Abstract
Background
Fibroblasts produce many components of the extracellular matrix (ECM) and so they contribute to the maintenance of connective tissue integrity.
Objective
The aim of this study is to evaluate the effect of velvet antler extract (VAE) on the ECM production of dermal fibroblasts cultured in vitro.
Methods
Primary cultured human dermal fibroblasts were treated with VAE, and then the ECM production was determined by RT-PCR, ELISA and Western blot analysis. Furthermore, the change of gene expression according to VAE treatment was evaluated by cDNA microarray.
Results
VAE accelerated the growth of fibroblasts in a dose-dependent manner. VAE increased the production of several ECM components, including type 1 collagen, fibronectin and elastin. In line with these results, the phosphorylations of p42/44 ERK and p38 mitogen-activated protein kinase were markedly increased by VAE, suggesting that the enhancement of ECM production may be linked to the activation of intracellular signaling cascades. VAE also significantly increased cell migration on an in vitro scratch wound test. In cDNA microarray, many genes related with connective tissue integrity were identified to be up-regulated by VAE.
Conclusion
These results suggest that VAE has a potential to stimulate ECM production, and VAE may be applicable for maintaining the skin's texture.
Keywords: cDNA microarray, Extracellular matrix, Fibroblast, Velvet antler extract
INTRODUCTION
Fibroblasts are the major cells in the dermis, and these cells provide tensile strength and elasticity through the production and secretion of various components of the extracellular matrix (ECM), including collagens, proelastin, glycoproteins and proteoglycans1. Intracellular signaling cascades such as transforming growth factor-β (TGF-β), extracellular signal-regulated kinases (ERKs), p38 mitogen-activated protein kinase (MAPK) and the Wnt/β-catenin pathway have been implicated in the regulation of ECM production in fibroblasts. With ageing, fibroblasts lose their proliferative potential and they show diminished ECM biosynthesis, resulting in dermal atrophy and wrinkle formation2. In addition to their role for maintaining the mechanical properties of skin, fibroblasts also play a pivotal role during the wound healing process. Wound healing is a complex process that involves inflammation, granulation tissue formation and tissue remodeling, and this is all regulated by a number of cytokines and growth factors3. During this process, fibroblasts proliferate and produce many of the ECM molecules in a TGF-β-dependent manner, and this contributes to the recovery of connective tissue integrity4. Thus, it has long been believed that enhancing the activity of fibroblasts, in the context of ECM production, may have beneficial effects on maintenance of skin texture.
Velvet antler is one of the most famous Korean traditional medicines, and it has been considered to contain some functional components for health promotion. It has been used for the treatment of bone disease, liver damage and anemia5. Several studies have indicated that velvet antler extract (VAE) has immunosuppressive activity, an inhibitory potential for bone resorption and stimulatory effects on hematopoietic stem cells6-8. Although the diverse activities of VAE have been implicated in various biological systems, the potential effects of VAE on the skin have not yet been precisely determined. At present, there is only limited data available to support the potential effects of VAE on skin cells. For instance, a water extract and the polypeptides of velvet antler have a stimulatory effect on the proliferation of epidermal cells9, and topical treatment with VAE accelerated the repair of cutaneous wounds in diabetic rats10.
In the present study, we demonstrate that VAE stimulates the ECM production in dermal fibroblasts cultured in vitro, and that many genes related to connective tissue integrity were up-regulated by VAE.
MATERIALS AND METHODS
Cell culture
Normal human skin samples were obtained from circumcisions, in accordance with the ethical committee approval process of Chungnam National University Hospital. The specimens were briefly sterilized in 70% ethanol, minced and then incubated in DMEM supplemented with 10% FBS and antibiotics (Gibco BRL, Rockville, MD, USA). Dermal fibroblasts normally outgrew from the explants after 5~7 days. At confluence, the cells were routinely passaged using a 1:4 split ratio and were used between passages 4 and 16. For treatment with VAE, approximately 1×106 cells were seeded on 100-mm culture dishes and grown to sub-confluence. The cells were starved of serum for 24 h, and then treated with VAE in serum-free medium.
Preparation of VAE
The dried velvet antler was crushed and extracted with cold ethanol. The ethanol-extract was concentrated in a vacuum evaporator (Buchi, Switzerland) and the resulting residue was weighed and dissolved to a 1% solution in 70% ethanol.
Cell growth analysis
For the [3H]thymidine uptake assay, fibroblasts were seeded in a 60-mm culture dish and treated with 1 µCi of [3H]thymidine (Amersham, Buckinghamshire, UK). Following incubation for the indicated time points, the cells were washed twice with PBS and incubated with 0.1 N NaOH at room temperature. The radioactivity in the cell lysates was measured using a liquid scintillation counter.
ELISA
The ELISA kit for type 1 procollagen was purchased from Takara Bio Inc. (Shiga, Japan), and the ELISA kit for fibronectin was purchased from American Diagnostica (Greenwich, CT, USA). The levels of type 1 procollagen and fibronectin secreted from the fibroblasts were quantified according to the manufacturer's recommended protocols. The measurements were repeated at least 3 times, with independent cell batches.
Western blot analysis
The cells were lysed in Proprep solution (Intron, Daejeon, Korea). After vigorous pipetting, the extracts were centrifuged for 15 min at 13,000 rpm. The total protein was measured using a Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). The samples were run on SDS-polyacrylamide gels and transferred onto nitrocellulose membranes and then the proteins on the membranes were incubated with the appropriate antibodies for overnight at 4℃ with gentle agitation. The blots were then incubated with the peroxidase-conjugated secondary antibodies for 30 minutes at room temperature, and visualized by enhanced chemiluminescence (Intron, Daejeon, Korea). The following primary antibodies were used in this study: collagen type 1 α1 and elastin (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), phospho-p38 MAPK, total-p38 MAPK, phospho-p42/44 ERK and total-p42/44 ERK (Cell Signaling Technology, Danvers, MA, USA) and actin (Sigma, St. Louis, MO, USA).
In vitro wound healing assay
When a new artificial gap is created on a confluent cell monolayer, the cells on the edge of the gap move toward the opening to close the gap until new cell-cell contacts are again established11. We performed an in vitro wound healing assay to evaluate the migration potency of the fibroblasts. Fibroblasts were grown to confluency and they were incubated with 10 µg/ml mitomycin C (Sigma) for 2 h to rule out the proliferative effect. A cell-free area was introduced by scraping the monolayer with a pipette tip. After incubation, the cells were photographed by using an inverted phase-contrast microscope.
Total RNA extraction and cDNA microarray analysis
Total RNA was extracted from the cells using the Trizol Reagent Kit (Invitrogen) according to the manufacturer's instructions. For the microarray analysis, a 22K Human Genome Array was used (Genomictree, Daejeon, Korea). One hundred µg total RNA was reverse transcribed in the presence of Cy3 and/or Cy5 monoreactive dye (Amersham, Little Chalfont, UK) using a Superscript cDNA synthesis system (Gibco BRL, Rockville, MD, USA). After hybridization, microarray scanning and data normalization were performed using a GenePix 4000B scanner and GenePix Pro 3.0 software (Axon Instruments, Union City, CA, USA). The differentially expressed genes were selected based on the fold-change and Welch's t-test.
RT-PCR
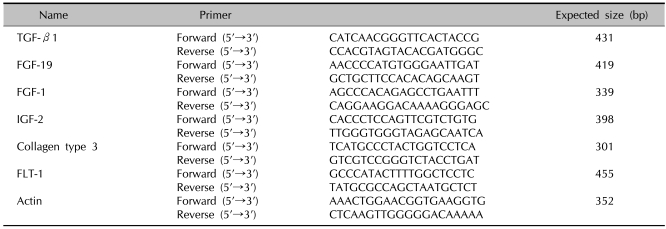
Two µg of total RNA were reverse-transcribed using M-MLV reverse transcriptase (ELPIS Biotech, Daejeon, Korea). Aliquots of the RT mixture were subjected to PCR with specific primer sets (Table 1).
Table 1.
Nucleotide sequence of the primers
RESULTS
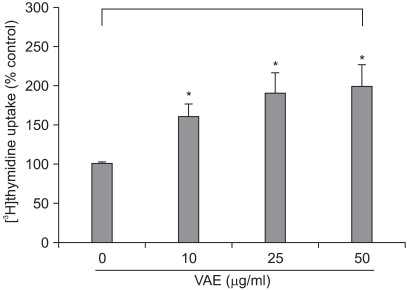
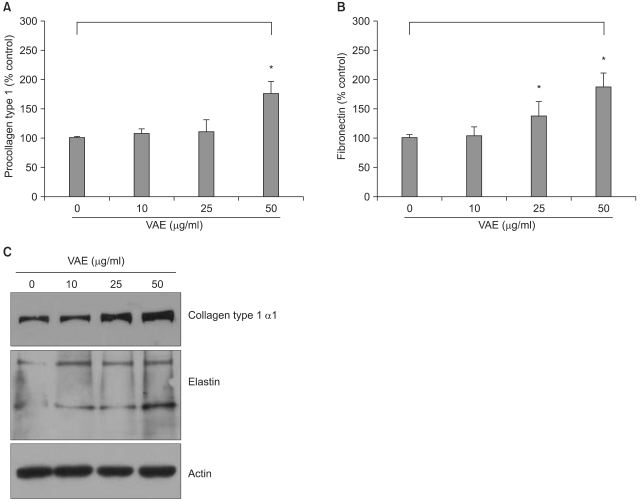
It has been previously reported that the polypeptides from velvet antler have a stimulatory effect on the proliferation of epidermal cells9. To investigate whether VAE has a potential for stimulating cell growth, we first performed a [3H]thymidine uptake assay. Dermal fibroblasts were serially treated with various concentrations of VAE and incubated for 2 days. Consistent with the previous reports, VAE increased the [3H]thymidine uptake of the fibroblasts in a dose-dependent manner (Fig. 1). Since the proliferative property of fibroblasts is frequently linked to the ECM production capability, we next determined the effect of VAE on the production of several ECM components. ELISA assay showed that VAE increased the secretion of type 1 procollagen and fibronectin in a dose-dependent manner (Fig. 2A, B). Concomitantly, Western blotting demonstrated that VAE increased the intracellular protein levels of collagen type 1 α1 and other connective tissue components of elastin (Fig. 2C).
Fig. 1.
Effect of velvet antler extract (VAE) on the growth of dermal fibroblasts. The cells were treated with VAE at the indicated concentrations for 2 days in the presence of [3H]thymidine. The radioactivity was measured by a liquid scintillation counter. The results are shown as percentage of the control±standard deviation (SD) (*p<0.05 vs. control).
Fig. 2.
Effect of velvet antler extract (VAE) on the extracellular matrix (ECM) production of dermal fibroblasts. The cells were treated with VAE at the indicated concentrations for 2 days. The conditioned medium was collected, and the secreted procollagen type 1 (A) and fibronectin (B) were measured by ELISA. The results are shown as percentage of the control±standard deviation (SD) (*p<0.05 vs. control). (C) The cellular proteins were harvested and the protein levels for collagen type 1 α1 and elastin were verified by Western blot analysis.
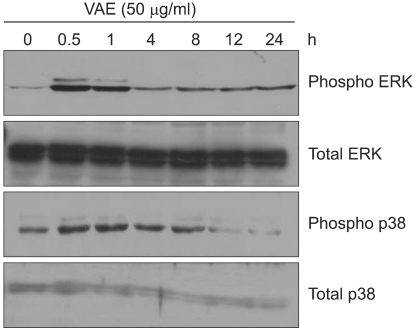
As the intracellular signaling cascades such as p42/44 ERK and p38 MAPK cascades have been implicated in the regulation of ECM production12,13, we investigated the effect of VAE on MAPK activation. As shown in Fig. 3, VAE treatment led to quick phosphorylation of p42/44 ERK and p38 MAPK. The phosphorylation then gradually diminished in a time-dependent manner. These results suggest that VAE affects the intracellular signaling cascades and thereby has an influence on the expression of ECM components.
Fig. 3.
Effect of velvet antler extract (VAE) on intracellular signaling pathway. The cells were treated with VAE for the indicated time points. The cellular proteins were prepared and the phosphorylations of ERK and p38 MAPK were determined by Western blot analysis.
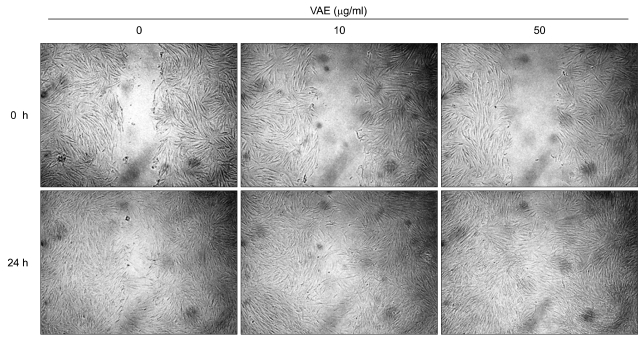
Previous data indicates that VAE accelerated the wound healing in diabetic rats10. During the wound closure, fibroblasts extensively proliferate and migrate, and then they fill the wound gap. Thus, we next determined whether VAE has a potential for enhancing cell migration. We carried out an in vitro scratch assay because it is considered to be a straightforward and economical method to study cell migration. Faster closure of the scratch wound was observed in the VAE-treated group as compared with that of the control group (Fig. 4).
Fig. 4.
Effect of velvet antler extract (VAE) on cell migration. Confluent monolayers of dermal fibroblasts were wounded using a pipette tip, and the monolayer cells were treated with VAE at the indicated concentrations. The areas of cell-free wounds were photographed after 24 h treatment.
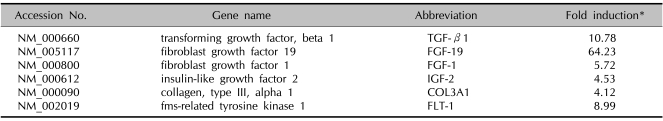
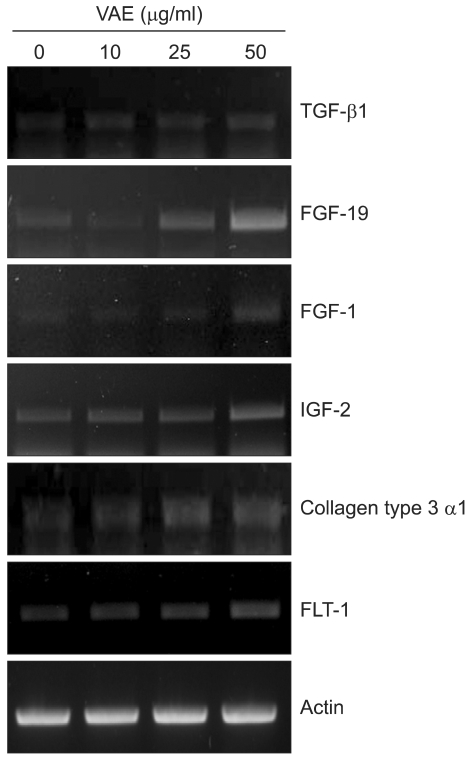
We carried out cDNA microarray analysis to further delineate the effects of VAE on fibroblasts. As a result, we obtained about 1,200 differentially expressed genes by VAE treatment. We selected several genes that may be linked to the wound healing process and that showed their fold inductions in cDNA microarray (Table 2). We then performed RT-PCR analysis to confirm the cDNA microarray results. The expression levels for TGF-β1, FGF-19, FGF-1, IGF-2, collagen type 3 α1 and FLT-1 were significantly increased by VAE in a dose-dependent manner (Fig. 5), which well-matched the results of the cDNA microarray.
Table 2.
Representative genes that are highly up-regulated by VAE in fibroblasts
*Dermal fibroblasts were treated with 50 µg/ml velvet antler extract (VAE) for 2 days. The total RNAs were isolated and used for preparation of the Cy3- or Cy5-labeled probes.
Fig. 5.
Dermal fibroblasts were treated with velvet antler extract (VAE) at the indicated concentrations for 2 days. The levels of expression of selected genes were verified by RT-PCR.
DISCUSSION
The maintenance of skin texture in the context of mechanical properties, including tensile strength and elasticity, is largely dependent on the activities of fibroblasts. This notion is clearly supported by the fact that biosynthesis of the ECM by fibroblasts is markedly diminished in aged persons2. Therefore, the approach that enhances ECM production and thereby strengthens the skin texture is an attractive method for curing connective tissue-related skin phenotypes such as wrinkle formation. In this study, we attempted to validate the potential effect of VAE on ECM production by using primary cultured dermal fibroblasts.
We demonstrated that VAE, a frequently used traditional medicine, has a potential for enhancing fibroblasts' proliferation and cell migration, and also that VAE increases the production of fibronectin, type 1 collagen and elastin. In addition, the cDNA microarray showed that several growth factors were greatly increased by VAE treatment of fibroblasts. Fibronectin, type 1 collagen and elastin are important constituents in connective tissue. Fibronectin is a multifunctional glycoprotein that is involved in the interaction between fibroblasts and the ECM. It is secreted from various cells, including fibroblasts, in the early stage of wound healing, and fibronectin induces cell migration, suggesting that it plays a critical role in the wound healing process14. Type I collagen plays a critical role in maintenance of the tensile strength of skin, and the dermis predominantly contains type 1 collagen, which is synthesized from dermal fibroblasts as precursor molecules called procollagen15. Elastin is physiologically important for the elasticity of skin, and it is also secreted from skin fibroblasts as a soluble precursor tropoelastin, which is subsequently cross-linked into insoluble elastin16. Based on the potential effect of VAE on those ECM components, it is expected that VAE can be used for strengthening of skin texture and/or curing of connective tissue-related skin phenotypes.
Interestingly, the effects of VAE on fibroblasts are somewhat similar to what can be seen in the wound healing process; cell proliferation, migration and ECM production. It has been well established that several growth factors, including TGF-β1, IGF-2 and platelet-derived growth factor, are up-regulated during the growth phase of cutaneous repair17. Among them, TGF-β is recognized as the most important modulator that evokes activation of the Smad signaling cascades. In our study, VAE treatment resulted in significant increases of TGF-β1, together with several growth factors such as FGF-19, FGF-1 and IGF-2. Furthermore, we found that the expression of FLT-1 (fms-like tyrosine kinase) was up-regulated by VAE. The FLT-1 is a receptor for vascular endothelial growth factor (VEGF)18, which participates in angiogenesis. Taken together, our data suggest that VAE may lead the fibroblasts towards the activated status that can be seen in the wound healing process, and this raises the possibility of therapeutically using VAE for fast wound closure.
In summary, we demonstrated that VAE has a potential for stimulating ECM production in dermal fibroblasts. Since the effect of VAE on the ECM production in fibroblasts had not been previously investigated, our data provides novel clues on which to base further investigations on the potential applications of VAE. Our results suggest that VAE could be used for connective tissue-related skin phenotypes as a co-modality together with first-line treatments.
Footnotes
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A080309). This study was also supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (01-PJ3-PG6-01GN12-0001).
References
- 1.Pieraggi MT, Bouissou H, Angelier C, Uhart D, Magnol JP, Kokolo J. The fibroblast. Ann Pathol. 1985;5:65–76. [PubMed] [Google Scholar]
- 2.Uitto J. Connective tissue biochemistry of the aging dermis. Age-related alterations in collagen and elastin. Dermatol Clin. 1986;4:433–446. [PubMed] [Google Scholar]
- 3.Chan JC, Duszczyszyn DA, Castellino FJ, Ploplis VA. Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am J Pathol. 2001;159:1681–1688. doi: 10.1016/S0002-9440(10)63015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- 5.Jhon GJ, Park SY, Han SY, Lee S, Kim Y, Chang YS. Studies of the chemical structure of gangliosides in deer antler, Cervus nippon. Chem Pharm Bull (Tokyo) 1999;47:123–127. doi: 10.1248/cpb.47.123. [DOI] [PubMed] [Google Scholar]
- 6.Kang SK, Kim KS, Kim SI, Chung KH, Lee IS, Kim CH. Immunosuppressive activity of deer antler extracts of Cervus korean TEMMINCK var. mantchuricus Swinhoe, on type II collagen-induced arthritis. In Vitro Cell Dev Biol Anim. 2006;42:100–107. doi: 10.1290/0510067.1. [DOI] [PubMed] [Google Scholar]
- 7.Li YJ, Kim TH, Kwak HB, Lee ZH, Lee SY, Jhon GJ. Chloroform extract of deer antler inhibits osteoclast differentiation and bone resorption. J Ethnopharmacol. 2007;113:191–198. doi: 10.1016/j.jep.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Yang HO, Kim SH, Cho SH, Kim MG, Seo JY, Park JS, et al. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler) Chem Pharm Bull (Tokyo) 2004;52:874–878. doi: 10.1248/cpb.52.874. [DOI] [PubMed] [Google Scholar]
- 9.Guan SW, Duan LX, Li YY, Wang BX, Zhou QL. A novel polypeptide from Cervus nippon Temminck proliferation of epidermal cells and NIH3T3 cell line. Acta Biochim Pol. 2006;53:395–397. [PubMed] [Google Scholar]
- 10.Mikler JR, Theoret CL, High JC. Effects of topical elk velvet antler on cutaneous wound healing in streptozotocin-induced diabetic rats. J Altern Complement Med. 2004;10:835–840. doi: 10.1089/acm.2004.10.835. [DOI] [PubMed] [Google Scholar]
- 11.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 12.Lim IJ, Phan TT, Tan EK, Nguyen TT, Tran E, Longaker MT, et al. Synchronous activation of ERK and phosphatidylinositol 3-kinase pathways is required for collagen and extracellular matrix production in keloids. J Biol Chem. 2003;278:40851–40858. doi: 10.1074/jbc.M305759200. [DOI] [PubMed] [Google Scholar]
- 13.Lee DJ, Rosenfeldt H, Grinnell F. Activation of ERK and p38 MAP kinases in human fibroblasts during collagen matrix contraction. Exp Cell Res. 2000;257:190–197. doi: 10.1006/excr.2000.4866. [DOI] [PubMed] [Google Scholar]
- 14.Kanzaki T, Morisaki N, Shiina R, Saito Y. Role of transforming growth factor-beta pathway in the mechanism of wound healing by saponin from Ginseng Radix rubra. Br J Pharmacol. 1998;125:255–262. doi: 10.1038/sj.bjp.0702052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol. 2004;165:741–751. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sephel GC, Davidson JM. Elastin production in human skin fibroblast cultures and its decline with age. J Invest Dermatol. 1986;86:279–285. doi: 10.1111/1523-1747.ep12285424. [DOI] [PubMed] [Google Scholar]
- 17.Falanga V, Isaacs C, Paquette D, Downing G, Kouttab N, Butmarc J, et al. Wounding of bioengineered skin: cellular and molecular aspects after injury. J Invest Dermatol. 2002;119:653–660. doi: 10.1046/j.1523-1747.2002.01865.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]