Abstract
Background: Bacterial biofilms play a major role in chronic orthopaedic infections. Recently, farnesol (an antifungal agent) has been shown to express antimicrobial activities against Staphylococcus aureus and Streptococcus mutans. However, the effects of farnesol on the formation of bacterial biofilms on orthopaedic biomaterials and its effects on osteoblasts have not been investigated, to our knowledge, and are therefore the focus of this study.
Methods: Biofilms of Staphylococcus aureus (Seattle 1945GFPuvr) were grown on titanium alloy discs. The effects of soluble farnesol on biofilm formation with or without gentamicin were examined with fluorescence microscopy and in quantitative cultures. The effect of farnesol coated on titanium alloy discs was also investigated, as was the effect of the agent on MC3T3-E1 pre-osteoblastic cells cultured on titanium alloy discs.
Results: Soluble farnesol at a 30-mM concentration reduced the number of viable bacteria 104-fold and completely inhibited biofilm formation. Low concentrations of soluble farnesol (0.03 to 3 mM) did not inhibit biofilm formation and did not potentiate the effect of a submaximal concentration of gentamicin. Dried farnesol on titanium alloy discs reduced the number of viable bacteria fiftyfold. The effect of farnesol on bacterial biofilm formation lasted for at least three days. Soluble farnesol added after the biofilm had already formed also reduced the final number of viable bacteria, by fifty-six-fold. Soluble farnesol (3-mM and 30-mM concentrations) inhibited spreading of the MC3T3-E1 cells.
Conclusions: In vitro, a high concentration of farnesol (30 mM) shows antimicrobial properties against bacterial biofilms; however, it also has a negative effect on pre-osteoblasts. Farnesol can also express antimicrobial activity when predried on titanium discs and when added to preformed biofilms.
Clinical Relevance: As musculoskeletal infections remain a complicated problem, development of novel interventions to enhance prevention or treatment is necessary. Our study provides a basic knowledge of the effectiveness of farnesol against Staphylococcus aureus biofilms on titanium alloy surfaces. Although farnesol may have a clinical role, additional investigations, both in vitro and in vivo, are needed.
Infections at the sites of orthopaedic implants are devastating complications that frequently require prolonged management and multiple additional surgical procedures1. Despite the use of universal sterile precautions and prophylactic systemic antibiotics, infections still occur and lead to common complications such as septic loosening and osteomyelitis2. Staphylococcus aureus is one of the most common organisms associated with musculoskeletal infection and causes substantial morbidity, mortality, and overall health-care costs3,4.
Bacterial biofilms develop on many medical devices, including orthopaedic implants, and they play a major role in chronic recalcitrant infection5-7. Because bacteria embedded in the biofilm are protected by a self-produced polymeric matrix, they are often less responsive to both antibiotic treatment and the host immune system8,9. Application of antibiotics to orthopaedic biomaterials or bone cement is the method most commonly used to prevent and treat orthopaedic infections. However, antibiotic usage potentially causes adverse effects, such as allergic reactions, toxicity to the surrounding cells, and, most importantly, antibiotic resistance10-15. In addition, antibiotic loading reduces but does not necessarily eliminate production of biofilms on bone cement16-18.
Farnesol is a sesquiterpene alcohol found in essential oils of citrus fruits. It inhibits the filamentation process and the cell-cell communications known as “quorum-sensing systems” in Candida albicans and subsequently compromises biofilm formation by this fungus19-26. Although numerous studies have demonstrated inhibition of Candida albicans biofilm formation by farnesol, its effect on bacteria has not been fully investigated. Recently, it has been reported that farnesol also reduces bacterial biofilm formation by Streptococcus mutans and Staphylococcus aureus27-30. However, to the best of our knowledge, no investigators have examined the effect of farnesol on the formation of bacterial biofilms on orthopaedic biomaterials or its effect on osteoblasts.
Our objective was to evaluate farnesol's potential to prevent and treat orthopaedic infection. We therefore investigated its effects on the formation of Staphylococcus aureus biofilms on orthopaedic biomaterials as well as its effects on osteoblasts. Our hypothesis was that farnesol is a safe and effective non-antibiotic agent that can inhibit the formation of bacterial biofilms on orthopaedic biomaterials without impairing osseointegration.
Materials and Methods
Orthopaedic Biomaterials
Titanium alloy (Ti6Al4V) discs (diameter = 12.7 mm, height = 4.7 mm) were obtained from Titanium Industries (Wood Dale, Illinois). The titanium alloy discs were passivated in 25% nitric acid for twenty-four hours and washed with Dulbecco phosphate-buffered saline solution (Mediatech, Herndon, Virginia) until a pH of 7.0 to 7.4 was achieved. The discs were washed with sterile water, air-dried, and heat-treated (at 180°C) overnight.
Bacterial Biofilm Formation
The Staphylococcus aureus strain used in this study was Seattle 1945GFPuvr, which was engineered to constitutively express green fluorescent protein31. The Staphylococcus aureus was prepared by adding a 1-mL aliquot of material from an overnight culture to 100 mL of brain-heart infusion broth and incubating it at 37°C in a bacterial shaker for 1.5 to two hours until the early log phase of growth was reached (absorbance at 600 nm between 0.4 and 0.6). Ten milliliters of the suspension was then centrifuged at 3700 times gravity for ten minutes. The bacterial pellet was resuspended in 10 mL of cell culture medium (minimum essential medium, alpha modification [HyClone, Logan, Utah] supplemented with 10% fetal bovine serum, non-essential amino acids, L-glutamine, and pyruvate). Serial dilution of the bacteria was performed in the cell culture medium to achieve a final bacterial cell suspension of ∼1 × 106 cells/mL. One milliliter of the bacterial suspension was added to a titanium alloy disc in a twenty-four-well tissue-culture plate (Corning, Corning, New York). Cultures were incubated at 37°C, 5% CO2, for the indicated times. Following incubation, biofilms were examined with fluorescence microscopy (Leica MZ 16F; Spectronic Analytical Instruments, Garforth, Leeds, United Kingdom).
After microscopic examination, each titanium alloy disc was sonicated in 10 mL of Dulbecco phosphate-buffered saline solution for five minutes in a 50-W ultrasonic bath (Fisher Scientific, Pittsburgh, Pennsylvania) at a nominal frequency of 43,000 Hz followed by vortexing for thirty seconds to remove the adherent bacteria. Serial tenfold dilutions were performed with Dulbecco phosphate-buffered saline solution. Each dilution was cultured on brain-heart infusion-broth agar plates. The plates were incubated for twenty-four hours at 37°C, and the number of viable bacteria (colony-forming units [CFU] per disc) was determined.
Effect of Farnesol on Bacterial Biofilm Formation
Trans,trans-farnesol (96% 3,7,11-trimethyl-2,6,10-dodecatrien-1-ol, catalog number 277541) was purchased from Sigma-Aldrich (St. Louis, Missouri). Immediately prior to use, the farnesol (3.95 M) was diluted 131.8-fold to obtain an emulsion consisting of 30 mM of farnesol per liter of cell culture medium (minimum essential medium, alpha modification [HyClone] supplemented with 10% fetal bovine serum, non-essential amino acids, L-glutamine, and pyruvate). The farnesol emulsion was diluted tenfold further in cell culture media to obtain a solution of approximately 3 mM, which was further diluted to obtain the indicated final concentrations. Soluble farnesol was added to the bacterial cultures with the titanium alloy discs. In selected experiments, bacteria were pre-cultured on the disc for one or twenty-four hours prior to the addition of soluble farnesol. Soluble farnesol was also tested in the presence or absence of 1 μg/mL gentamicin sulfate (Sigma-Aldrich). In addition, farnesol was tested after it had been applied to the titanium alloy discs themselves. For this purpose, one group of titanium alloy discs (referred to as “wet farnesol on titanium discs”) was incubated with shaking for ten minutes in 30-mM farnesol in sterile water. The discs were then transferred to twenty-four-well tissue-culture plates, and the bacterial suspensions were added immediately. An additional group of titanium alloy discs (referred to as “dried farnesol on titanium discs”) was incubated in 30-mM farnesol in sterile water with shaking for twenty-four hours, transferred to six-well tissue-culture plates, air-dried, and then transferred to twenty-four-well tissue-culture plates prior to the addition of bacterial suspensions. All experiments were performed three times with triplicate cultures in each group.
Effect of Farnesol on Osteoblasts
MC3T3-E1 pre-osteoblastic cells were grown in cell culture medium (minimum essential medium, alpha modification [HyClone] supplemented with 10% fetal bovine serum, non-essential amino acids, L-glutamine, pyruvate, and penicillin-streptomycin). The cells were added to a twenty-four-well tissue-culture plate that contained titanium alloy discs at a concentration of 5.16 × 104 cells/well. The MC3T3-E1 cells were incubated with or without farnesol at 37°C, 5% CO2, for two days. In selected experiments, MC3T3-E1 cells were incubated in the presence or absence of farnesol for two hours followed by washing the cells twice with Dulbecco phosphate-buffered saline solution and further incubated in cell culture medium for two or five days. Following incubation, Texas Red-X phalloidin (Molecular Probes, Eugene, Oregon) and 4′-6-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes) were used to stain the cells' cytoskeleton and nuclei, respectively. The morphology of the attached pre-osteoblasts on the titanium alloy discs was evaluated with fluorescence microscopy. The experiments were performed three times with triplicate cultures in each group.
Statistical Analysis
The quantitative results are presented as the mean and standard deviation of three experiments. The level of significance was set at p < 0.05. To better meet assumptions of normality and equal variance in analysis of variance, the log-10 transformed values of bacterial counts were analyzed. Since the lowest possible value for a single count was 100, undetectable counts were set to fifty prior to taking logarithms. The mean of the log-transformed counts across the three replicates per experiment was then calculated for each experiment and treatment. These means were then analyzed with use of a randomized block analysis of variance, with experiments as the blocks. This analysis controls for systematic variability due to multiple experiments. Pairwise comparisons among treatment group means were carried out with use of the Tukey multiple-comparisons procedure. If the only comparisons of interest were those comparing each treatment with a single control group, then the Dunnett multiple-comparisons method was used instead.
Source of Funding
This study was supported by a grant from the Sulzer Settlement Medical Research Trust Fund and the Department of Orthopaedics, University Hospitals Case Medical Center, Case Western Reserve University.
Results
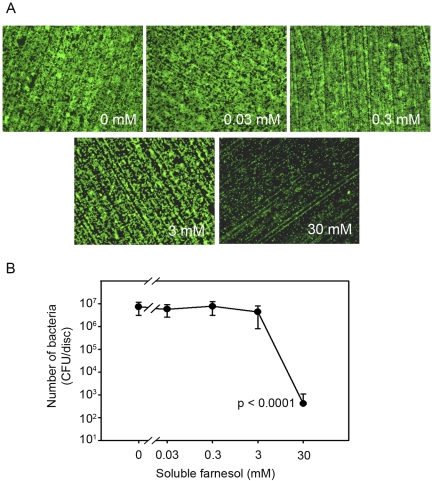
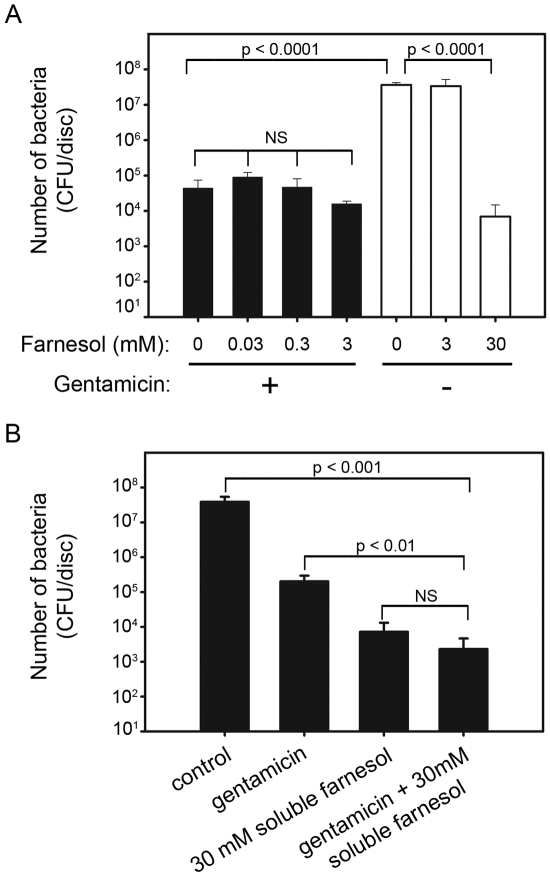
Fluorescence microscopy demonstrated that green fluorescent protein-expressing Staphylococcus aureus formed a homogeneous biofilm on the titanium alloy discs after culture for three hours in the absence of farnesol (Fig. 1, A). A similar amount of biofilm production was observed in the presence of soluble farnesol at concentrations of ≤3 mM (Fig. 1, A). However, 30-mM soluble farnesol resulted in complete inhibition of biofilm formation (Fig. 1, A). The microscopic results were confirmed by determining the number of viable bacteria attached to each disc by plate dilution. The 30-mM soluble farnesol reduced the number of viable bacteria 104-fold (p < 0.0001), and the lower concentrations of farnesol had no detectable effect (Fig. 1, B). Therefore, 30-mM farnesol was used for all subsequent experiments.
Fig. 1.
Soluble farnesol (30 mM) inhibits biofilm formation by Staphylococcus aureus. Staphylococcus aureus was incubated for three hours on titanium alloy discs in the presence of the indicated concentrations of farnesol. Biofilm formation was observed with fluorescence microscopy (magnification, 8×) (A), and the number of viable bacteria was quantified with plate dilution (B). CFU = colony-forming units. Error bars indicate the standard deviation.
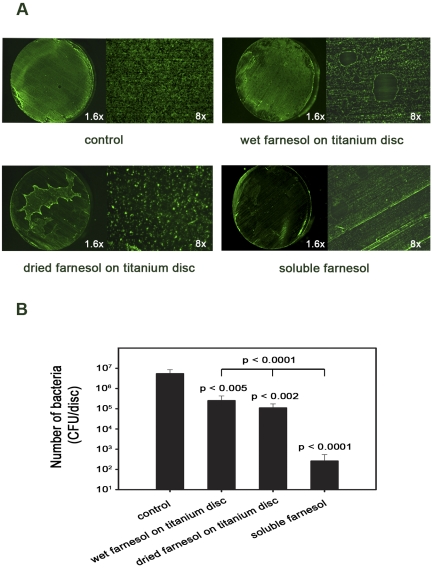
When the farnesol was applied to the titanium alloy discs rather than to the culture medium, it was found that both application methods (culture of the bacterial suspensions with the titanium alloy discs immediately after incubation of the discs in the 30-mM farnesol solution [wet farnesol on titanium disc] or after incubation in the farnesol followed by air-drying [dried farnesol on titanium disc]) significantly reduced the number of viable bacteria when compared with that in the control group (p < 0.005) (Fig. 2, B) and reduced biofilm formation (right top and left bottom panels of Fig. 2, A). Biofilm formation was extremely non-homogeneous in the dried farnesol-on-titanium disc group (left bottom panel in Fig. 2, A). This was presumably due to nonuniform air-drying and concentration of the farnesol in the areas that dried last. However, the magnitude of the reduction in biofilm formation and in the number of viable bacteria was much lower in the wet and dried-farnesol groups than in the soluble-farnesol group (p < 0.0001) (Fig. 2, A and B).
Fig. 2.
Farnesol directly applied to titanium alloy discs inhibits biofilm formation by Staphylococcus aureus. In the “wet farnesol on titanium disc” group, the discs were incubated in 30-mM farnesol and transferred to tissue-culture plates, and the bacterial suspension was added immediately. In the “dried farnesol on titanium disc” group, the discs were incubated in 30-mM farnesol and then air-dried prior to transfer to the tissue-culture plates. The number of viable bacteria was quantified after three hours of incubation. The effect of farnesol was evaluated with fluorescence microscopy with both low (1.6×) and high (8×) magnification (A), and the number of viable bacteria was quantified (B). CFU = colony-forming units.
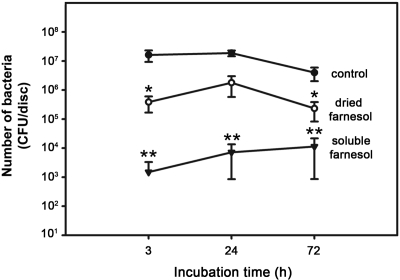
Time-course experiments were performed to determine whether the effects of farnesol are long-lasting. As observed previously (Figs. 1, B, and 2, B), soluble farnesol reduced the number of viable bacteria 104-fold (p < 0.0001) at three hours (Fig. 3), whereas it reduced the number of viable bacteria 2600-fold and 350-fold (p < 0.0001) at twenty-four hours and seventy-two hours, respectively (Fig. 3). The smaller reductions at twenty-four and seventy-two hours were due to the combination of a decrease in the number of viable bacteria in the control group and an increase in the number of viable bacteria in the group with soluble farnesol. Dried farnesol on titanium discs had an intermediate effect on the number of viable bacteria at each time point (Fig. 3).
Fig. 3.
Farnesol inhibits biofilm formation by Staphylococcus aureus for at least three days. Staphylococcus aureus was incubated on titanium alloy discs in the absence of farnesol or in the presence of soluble farnesol or dried farnesol on the titanium disc. The number of viable bacteria was quantified after the indicated time periods. The single asterisk denotes p < 0.05 and the double asterisks denote p < 0.0001 compared with the control group at the same time point. CFU = colony-forming units.
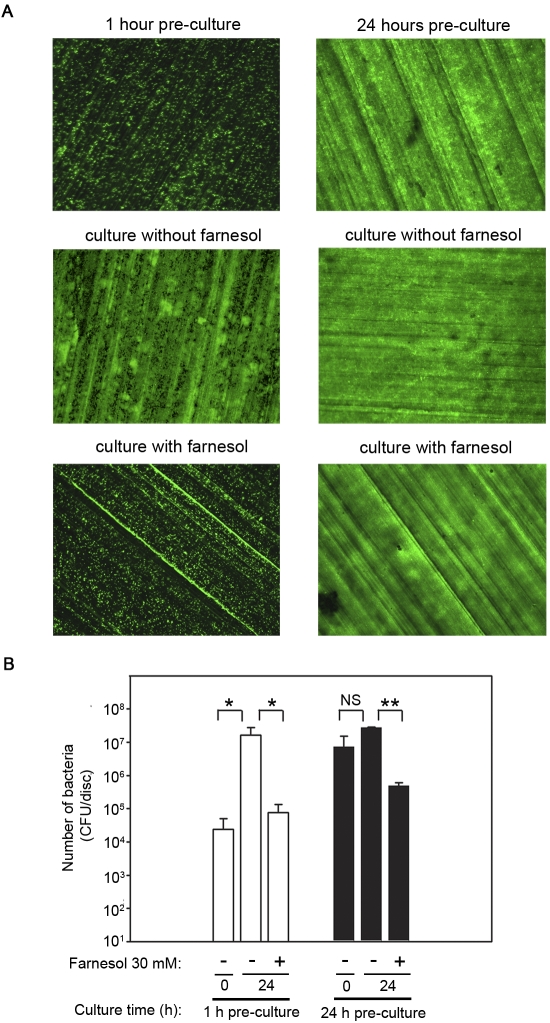
To determine whether farnesol affects bacteria that have already attached to titanium alloy discs, bacteria were pre-cultured on the discs for one or twenty-four hours prior to the addition of farnesol. After one hour of pre-culture, bacteria were attached to the titanium alloy discs either individually or in small colonies (upper left panel in Fig. 4, A). An additional twenty-four hours of culture in the absence of farnesol resulted in a 670-fold increase (p < 0.0001) in the number of viable bacteria (white bars in Fig. 4, B) and formation of a bacterial biofilm (middle left panel in Fig. 4, A). Farnesol, however, reduced the number of viable bacteria 210-fold compared with the number in the group without farnesol (p < 0.0001) and prevented formation of the biofilm (bottom left panel in Fig. 4, A, and white bars in Fig. 4, B). In contrast, after twenty-four hours of pre-culture, the bacteria had already formed a biofilm (top right panel in Fig. 4, A), and an additional twenty-four hours of culture in the absence of farnesol did not lead to detectable changes (middle right panel in Fig. 4, A, and black bars in Fig. 4, B). Farnesol, however, reduced the number of viable bacteria fifty-six-fold (p < 0.001) (black bars in Fig. 4, B). Although farnesol did not detectably affect the morphology of the biofilm in this group (bottom right panel in Fig. 4, A), the green fluorescent protein fluorescence is unlikely to distinguish between live and killed bacteria.
Fig. 4.
Farnesol reduces the viability of bacteria that have already attached to titanium alloy discs. Staphylococcus aureus was pre-cultured with titanium discs for one hour or twenty-four hours. Following the pre-culture periods, the media were changed and the discs were cultured for twenty-four hours in the presence or absence of 30-mM farnesol. The effect of farnesol was evaluated with fluorescence microscopy (magnification, 8×) (A), and the number of viable bacteria was quantified (B). The single asterisk denotes p < 0.0001, the double asterisks denote p < 0.001, and NS indicates not significant. CFU = colony-forming units.
To determine whether farnesol would have an additive effect with antibiotics, bacteria were cultured on titanium alloy discs with or without farnesol in the presence of a submaximal level of gentamicin sulfate (1 μg/mL). The gentamicin sulfate reduced the number of viable bacteria 825-fold (p < 0.0001, compare first black bar with first white bar in Fig. 5, A). However, 0.03 to 3 mM of farnesol did not detectably increase the antibacterial effect of this concentration of gentamicin (black bars in Fig. 5, A). A finding that was similar to those in previous experiments was that 3-mM farnesol, as a negative control, had no detectable effect (white bars in Fig. 5, A), while 30-mM farnesol, as a positive control, significantly reduced the number of viable bacteria (p < 0.0001). Therefore, 30-mM farnesol was tested in combination with gentamicin. The number of viable bacteria observed in the presence of both 30-mM soluble farnesol and gentamicin (1 μg/mL), however, was not significantly different from the number in the group with soluble farnesol alone (Fig. 5, B).
Fig. 5.
Neither low concentrations of farnesol nor gentamicin potentiated the effect of each other. Farnesol concentrations of 0.03 to 3 mM (A) and 30 mM (B) were tested in combination with 1 μg/mL gentamicin. Staphylococcus aureus was incubated with the indicated concentrations of farnesol in the presence or absence of gentamicin. The number of viable bacteria was determined after three hours. NS indicates not significant. CFU = colony-forming units.
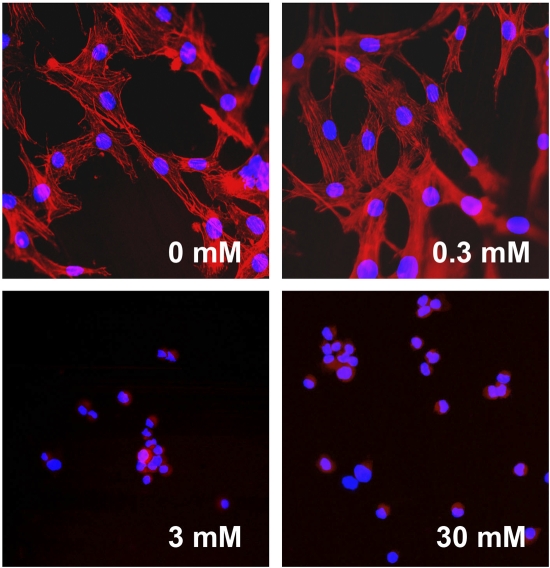
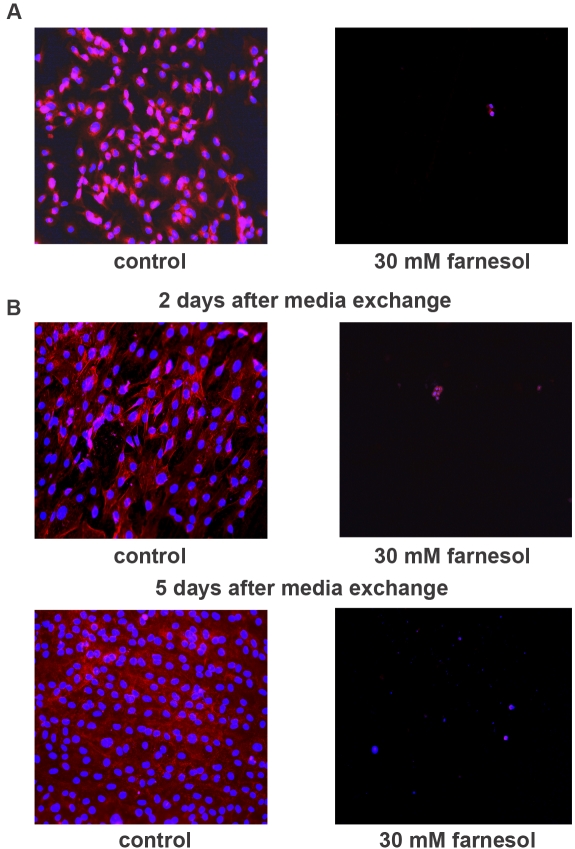
To investigate the effects of farnesol on osteoblasts, MC3T3-E1 pre-osteoblastic cells were grown on titanium alloy discs for two days with or without farnesol. As demonstrated in Figure 6, MC3T3-E1 cells attached and spread homogeneously on titanium alloy discs in the absence of farnesol or in the presence of 0.3-mM farnesol. However, in the 3-mM and 30-mM farnesol groups, the pre-osteoblasts attached but did not spread. Instead, they formed aggregates consisting of three to ten rounded cells (Fig. 6). To determine whether these effects were reversible, MC3T3-E1 cells were exposed to farnesol for two hours and then cultured in the absence of farnesol. The brief two-hour exposure period reduced both attachment and spreading (Fig. 7, A), and the pre-osteoblastic cells did not recover during culture in the absence of farnesol for an additional two or five days (Fig. 7, B).
Fig. 6.
High concentrations of soluble farnesol (3 and 30 mM) inhibit spreading of MC3T3-E1 pre-osteoblastic cells. MC3T3-E1 cells were incubated on titanium alloy discs without or with the indicated concentrations of farnesol for two days. The cellular morphology was assessed with fluorescence microscopy following staining with Texas Red-X phalloidin and DAPI (magnification, 20×).
Fig. 7.
The effects of farnesol on MC3T3-E1 pre-osteoblastic cells are not reversed by subsequent culture in the absence of farnesol. MC3T3-E1 cells were incubated on titanium alloy discs in the presence or absence of 30-mM farnesol for two hours (A). Following the incubation, the cells were washed twice with Dulbecco phosphate-buffered saline solution and further incubated in cell culture media without farnesol for two or five days (B). The cellular morphology was assessed with fluorescence microscopy following staining with Texas Red-X phalloidin and DAPI (magnification, 20×).
Discussion
Bacterial biofilm is defined as a microbially derived sessile community, typified by cells that are embedded in a matrix of extracellular polymeric substance and exhibit an altered phenotype with regard to growth, gene expression, and protein production32. A benefit for bacteria of growing as a biofilm is the facilitation of resistance to a number of removal tactics, such as elimination by antimicrobial agents, host phagocytic clearance, and host production of oxygen radicals. As a result, bacteria in the biofilm become 1000 to 1500 times more resistant to antibiotics, which make them difficult to eradicate33-37. Currently, coating the implant with antibiotic-loaded bone cement has been the only practical method for the local delivery of antibiotics around the prosthetic surface.
Our results showed that only a high concentration of farnesol (30 mM) exhibited an antibacterial effect. This result was different from that in previous studies, which showed lower concentrations of farnesol (300 μM to 5 mM) to have a significant antibacterial effect27-30. The difference is likely due to our suspending the farnesol directly in cell culture media rather than initially diluting it 100-fold in methanol as was done in most other studies20,22,38. In addition, the authors of the other studies used different surface materials, different types of bacteria, and bacterial culture media rather than the tissue culture media with 10% fetal bovine serum that we used to better replicate human body fluids27-30. It is possible, for example, that serum constituents, such as albumin, may bind to farnesol and thus reduce its antibacterial efficacy.
When bacteria were incubated with farnesol for longer periods of time than used in previous experiments, which were performed at three hours, the antibacterial effect of the farnesol was observed for at least seventy-two hours. The magnitude of the reduction of viable bacteria at seventy-two hours, however, was lower than that at three hours. This is due to the combination of a decrease in the number of viable bacteria in the control group and an increase in the number of viable bacteria in the group with soluble farnesol. The increase in bacterial number in the presence of farnesol is likely due to proliferation of the bacteria that were not killed by the farnesol. It is also possible that farnesol may have a direct stimulatory effect on proliferation of bacteria that are not killed initially. This possibility, however, is unlikely since the increase in the number of bacteria was quite modest. The reduction in viable bacteria in the control group is likely due to nutrient limitation or the accumulation of waste products.
Coating of the metal implants with anti-infective agents has been used as one modality to prevent orthopaedic infection. In recent years, there have been several advances in anti-infective technology in orthopaedics, including silver-coated pins, nitric oxide-releasing implants, gentamicin or vancomycin-coated rods, and antiseptic dye-coated devices39-43. In the current study, we used two methods for coating titanium alloy discs with farnesol: incubating the discs in farnesol solution and then adding the bacterial suspensions either immediately or after air-drying. Both types of farnesol-coated titanium alloy discs exhibited significant antibacterial activity; however, the magnitudes of the effects were much lower than that seen in the soluble-farnesol group. This may be due to the higher total amount of farnesol in the soluble-farnesol group. Although the results of the use of farnesol coatings are intriguing, it is not known whether a farnesol coating on an orthopaedic device would be stable during drilling or implanting into bone. Additional in vitro studies should also be carried out to examine the elution characteristics of farnesol from titanium alloy discs coated with farnesol.
We hypothesized that farnesol might synergize with a low concentration of antibiotic, thereby reducing the potential for adverse effects of either agent. However, when we tested farnesol in the presence or absence of gentamicin, we found that neither low concentrations of farnesol nor gentamicin potentiated the effect of the other agent.
The mechanism of farnesol's effect on the formation of a bacterial biofilm remains unclear. Farnesol was reported to inhibit the synthesis of biofilm matrix polysaccharides by Streptococcus mutans29,30, to disrupt the cell membranes of Staphylococcus aureus27, and to prevent the formation of the fibrin matrix by Staphylococcus aureus28. Although the exact mechanism is not well understood, the results from these studies as well as ours showed that farnesol can inhibit bacterial biofilm formation.
Local toxicity is always a major concern when applying any agent directly to the bone. McLaren showed that bone cement loaded with a high dose of antibiotics causes local levels of antibiotics inside the joint to exceed 2000 μg/mL44, and the local application of high-dose antibiotics has negative effects on both osteoblasts and osteoclasts11,45,46. However, to the best of our knowledge, there is no evidence that a high concentration of antibiotics jeopardizes clinical bone-healing or causes osteonecrosis. Similarly, our study showed that farnesol at concentrations of 3 and 30 mM had negative effects on pre-osteoblasts. In addition, the pre-osteoblasts did not recover during subsequent culture in the absence of farnesol. Our finding that farnesol prevents spreading of pre-osteoblasts is consistent with those of studies showing that farnesol causes cytoskeletal disorganization and apoptosis of other types of mammalian cells38,47,48. Nonetheless, it is unknown whether there would be any long-term local or systemic toxicity if farnesol were used in vivo.
One limitation of this study was that our quantitative culture technique had a detection limit for viable bacteria of 100 CFU/disc; therefore, if the number of viable bacteria per disc was <100 CFU it was undetectable. Another limitation of this study was its in vitro nature. In vivo studies need to be performed to determine farnesol's efficacy in an animal model as well as its toxicity to osteoblasts and surrounding soft tissues.
In conclusion, the results of our study provide a basic knowledge of the activities of farnesol against Staphylococcus aureus biofilms on titanium alloy surfaces. A high concentration of farnesol showed antibiotic properties against bacterial biofilms; however, it also had a negative effect on pre-osteoblasts. Farnesol may nonetheless be clinically useful if, for example, short-term treatments are successful at eradicating infections but have relatively minor negative effects on osteoblasts and other surrounding cells. Additional investigations are therefore warranted to compare the antibacterial effects of farnesol with its effects on mammalian cells.
Acknowledgments
Note: The authors thank Michelle Beidelschies, BS, and Mark Schluchter, PhD, Professor of Epidemiology and Biostatistics, for their assistance with the study.
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the Sulzer Settlement Medical Research Trust Fund and the Department of Orthopaedics, University Hospitals Case Medical Center, Case Western Reserve University. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
Investigation performed at University Hospitals Case Medical Center, Case Western Reserve University School of Medicine, Cleveland, Ohio
References
- 1.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-54. [DOI] [PubMed] [Google Scholar]
- 2.Court-Brown CM, Keating JF, McQueen MM. Infection after intramedullary nailing of the tibia. Incidence and protocol for management. J Bone Joint Surg Br. 1992;74:770-4. [DOI] [PubMed] [Google Scholar]
- 3.Hebert CK, Williams RE, Levy RS, Barrack RL. Cost of treating an infected total knee replacement. Clin Orthop Relat Res. 1996;331:140-5. [DOI] [PubMed] [Google Scholar]
- 4.Sanderson PJ. Infection in orthopaedic implants. J Hosp Infect. 1991;18 Suppl A:367-75. [DOI] [PubMed] [Google Scholar]
- 5.Benoit JL, Carandang G, Sitrin M, Arnow P. Intraluminal antibiotic treatment of central venous catheter infections in patients receiving parenteral nutrition at home. Clin Infect Dis. 1997;24:743-4. [DOI] [PubMed] [Google Scholar]
- 6.Raad I. Intravascular-catheter-related infections. Lancet. 1998;351:893-8. [DOI] [PubMed] [Google Scholar]
- 7.Ramage G, Tunney MM, Patrick S, Gorman SP, Nixon JR. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials. 2003;24:3221-7. [DOI] [PubMed] [Google Scholar]
- 8.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711-45. [DOI] [PubMed] [Google Scholar]
- 9.Gristina AG, Shibata Y, Giridhar G, Kreger A, Myrvik QN. The glycocalyx, biofilm, microbes, and resistant infection. Semin Arthroplasty. 1994;5:160-70. [PubMed] [Google Scholar]
- 10.Hope PG, Kristinsson KG, Norman P, Elson RA. Deep infection of cemented total hip arthroplasties caused by coagulase-negative staphylococci. J Bone Joint Surg Br. 1989;71:851-5. [DOI] [PubMed] [Google Scholar]
- 11.Isefuku S, Joyner CJ, Simpson AH. Gentamicin may have an adverse effect on osteogenesis. J Orthop Trauma. 2003;17:212-6. [DOI] [PubMed] [Google Scholar]
- 12.Richter-Hintz D, Rieker J, Rauch L, Homey B. [Sensitivity to constituents of bone cement in a patient with joint prosthesis]. Hautarzt. 2004;55:987-9. German. [DOI] [PubMed] [Google Scholar]
- 13.Sanzén L, Walder M. Antibiotic resistance of coagulase-negative staphylococci in an orthopaedic department. J Hosp Infect. 1988;12:103-8. [DOI] [PubMed] [Google Scholar]
- 14.Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br. 2003;85:646-9. [PubMed] [Google Scholar]
- 15.Thomes B, Murray P, Bouchier-Hayes D. Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J Bone Joint Surg Br. 2002;84:758-60. [DOI] [PubMed] [Google Scholar]
- 16.Anagnostakos K, Hitzler P, Pape D, Kohn D, Kelm J. Persistence of bacterial growth on antibiotic-loaded beads: is it actually a problem? Acta Orthop. 2008;79:302-7. [DOI] [PubMed] [Google Scholar]
- 17.Oga M, Arizono T, Sugioka Y, Naylor PT, Myrvik QN, Gristina AG. The inhibition of bacterial adhesion to a tobramycin-impregnated polymethylmethacrylate substratum. J Long Term Eff Med Implants. 1992;1:321-8. [PubMed] [Google Scholar]
- 18.van de Belt H, Neut D, Schenk W, van Horn JR, van der Mei HC, Busscher HJ. Gentamicin release from polymethylmethacrylate bone cements and Staphylococcus aureus biofilm formation. Acta Orthop Scand. 2000;71:625-9. [DOI] [PubMed] [Google Scholar]
- 19.Alem MA, Oteef MD, Flowers TH, Douglas LJ. Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development. Eukaryot Cell. 2006;5:1770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao YY, Cao YB, Xu Z, Ying K, Li Y, Xie Y, Zhu ZY, Chen WS, Jiang YY. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob Agents Chemother. 2005;49:584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogan DA. Talking to themselves: autoregulation and quorum sensing in fungi. Eukaryot Cell. 2006;5:613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001;67:2982-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabra-Rizk MA, Shirtliff M, James C, Meiller T. Effect of farnesol on Candida dubliniensis biofilm formation and fluconazole resistance. FEMS Yeast Res. 2006;6:1063-73. [DOI] [PubMed] [Google Scholar]
- 24.Martins M, Henriques M, Azeredo J, Rocha SM, Coimbra MA, Oliveira R. Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryot Cell. 2007;6:2429-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perumal P, Mekala S, Chaffin WL. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob Agents Chemother. 2007;51:2454-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramage G, Saville SP, Wickes BL, López-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jabra-Rizk MA, Meiller TF, James CE, Shirtliff ME. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob Agents Chemother. 2006;50:1463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsuyama M, Ichikawa H, Ogawa S, Ikezawa Z. A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J Dermatol Sci. 2005;38:197-205. [DOI] [PubMed] [Google Scholar]
- 29.Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, Vacca-Smith AM, Bowen WH. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother. 2003;52:782-9. [DOI] [PubMed] [Google Scholar]
- 30.Koo H, Schobel B, Scott-Anne K, Watson G, Bowen WH, Cury JA, Rosalen PL, Park YK. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J Dent Res. 2005;84:1016-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leid JG, Shirtliff ME, Costerton JW, Stoodley AP. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70:6339-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beveridge TJ, Makin SA, Kadurugamuwa JL, Li Z. Interactions between biofilms and the environment. FEMS Microbiol Rev. 1997;20:291-303. [DOI] [PubMed] [Google Scholar]
- 34.Chan C, Burrows LL, Deber CM. Helix induction in antimicrobial peptides by alginate in biofilms. J Biol Chem. 2004;279:38749-54. [DOI] [PubMed] [Google Scholar]
- 35.De Beer D, Srinivasan R, Stewart PS. Direct measurement of chlorine penetration into biofilms during disinfection. Appl Environ Microbiol. 1994;60:4339-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34-9. [DOI] [PubMed] [Google Scholar]
- 37.Xu KD, McFeters GA, Stewart PS. Biofilm resistance to antimicrobial agents. Microbiology. 2000;146:547-9. [DOI] [PubMed] [Google Scholar]
- 38.Scheper MA, Shirtliff ME, Meiller TF, Peters BM, Jabra-Rizk MA. Farnesol, a fungal quorum-sensing molecule triggers apoptosis in human oral squamous carcinoma cells. Neoplasia. 2008;10:954-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antoci V Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Vancomycin bound to Ti rods reduces periprosthetic infection: preliminary study. Clin Orthop Relat Res. 2007;461:88-95. [DOI] [PubMed] [Google Scholar]
- 40.Bahna P, Dvorak T, Hanna H, Yasko AW, Hachem R, Raad I. Orthopaedic metal devices coated with a novel antiseptic dye for the prevention of bacterial infections. Int J Antimicrob Agents. 2007;29:593-6. [DOI] [PubMed] [Google Scholar]
- 41.Darouiche RO. Anti-infective efficacy of silver-coated medical prostheses. Clin Infect Dis. 1999;29:1371-8. [DOI] [PubMed] [Google Scholar]
- 42.Lucke M, Schmidmaier G, Sadoni S, Wildemann B, Schiller R, Haas NP, Raschke M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone. 2003;32:521-31. [DOI] [PubMed] [Google Scholar]
- 43.Nablo BJ, Rothrock AR, Schoenfisch MH. Nitric oxide-releasing sol-gels as antibacterial coatings for orthopedic implants. Biomaterials. 2005;26:917-24. [DOI] [PubMed] [Google Scholar]
- 44.McLaren AC. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin Orthop Relat Res. 2004;427:101-6. [DOI] [PubMed] [Google Scholar]
- 45.Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res. 1996;333:245-51. [PubMed] [Google Scholar]
- 46.Miclau T, Edin ML, Lester GE, Lindsey RW, Dahners LE. Bone toxicity of locally applied aminoglycosides. J Orthop Trauma. 1995;9:401-6. [DOI] [PubMed] [Google Scholar]
- 47.Miquel K, Pradines A, Favre G. Farnesol and geranylgeraniol induce actin cytoskeleton disorganization and apoptosis in A549 lung adenocarcinoma cells. Biochem Biophys Res Commun. 1996;225:869-76. [DOI] [PubMed] [Google Scholar]
- 48.Joo JH, Liao G, Collins JB, Grissom SF, Jetten AM. Farnesol-induced apoptosis in human lung carcinoma cells is coupled to the endoplasmic reticulum stress response. Cancer Res. 2007;67:7929-36. [DOI] [PubMed] [Google Scholar]