Abstract
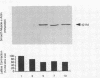
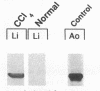
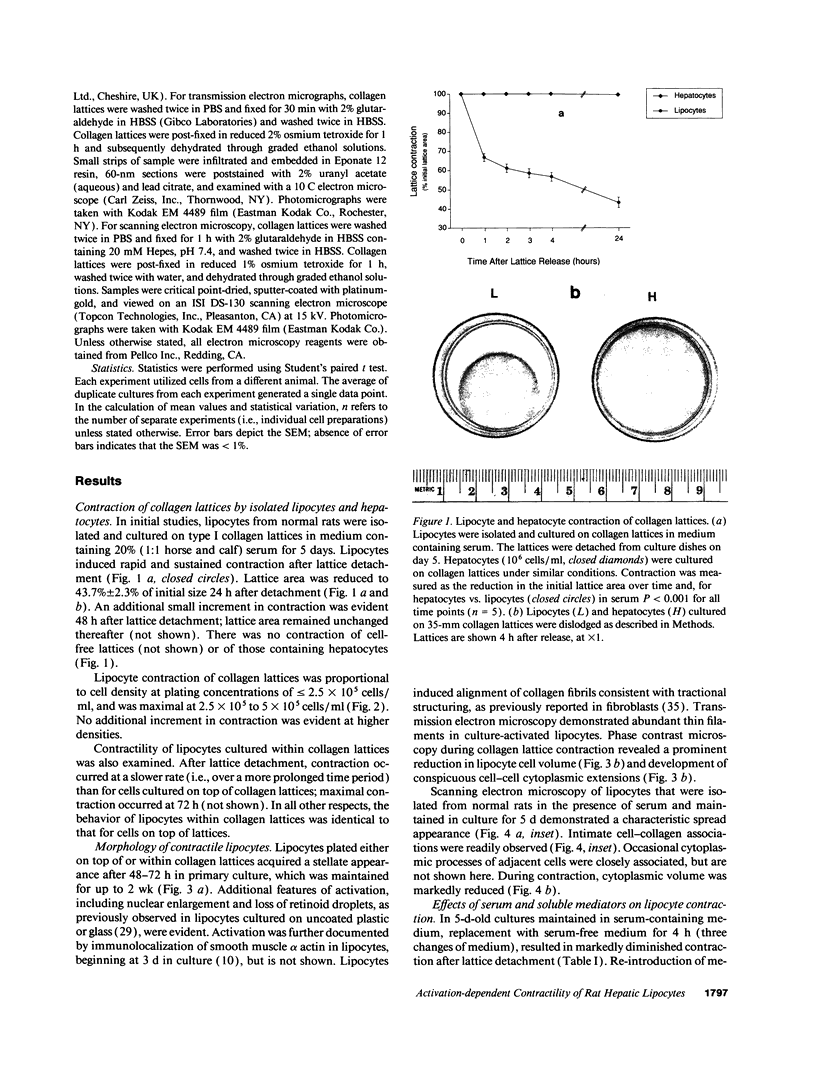
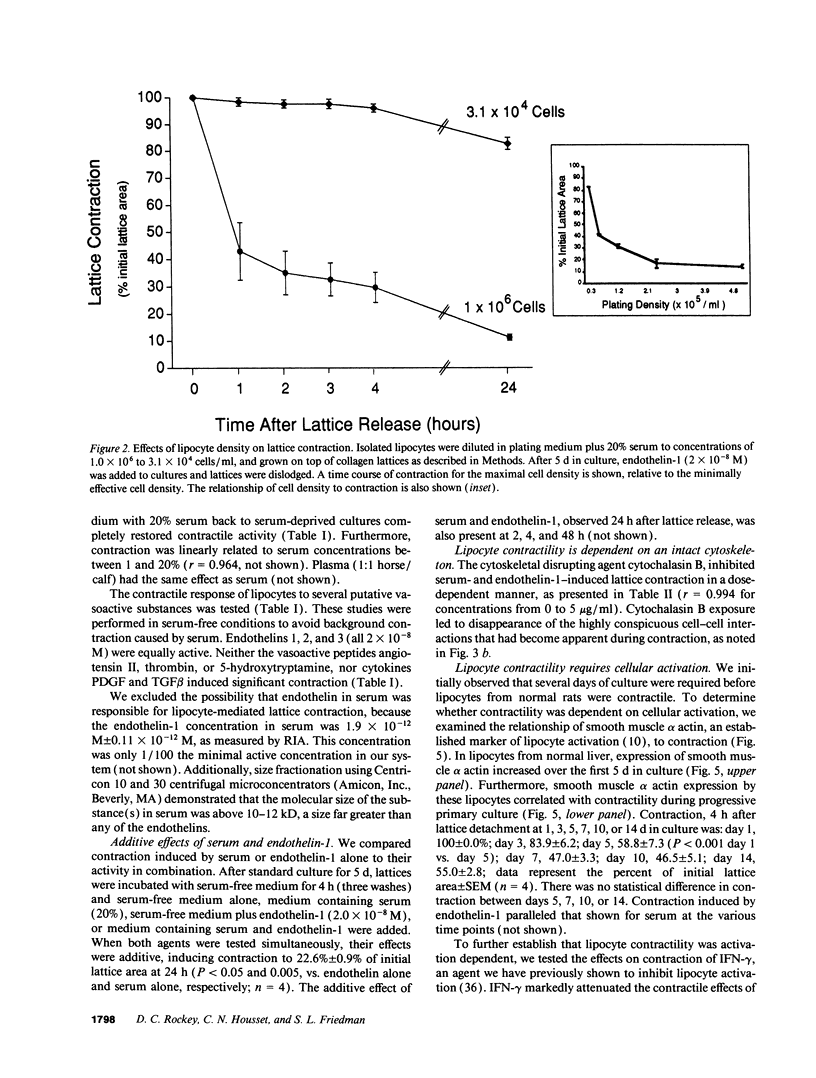
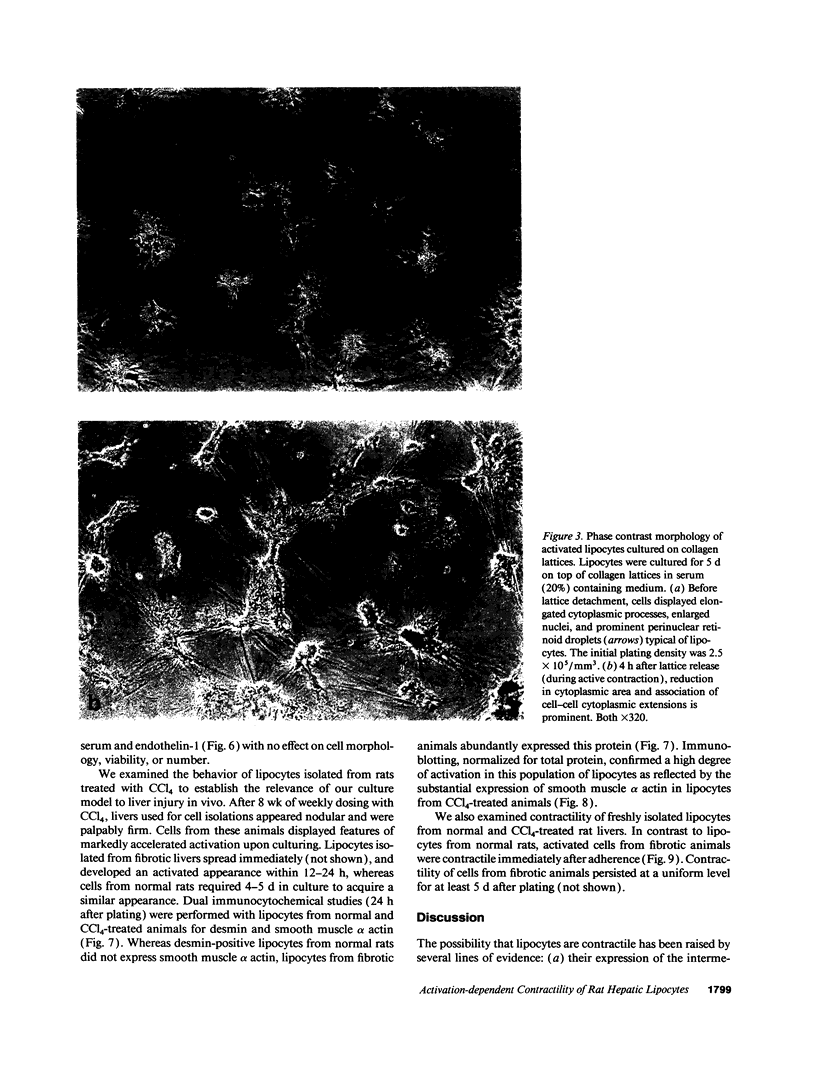
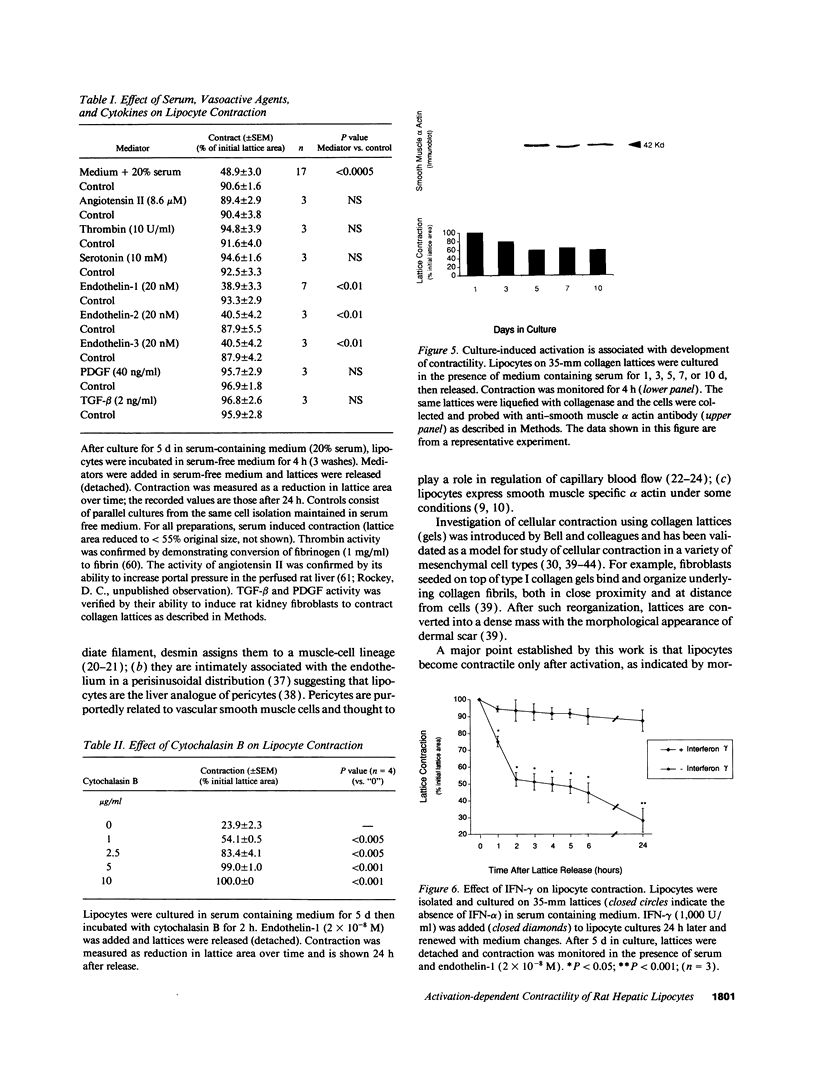
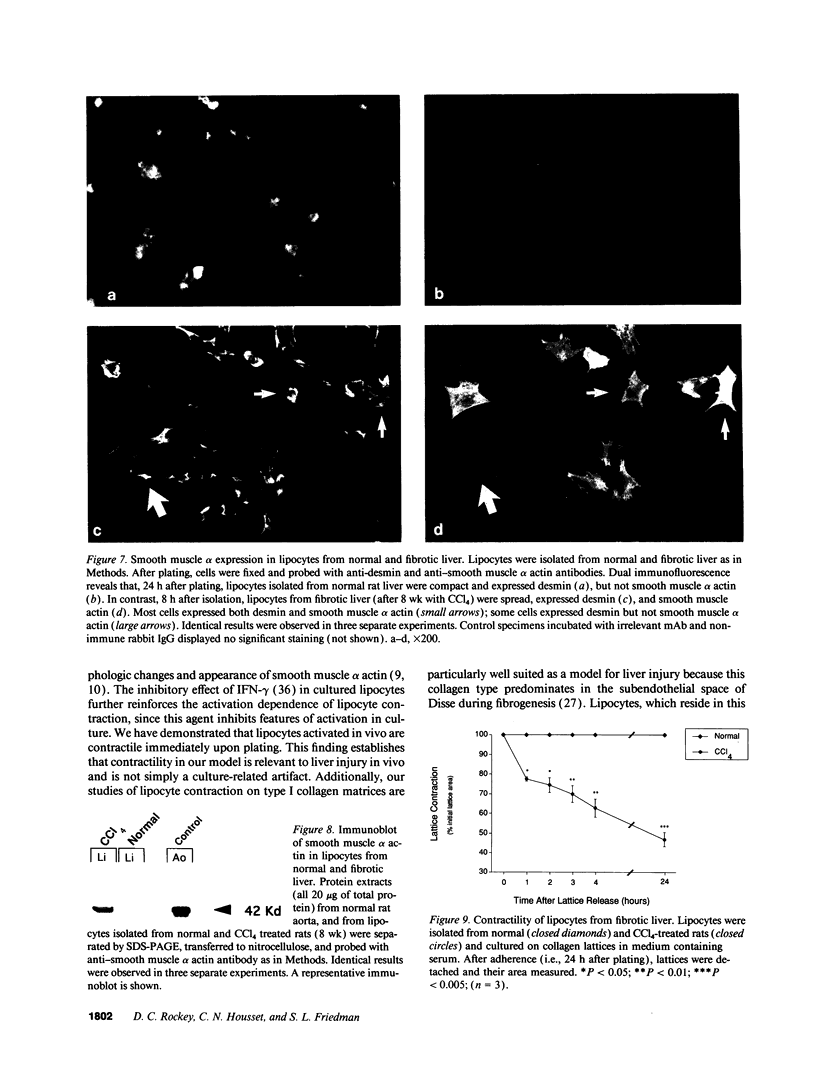
Hepatic lipocytes are perisinusoidal cells that have been thought to be analogous to tissue pericytes, a cell type with purported vasoregulatory properties. However, we and others have recently demonstrated that lipocytes acquire markers of smooth muscle cells or myofibroblasts only after liver injury, via a process termed "activation." In this study, we document lipocyte contractility on collagen lattices and examine the importance of activation in this process. In culture, lipocytes became contractile only after spreading and activating, coincident with expression of smooth muscle alpha actin, a marker of activation (1990. Virchows Arch. B Cell Pathol. 59:349). After 5 d in culture, lipocytes induced rapid and sustained contraction of collagen lattices (to 43.7 +/- 2.3% of their original size 24 h after detachment). There was no contraction of lattices containing hepatocytes. Scanning electron microscopy demonstrated intimate associations of lipocyte cell membranes and collagen fibrils. Reduction in cell volume during contraction was also prominent. Lattice contraction by lipocytes was proportional to cell number. Serum was a potent stimulator of lipocyte contraction, as were endothelin types 1, 2, and 3; the effect of serum and endothelin 1 were additive. Neither thrombin, angiotensin-II, serotonin, nor the cytokines PDGF and TGF beta induced contraction. Cytochalasin B treatment resulted in concentration-dependent inhibition of contraction. As a test of the in vivo relevance of the culture findings, lipocytes were isolated from fibrotic animals and examined immediately after adherence. Whereas lipocytes from normal liver were initially compact, smooth muscle alpha actin negative and noncontractile, cells from animals with hepatic injury due to CCl4 displayed an activated appearance, expressed smooth muscle alpha actin, and were contractile immediately after adherence. Additionally, IFN-gamma, an agent which blocks lipocyte activation (1992. Hepatology. 16:776), inhibited lipocyte contraction. The data document that normal (i.e., quiescent) lipocytes are not contractile, but that activation is associated with the development of contractility. These findings suggest that a role for lipocytes in organ contraction or vasoregulation may be confined to injured, not normal liver.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. N., Ruben Z., Fuller G. C. Cell-mediated contraction of collagen lattices in serum-free medium: effect of serum and nonserum factors. In Vitro Cell Dev Biol. 1990 Jan;26(1):61–66. doi: 10.1007/BF02624156. [DOI] [PubMed] [Google Scholar]
- Ballardini G., Fallani M., Biagini G., Bianchi F. B., Pisi E. Desmin and actin in the identification of Ito cells and in monitoring their evolution to myofibroblasts in experimental liver fibrosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56(1):45–49. doi: 10.1007/BF02890000. [DOI] [PubMed] [Google Scholar]
- Bell E., Ivarsson B., Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows C. G., Melcher A. H., Bhargava U., Aubin J. E. Fibroblasts contracting three-dimensional collagen gels exhibit ultrastructure consistent with either contraction or protein secretion. J Ultrastruct Res. 1982 Feb;78(2):178–192. doi: 10.1016/s0022-5320(82)80022-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bhathal P. S., Grossman H. J. Reduction of the increased portal vascular resistance of the isolated perfused cirrhotic rat liver by vasodilators. J Hepatol. 1985;1(4):325–337. doi: 10.1016/s0168-8278(85)80770-4. [DOI] [PubMed] [Google Scholar]
- Bioulac-Sage P., Lafon M. E., Saric J., Balabaud C. Nerves and perisinusoidal cells in human liver. J Hepatol. 1990 Jan;10(1):105–112. doi: 10.1016/0168-8278(90)90080-b. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991 Mar 1;5(3):271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- Boswell C. A., Majno G., Joris I., Ostrom K. A. Acute endothelial cell contraction in vitro: a comparison with vascular smooth muscle cells and fibroblasts. Microvasc Res. 1992 Mar;43(2):178–191. doi: 10.1016/0026-2862(92)90015-h. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Ehrlich H. P. Comparative studies of collagen lattice contraction utilizing a normal and a transformed cell line. J Cell Physiol. 1983 Aug;116(2):159–166. doi: 10.1002/jcp.1041160206. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. What distinguishes tenascin from fibronectin? FASEB J. 1990 Jun;4(9):2598–2604. doi: 10.1096/fasebj.4.9.1693347. [DOI] [PubMed] [Google Scholar]
- Davis B. H., Vucic A. The effect of retinol on Ito cell proliferation in vitro. Hepatology. 1988 Jul-Aug;8(4):788–793. doi: 10.1002/hep.1840080416. [DOI] [PubMed] [Google Scholar]
- Demaurex N., Schlegel W., Varnai P., Mayr G., Lew D. P., Krause K. H. Regulation of Ca2+ influx in myeloid cells. Role of plasma membrane potential, inositol phosphates, cytosolic free [Ca2+], and filling state of intracellular Ca2+ stores. J Clin Invest. 1992 Sep;90(3):830–839. doi: 10.1172/JCI115958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge A. B., Hechtman H. B., Shepro D. Microvascular endothelial-derived autacoids regulate pericyte contractility. Cell Motil Cytoskeleton. 1991;18(3):180–188. doi: 10.1002/cm.970180304. [DOI] [PubMed] [Google Scholar]
- Díaz-Flores L., Gutiérrez R., Varela H., Rancel N., Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol. 1991 Apr;6(2):269–286. [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Arenson D. M., Bissell D. M. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989 Jun 25;264(18):10756–10762. [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Geerts A., Lazou J. M., De Bleser P., Wisse E. Tissue distribution, quantitation and proliferation kinetics of fat-storing cells in carbon tetrachloride-injured rat liver. Hepatology. 1991 Jun;13(6):1193–1202. [PubMed] [Google Scholar]
- Greenwel P., Schwartz M., Rosas M., Peyrol S., Grimaud J. A., Rojkind M. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest. 1991 Dec;65(6):644–653. [PubMed] [Google Scholar]
- Gressner A. M., Bachem M. G. Cellular sources of noncollagenous matrix proteins: role of fat-storing cells in fibrogenesis. Semin Liver Dis. 1990 Feb;10(1):30–46. doi: 10.1055/s-2008-1040455. [DOI] [PubMed] [Google Scholar]
- Guidry C., Grinnell F. Contraction of hydrated collagen gels by fibroblasts: evidence for two mechanisms by which collagen fibrils are stabilized. Coll Relat Res. 1987 Feb;6(6):515–529. doi: 10.1016/s0174-173x(87)80050-x. [DOI] [PubMed] [Google Scholar]
- Horn T., Junge J., Christoffersen P. Early alcoholic liver injury: changes of the Disse space in acinar zone 3. Liver. 1985 Dec;5(6):301–310. doi: 10.1111/j.1600-0676.1985.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Irving M. G., Roll F. J., Huang S., Bissell D. M. Characterization and culture of sinusoidal endothelium from normal rat liver: lipoprotein uptake and collagen phenotype. Gastroenterology. 1984 Dec;87(6):1233–1247. [PubMed] [Google Scholar]
- Joyce N. C., DeCamilli P., Boyles J. Pericytes, like vascular smooth muscle cells, are immunocytochemically positive for cyclic GMP-dependent protein kinase. Microvasc Res. 1984 Sep;28(2):206–219. doi: 10.1016/0026-2862(84)90018-9. [DOI] [PubMed] [Google Scholar]
- Kawada N., Klein H., Decker K. Eicosanoid-mediated contractility of hepatic stellate cells. Biochem J. 1992 Jul 15;285(Pt 2):367–371. doi: 10.1042/bj2850367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley C., D'Amore P., Hechtman H. B., Shepro D. Microvascular pericyte contractility in vitro: comparison with other cells of the vascular wall. J Cell Biol. 1987 Mar;104(3):483–490. doi: 10.1083/jcb.104.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent G., Gay S., Inouye T., Bahu R., Minick O. T., Popper H. Vitamin A-containing lipocytes and formation of type III collagen in liver injury. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3719–3722. doi: 10.1073/pnas.73.10.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Maher J. J., McGuire R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak K. M., Leo M. A., Lieber C. S. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984 Jul;87(1):188–200. [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Failli P., Ruocco C., Casini A., Milani S., Baldi E., Giotti A., Gentilini P. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992 Aug;90(2):642–646. doi: 10.1172/JCI115905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor E., Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982 Dec;83(6):1183–1190. [PubMed] [Google Scholar]
- Rhodin J. A. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968 Dec;25(5):452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rockey D. C., Boyles J. K., Gabbiani G., Friedman S. L. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992 Apr;24(2):193–203. [PubMed] [Google Scholar]
- Rockey D. C., Maher J. J., Jarnagin W. R., Gabbiani G., Friedman S. L. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992 Sep;16(3):776–784. doi: 10.1002/hep.1840160325. [DOI] [PubMed] [Google Scholar]
- Rudolph R., McClure W. J., Woodward M. Contractile fibroblasts in chronic alcoholic cirrhosis. Gastroenterology. 1979 Apr;76(4):704–709. [PubMed] [Google Scholar]
- Schmitt-Gräff A., Krüger S., Bochard F., Gabbiani G., Denk H. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am J Pathol. 1991 May;138(5):1233–1242. [PMC free article] [PubMed] [Google Scholar]
- Sims D. E. Recent advances in pericyte biology--implications for health and disease. Can J Cardiol. 1991 Dec;7(10):431–443. [PubMed] [Google Scholar]
- Sims D. E. The pericyte--a review. Tissue Cell. 1986;18(2):153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg B. M., Smith K., Colozzo M., Pollack R. Establishment and transformation diminish the ability of fibroblasts to contract a native collagen gel. J Cell Biol. 1980 Oct;87(1):304–308. doi: 10.1083/jcb.87.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Jones J. C., Goldman R. D. Intermediate filaments. J Cell Biol. 1984 Jul;99(1 Pt 2):22s–27s. doi: 10.1083/jcb.99.1.22s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopak D., Harris A. K. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev Biol. 1982 Apr;90(2):383–398. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Nouchi T., Yamane M., Irie T., Miyakawa H., Sato C., Marumo F. Phenotypic modulation in lipocytes in experimental liver fibrosis. J Pathol. 1991 Jul;164(3):273–278. doi: 10.1002/path.1711640314. [DOI] [PubMed] [Google Scholar]
- Tilton R. G., Kilo C., Williamson J. R., Murch D. W. Differences in pericyte contractile function in rat cardiac and skeletal muscle microvasculatures. Microvasc Res. 1979 Nov;18(3):336–352. doi: 10.1016/0026-2862(79)90042-6. [DOI] [PubMed] [Google Scholar]
- Tilton R. G., Kilo C., Williamson J. R. Pericyte-endothelial relationships in cardiac and skeletal muscle capillaries. Microvasc Res. 1979 Nov;18(3):325–335. doi: 10.1016/0026-2862(79)90041-4. [DOI] [PubMed] [Google Scholar]
- Wake K. "Sternzellen" in the liver: perisinusoidal cells with special reference to storage of vitamin A. Am J Anat. 1971 Dec;132(4):429–462. doi: 10.1002/aja.1001320404. [DOI] [PubMed] [Google Scholar]
- Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol. 1980;66:303–353. doi: 10.1016/s0074-7696(08)61977-4. [DOI] [PubMed] [Google Scholar]
- Yokoi Y., Namihisa T., Kuroda H., Komatsu I., Miyazaki A., Watanabe S., Usui K. Immunocytochemical detection of desmin in fat-storing cells (Ito cells). Hepatology. 1984 Jul-Aug;4(4):709–714. doi: 10.1002/hep.1840040425. [DOI] [PubMed] [Google Scholar]
- de Leeuw A. M., McCarthy S. P., Geerts A., Knook D. L. Purified rat liver fat-storing cells in culture divide and contain collagen. Hepatology. 1984 May-Jun;4(3):392–403. doi: 10.1002/hep.1840040307. [DOI] [PubMed] [Google Scholar]