Abstract
Cutaneous metastasis from breast cancer can occur by direct invasion, lymphatic and vascular spread as well as iatrogenic implantation. Metastasis that occurs by iatrogenic implantation after needle biopsy is very rare but the potential risk must be considered. In this report, we describe a case of breast cancer cutaneous metastasis that occurred by iatrogenic implantation following core needle biopsy. A 53-year-old woman presented with a 1×1 cm sized erythematous nodule at the biopsy site after breast conserving surgery for primary cancer. Histopathological findings confirmed cutaneous metastasis. The possibility of this consequence must be considered when performing needle biopsies.
Keywords: Breast cancer, Core needle biopsy, Cutaneous metastasis
INTRODUCTION
Fine-needle aspiration biopsy, core needle biopsy, stereotactic core needle biopsy and vacuum-assisted biopsy are widely used to confirm lesions with minimal invasion. However, the potential risk of malignant cell seeding and cancer recurrence has always been a concern when applying these methods to confirm malignant tumor, including breast cancer.
Breast cancer is the most common tumor to metastasize to the skin, excluding melanoma. In a meta-analysis, cutaneous metastasis from breast cancer has been reported in 24% of all cases1. Therefore clinicians should consider cutaneous metastasis when presented with skin lesions in patients with a prior history of breast cancer. The mechanisms of metastases are known to be direct extension, lymphatic, hematogenous spread and, less likely, iatrogenic implantation. Iatrogenic implantation is very rare, and in the Korean dermatologic literature, there has been only one report on cutaneous metastasis by this technique: a case of cutaneous metastasis of pancreatic carcinoma seeded by fine needle aspiration biopsy2. We document a case of breast cancer cutaneous metastasis following core needle biopsy, with a review of the relevant literature.
CASE REPORT
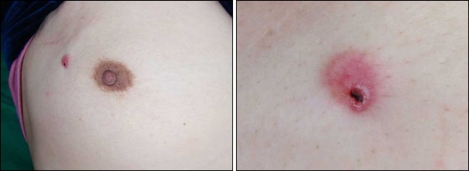
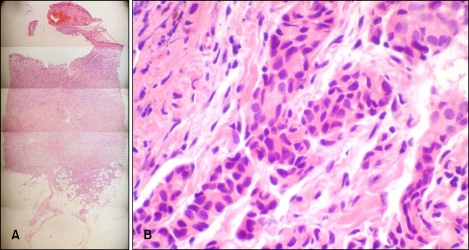
A 53-year-old woman presented with an asymptomatic, solitary, erythematous nodule in the upper outer quadrant of the right breast on 25 February 2009. The nodule had persisted for 1 year and was accompanied with bleeding. It was firm, crusted and 1×1 cm in size (Fig. 1). The patient had been diagnosed with an invasive ductal carcinoma of the right breast based on ultrasound-guided core needle biopsy using a 14-gauge needle, performed on 9 July 2005. Four specimens were obtained, and the patient underwent breast conserving surgery on 28 July 2005. The surgical specimens demonstrated a 0.8×0.7 cm sized tumor mass without lymphatic, vessel and perineural invasion. The patient subsequently received adjuvant radiotherapy and tamoxifen. No other notable findings were revealed based on family history. A cutaneous lesion occurred at the biopsy site in February 2008. Skin biopsy was performed at the central portion of the nodule on 25 February 2009. Histological examination of the lesion showed an elevated epidermis with separation of the dermo-epidermal junction. Hyperkeratosis, parakeratosis and red blood cell extravasation were observed within the crust. From the superficial dermis to the deep dermis, infiltration of the tumor cells was demonstrated (Fig. 2A). Tumor cells formed nests in an Indian file arrangement and showed glandular formations in other areas. The cells had large, polymorphous, hyperchromatic nuclei and invasion of the lymphatic ducts and blood vessels was not observed (Fig. 2B). Biopsy specimens were positive for estrogen and progesterone receptors that were also noted in specimens obtained during breast conserving surgery. Biopsy confirmed the diagnosis of breast cancer cutaneous metastasis. On 12 March 2009 the needle tract, including the cutaneous lesion was totally excised, which showed moderately differentiated metastatic invasive ductal carcinoma with clear resection margins.
Fig. 1.
A 1×1 cm sized, firm, crusted, erythematous nodule at the site of the previous core needle biopsy is shown. The operation scar of previous breast conserving surgery is also illustrated.
Fig. 2.
(A) Separation of the dermoepidermal junction and infiltration of tumor cells (H&E, ×25). (B) Tumor cells forming nests (H&E, ×100).
DISCUSSION
Cutaneous involvement of breast carcinoma has various characteristic morphologies. Nodules, as seen in our case, are the most common form of presentation. Cutaneous metastases can also be in telangiectatic, ulcerative, zosteriform, pigmented, purpuric and cicatrical patterns3. Alopecia neoplastica, en cuirasse, bullous and inflammatory carcinoma are other clinical manifestations. Cutaneous metastasis can occur by direct extension, via the lymphatics or blood vessels and, very rarely, by iatrogenic implantation such as after a needle biopsy.
Needle biopsies can provide minimally invasive, rapid and valuable diagnosis when breast carcinoma is suspected. However, the risk of malignant breast cell seeding following needle biopsy is of concern. Until Harter et al.4 first documented a case of malignant seeding of the needle tract during stereotactic core needle breast biopsy in 1992, seeding had only been considered a theoretical possibility. In a review article by Liebens et al.5, malignant epithelial cell displacement was found in needle tract specimens in 22% of patients (150/667), following large-gauge needle core biopsy. According to Uematsu and Kasami6, the risk of breast cancer needle tract seeding for an automated needle biopsy was estimated to be 69% of the cases when using core washing cytology. The wide reported range suggests the possibility of over-estimation when using core washing material, or an under-estimation in histological examinations5. In this setting, however, it is important that needle tract seeding is not necessarily indicative of tumor implantation and local recurrence6.
Local recurrence of breast cancer at the core needle biopsy site following definitive surgical procedures, such as conservative surgery, modified radical mastectomy and skin sparing mastectomy have been rarely described7-9. The true incidence of local recurrence from seeding of the needle tract is unknown. The low reported incidence can be explained by 1) unrecognized cases of biopsy site recurrence, and 2) adjuvant therapy, for example radiation therapy, which can inhibit the growth of seeded lesions, thereby preventing local recurrence7.
Risk factors of malignant cell seeding that have been suggested include the histological type of the tumor, tumor grade, time interval between the biopsy and surgery, type of surgery and adjuvant therapy, needle caliber and the number of needle passes. Nagi et al.10 found that cell displacement occurred predominantly in papillary lesions, i.e. lesions including at least one of pure intraductal papilloma, intraductal papilloma mixed with ductal carcinoma in situ (DCIS), micropapillary DCIS, papillary DCIS and invasive carcinoma. Invasive ductal carcinoma resulted in more tumor displacement compared to invasive lobular carcinoma in one study11. A high level of cellularity is unusual for invasive lobular carcinoma, and the tumor usually has diffuse infiltrative growth, thus resulting in less tumor displacement6. A shorter interval between core needle biopsy and surgical excision was found to be associated with an increased risk of cell displacement1. Needle size and the number of needle passes were not significantly associated with a higher risk of malignant cell displacement5,6,11.
No difference in local recurrence was found when core needle biopsies and excisional biopsies5 were compared. Fitzal et al.12 evaluated overall survival data and the incidence of local recurrence was not higher in patients who had undergone preoperative core needle biopsies.
In summary, we presented a case of breast cancer cutaneous metastasis at the core needle biopsy site thought to be due to needle tract seeding. It is important to place biopsy needle close to the primary tumor site. While there are strong recommendations for routine excision of the needle tract at the time of definitive surgery, large studies demonstrating consistent evidence in support of it are lacking6. Nonetheless, the potential consequence of needle biopsies must be taken into consideration throughout the follow-up period.
References
- 1.Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164–167. doi: 10.1097/01.SMJ.0000053676.73249.E5. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Lee MH, Haw CR. Cutaneous metastasis of pancreatic carcinoma by percutaneous fine needle aspiration biopsy. Ann Dermatol. 1995;7:206–209. [Google Scholar]
- 3.Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228–236. doi: 10.1016/0190-9622(93)70173-q. [DOI] [PubMed] [Google Scholar]
- 4.Harter LP, Curtis JS, Ponto G, Craig PH. Malignant seeding of the needle track during stereotaxic core needle breast biopsy. Radiology. 1992;185:713–714. doi: 10.1148/radiology.185.3.1343569. [DOI] [PubMed] [Google Scholar]
- 5.Liebens F, Carly B, Cusumano P, Van Beveren M, Beier B, Fastrez M, et al. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas. 2009;62:113–123. doi: 10.1016/j.maturitas.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Uematsu T, Kasami M. The use of positive core wash cytology to estimate potential risk of needle tract seeding of breast cancer: directional vacuum-assisted biopsy versus automated core needle biopsy. Breast Cancer. 2010;17:61–67. doi: 10.1007/s12282-009-0109-9. [DOI] [PubMed] [Google Scholar]
- 7.Chao C, Torosian MH, Boraas MC, Sigurdson ER, Hoffman JP, Eisenberg BL, et al. Local recurrence of breast cancer in the stereotactic core needle biopsy site: case reports and review of the literature. Breast J. 2001;7:124–127. doi: 10.1046/j.1524-4741.2001.007002124.x. [DOI] [PubMed] [Google Scholar]
- 8.Uriburu JL, Vuoto HD, Cogorno L, Isetta JA, Candas G, Imach GC, et al. Local recurrence of breast cancer after skin-sparing mastectomy following core needle biopsy: case reports and review of the literature. Breast J. 2006;12:194–198. doi: 10.1111/j.1075-122X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- 9.Intra M, Mazzarol G, Rietjens M, Diaz Brito JA, Gennari R, Soteldo J, et al. Extramammary recurrence of DCIS after total mastectomy: an iatrogenic displacement following needling procedures? Breast J. 2005;11:297–300. doi: 10.1111/j.1075-122x.2005.21695.x. [DOI] [PubMed] [Google Scholar]
- 10.Nagi C, Bleiweiss I, Jaffer S. Epithelial displacement in breast lesions: a papillary phenomenon. Arch Pathol Lab Med. 2005;129:1465–1469. doi: 10.5858/2005-129-1465-EDIBLA. [DOI] [PubMed] [Google Scholar]
- 11.Diaz LK, Wiley EL, Venta LA. Are malignant cells displaced by large-gauge needle core biopsy of the breast? AJR Am J Roentgenol. 1999;173:1303–1313. doi: 10.2214/ajr.173.5.10541110. [DOI] [PubMed] [Google Scholar]
- 12.Fitzal F, Sporn EP, Draxler W, Mittlbock M, Taucher S, Rudas M, et al. Preoperative core needle biopsy does not increase local recurrence rate in breast cancer patients. Breast Cancer Res Treat. 2006;97:9–15. doi: 10.1007/s10549-005-6935-3. [DOI] [PubMed] [Google Scholar]