiology, clinical features, and immunology of the "intrinsic" (non-IgE-mediated) type of atopic dermatitis (constitutional dermatitis) Allergy. 2001;56:841–849. doi: 10.1034/j.1398-9995.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- 16.Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10–19. doi: 10.1159/000245677. [DOI] [PubMed] [Google Scholar]
- 17.Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157:645–648. doi: 10.1111/j.1365-2133.2007.08112.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Yun SJ, Lee JB, Kim SJ, Won YH, Lee SC. The efficacy of cyclosporin in patients with atopic dermatitis. Korean J Dermatol. 2008;46:224–230. [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Leung DYM, Eichenfield LF, Boguniewicz M. Atopic dermatitis. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th ed. New York: McGraw-Hill; 2008. pp. 146–158. [Google Scholar]
- 21.Bunikowski R, Staab D, Kussebi F, Brautigam M, Weidinger G, Renz H, et al. Low-dose cyclosporin A microemulsion in children with severe atopic dermatitis: clinical and immunological effects. Pediatr Allergy Immunol. 2001;12:216–223. doi: 10.1034/j.1399-3038.2001.012004216.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee SS, Tan AW, Giam YC. Cyclosporin in the treatment of severe atopic dermatitis: a retrospective study. Ann Acad Med Singapore. 2004;33:311–313. [PubMed] [Google Scholar]
- 23.Granlund H, Erkko P, Remitz A, Langeland T, Helsing P, Nuutinen M, et al. Comparison of cyclosporin and UVAB phototherapy for intermittent one-year treatment of atopic dermatitis. Acta Derm Venereol. 2001;81:22–27. doi: 10.1080/00015550120235. [DOI] [PubMed] [Google Scholar]
- 24.Okudaira H, Sakurai Y, Terada K, Terada E, Ogita T, Miyamoto T. Cyclosporin A-induced suppression of ongoing IgE antibody formation in the mouse. Int Arch Allergy Appl Immunol. 1986;79:164–168. doi: 10.1159/000233965. [DOI] [PubMed] [Google Scholar]
- 25.Eynott PR, Salmon M, Huang TJ, Oates T, Nicklin PL, Chung KF. Effects of cyclosporin A and a rapamycin derivative (SAR943) on chronic allergic inflammation in sensitized rats. Immunology. 2003;109:461–467. doi: 10.1046/j.1365-2567.2003.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]