Abstract
Background
Many studies have shown that chronic ethanol exposure can enhance later self-administration of ethanol, but only a few studies have identified critical parameters for such exposure. The present studies examined temporal and other parameters of chronic ethanol exposure on subsequent intragastric (IG) self-infusion of ethanol.
Methods
Sprague-Dawley rats implanted with IG catheters were passively infused with ethanol for 5–6 days and then allowed to self-infuse ethanol or water using a procedure in which infusions were contingent upon licking fruit-flavored solutions. Experiment 1 examined the time interval between consecutive periods of passive infusion (Massed Group: 12 h vs. Spaced Group: 36 h). Experiment 2 studied the interval between the final passive infusion and onset of self-infusion (12 vs. 36 h). Finally, Experiment 3 tested the effect of inserting self-infusion days within the passive infusion phase.
Results
Passive ethanol exposure on consecutive days induced relatively large amounts of ethanol self-infusion (4.1 to 7.9 g/kg/d). Increasing the duration of the ethanol-free interval between periods of passive exposure to 36 h significantly reduced ethanol self-infusion (2.2 g/kg/d; Exp. 1). The time delay between the last passive ethanol exposure and onset of self-infusion had no effect on self-infusion (Exp. 2). Moreover, inserting no-choice self-infusion days between the last few passive exposure days did not increase self-infusion (Exp. 3).
Conclusions
Measurement of withdrawal signs indicated that Massed passive exposure produced stronger dependence than Spaced passive exposure, suggesting that enhanced ethanol self-infusion in Massed groups might be explained by the opportunity for greater negative reinforcement by ethanol. Although enhanced negative reinforcement might also explain why the Massed group showed a weaker aversion for the ethanol-paired flavor than the Spaced group, this observation could also be explained by the development of greater tolerance to ethanol’s aversive pharmacological effects in the Massed group.
Keywords: intragastric, self-administration, tolerance, dependence, rats
Introduction
Chronic forced exposure to high ethanol doses by various routes of administration has been shown to increase later self-administration of ethanol. Although one early study was notable for its failure to see an effect of repeated ethanol intubation on subsequent ethanol drinking (Begleiter, 1975), several other studies showed that rats receiving multiple intragastric (IG) infusions of ethanol later drank more 10% (v/v) ethanol than rats given water, saline or no pre-treatment (Deutsch & Koopmans, 1973; LeMagnen & Marfaing-Jallat, 1984; Marfaing-Jallat & LeMagnen, 1982; Marfaing-Jallat & LeMagnen, 1985). The generality of these findings was later extended in studies showing that chronic forced exposure to ethanol either in a liquid diet (Schulteis et al., 1996) or in a vapor chamber (O’Dell et al., 2004; Funk & Koob, 2007; Funk et al., 2006, 2007; Gilpin et al., 2008; Roberts et al., 1996, 2000; Valdez et al., 2002; Walker & Koob, 2007, 2008; Walker et al., 2008) increased subsequent responding for ethanol by rats in an operant procedure. Recently, this phenomenon has also been extended to mice in studies showing that chronic ethanol exposure in a vapor chamber will enhance later ethanol drinking in alcohol-preferring C57BL/6J mice (Becker & Lopez, 2004; Dhaher et al., 2008; Finn et al., 2007; Lopez & Becker, 2005).
The enhancing effect of chronic forced ethanol exposure on ethanol intake has also been seen in rats that are allowed to self-administer ethanol IG rather than orally. In a series of studies reported in the 1970s, Deutsch and colleagues found that forced IG exposure to ethanol produced a greater voluntary intake of ethanol when its infusion was subsequently coupled to drinking a distinctive flavored solution using a unique counter-injection pump system (e.g., Deutsch & Hardy, 1976; Deutsch & Walton, 1977a, 1977b; Hardy & Deutsch, 1977). In their most compelling demonstration, Deutsch and Cannis (1980) gave Sprague-Dawley rats a continuous choice between two different flavored solutions (which did not contain ethanol) after they had been passively exposed to high volumes of IG ethanol over a 72-h period. Consumption of one flavor was programmed to produce an IG infusion of ethanol whereas consumption of the other flavor had no consequence. Experimental rats consumed an average of 10.5 g/kg ethanol per day compared to only 2 g/kg/d in untreated control rats over a 6-day test period.
Although a variant of the IG self-infusion procedure developed by Deutsch and colleagues was later used a few times to study ethanol intake in rats that were selectively bred for alcohol preference (Murphy et al., 1988; Waller et al., 1984), the model has lain dormant as a tool for studying effects of chronic forced ethanol exposure until it was recently re-established in our laboratory (Fidler et al., 2006). In our version of the procedure, rats implanted with IG catheters received 3–6 days of passive exposure to 10% (v/v) ethanol followed by an opportunity to drink two flavored solutions, one of which was paired with IG infusion of ethanol. Like Deutsch and Cannis (1980), we found that chronic ethanol exposure subsequently produced greater ethanol self-infusion (4–7 g/kg/d) than was seen in control rats not previously exposed to ethanol (0–2.6 g/kg/d). However, our rats never achieved the consistently high daily intakes reported by those investigators. All of these IG experiments differ from other pre-exposure studies in that the route of administration for ethanol is the same (via IG catheter) across all phases of the experiment.
While the enhancing effect of chronic ethanol exposure on later self-administration is now well established, relatively little is known about the critical parameters of such exposure. One variable that has been reported to be important in procedures involving prior exposure to ethanol vapor is whether the exposure is continuous or intermittent. For example, O’Dell et al. (2004) compared operant oral self-administration of ethanol in groups of rats that had received continuous (24 h/d for 2 weeks) or intermittent (14 h on/10 h off each day for 4 weeks) ethanol vapor exposure before testing. Results showed that rats previously given intermittent exposure self-administered significantly more ethanol than rats that had previously received a similar total ethanol exposure in a continuous manner. In fact, self-administration of rats that received only 2 weeks of continuous ethanol vapor exposure did not differ from that of untreated control rats. Intermittent ethanol vapor exposure (16 h on/8 h off × 4 d) was also found to be better than continuous exposure (64 h) for enhancing ethanol intake of C57BL/6J mice trained in a limited-access (2 h/d) two-bottle choice drinking procedure (Lopez & Becker, 2005).
The experiments reported here were generally designed to extend examination of the parameters of chronic ethanol exposure to the IG self-infusion procedure. For Deutsch and Cannis (1980), the passive infusion was continuous unless body temperature dropped by more than 3°C and rats were restrained. The passive parameters in our previous work, Fidler et al. (2006), differed from Deutsch and Cannis in several important ways. In the Fidler er al. experiments, rats were tethered to an overhead fluid swivel, but were otherwise unrestrained during the passive infusion phase (and the rest of the experiment). Body temperature was monitored via an implanted biotelemetry device (Mini-Mitter Co.) rather than using an implanted thermistor with wire leads (as had been the case for Deutsch and Cannis). Perhaps due to the difference in restraint conditions, Fidler et al. found it necessary to use a different temperature criterion (a drop of 1°C) to avoid overdosing. In addition to the temperature cut-off, Fidler et al. placed limits on the total amount of ethanol that could be infused during 6-h blocks of the passive infusion phase. As a result of these changes, the passive infusion of ethanol in the Fidler et al. experiments might have been more intermittent than in the Deutsch and Cannis procedure. In fact, for two of their experiments (Exp. 2 and 3), Fidler et al. added a 6-h ethanol-free recovery period to all but the first passive infusion day.
In Experiment 1, we studied the timing of passive ethanol exposure by comparing rats that received ethanol infusions on 5 consecutive days (Massed Group) to rats that also received 5 days of infusion, but with a drug-free day after each day of infusion (Spaced Group, similar to the experimental groups in Experiments 2 and 3 of Fidler et al., 2006). Our intent was to make a comparison conceptually similar to that previously described in studies of continuous versus intermittent ethanol vapor exposure (Lopez & Becker, 2005; O’Dell et al., 2004). Based on the outcome of our first study, Experiment 2 was designed to examine the effect of the time delay between the end of passive exposure and the beginning of self-administration in groups that received the same passive phase treatment as the Massed Group. One group (Immediate Group) began self-administration after the same delay (12 h) experienced by the Massed Group in Experiment 1. A second group (Delayed Group) was exposed to a delay three times as long (36 h). Finally, in Experiment 3, we explored a procedure in which experimental rats (Master Group) initially received 3 consecutive days of passive exposure (massed procedure) followed by alternating days of no-choice ethanol self-infusion and 3 additional passive exposure days. These rats were compared to control rats (Yoked Group) that received identical treatment on passive exposure days, but had no control over the ethanol they received on self-infusion days (i.e., their schedule of ethanol infusions was determined by self-infusing rats in the Master Group).
General Materials and Methods
Subjects
Eighty male Sprague Dawley rats purchased from Harlan Sprague Dawley were used in these experiments. Experiments 1 and 2 were each run in two replications with 16 rats per replication. Experiment 3 was run in a single replication with 16 rats. The rats were individually housed in hanging wire cages until the start of the experiment. Food (Rodent Diet 5001, LabDiet, Richmond, IN) and water were freely available except during the 16–20 h prior to surgery (when food was removed from the cages). The colony was maintained on a 12h/12h light/dark schedule with the lights on at 7:00 am. The OHSU IACUC approved the experimental protocols, which complied with the NIH Guide for Care and Use of Laboratory Animals (1996).
Apparatus
Chambers
During the experiments, rats were housed in acrylic and aluminum chambers, 27.5 cm long by 25.5 cm wide and 31.0 cm high (Fidler et al., 2006 Control Experiment). Each chamber was fitted with a food cup (containing Rodent Diet 5001) on one end wall and two retractable sipper tubes (ENV-252M, MED Associates, St. Albans, VT) with lickometers (ENV-250B, MED Associates) mounted on the opposite end of the chamber 9 cm apart and 6 cm above the floor (16 gauge #2 woven stainless steel mesh). A fluid swivel (LTS-36 Infusion Swivel, Ledger Technical services, Kalamazoo, MI) was attached to a counter-balance arm assembly (PHM-110, MED Associates) and mounted over the chamber. A fluid line, ID 0.51 mm × OD 1.52 mm (Tygon® Flexible Plastic Tubing, Formulation S-54-HL, Saint-Gobain PPL Corp.), encased in a lightweight spring ran from the swivel to the animal and was connected to the backmount by means of a luer connector (Male luer lock with 1/16″ hose barb U-30504-02, Cole-Parmer, Vernon Hills, IL). Tygon® tubing (Formulation R-3603, ID 0.51 mm × OD 2.34 mm, Cole Parmer) connected the swivel to a Y-connector (U-TCY-22-10, Small Parts, Miramar, FL) that was attached to two fluid lines (of the same Tygon® Tubing) running from the Syringe Pumps (Model A, Razel Scientific Instruments, St. Albans, VT). An acrylic tray inserted under the floor was filled with fresh Aspen chips every day. Each chamber was placed in an individual, sound-attenuating, ventilated shell.
Pumps
The syringe pumps were fitted with either 0.83 or 1.5 RPM motors (Razel Scientific Instruments) and with either 35 mL or 60 mL syringes for reservoirs depending on the desired flow rate. A Macintosh G4 computer with Labview software collected data and controlled the infusions.
Solutions
Ethanol solutions were made using 95% ethanol diluted in sterile water to a concentration of 10% v/v (during the Passive infusion phase) or 20% v/v (for Self-Infusion phases). As in the Deutsch and Cannis (1980) study, ethanol concentration was increased during the self-infusion phases because self-infused ethanol was diluted in the stomach by intake of the S+ fluid, which occurred when completing the lick contingency (see below). Sterile water was used for water infusions. During the habituation phase, rats had access to 0.2 % w/v saccharin (Sigma-Aldrich, St. Louis, MO) in tap water. Flavored solutions were 0.05% w/v grape or cherry unsweetened Kool-Aid (Kraft Foods Inc., Northfield, IL) and 0.2% w/v saccharin in tap water. Infusions of 25% v/v STAT™ high calorie liquid diet (PRN Pharmacal, Pensacola, FL) in sterile water were given to rats that lost weight during the passive infusion phase. STAT™ is a concentrated high calorie liquid diet used to combat dehydration and maintain nutritive balance. All rats in each experiment received infusions of STAT™. There were no differences between groups in either the frequency or cumulative volume of STAT™ infusions in any experiment.
Procedure
The general structure of each of these experiments was the same. Rats were implanted with IG catheters and allowed to recover before the experiment began. Each experiment consisted of Habituation, Passive Infusion, No-Choice Self-infusion and Choice Self-infusion phases. During the Habituation phase, the rats had ad lib access to saccharin and lab chow. During the Passive infusion phase, non-contingent infusions of 10% ethanol were administered on an experimenter-determined schedule. In both self-infusion phases, ethanol infusions were contingent upon licking a flavored solution. During the no-choice phase, only the ethanol-paired flavor was available. During the choice phase, licks on a second flavored solution were paired with water infusions. Additional details on each phase of these experiments are provided below.
Surgery
Rats were food deprived 16–24 h prior to being implanted with an IG catheter under isoflurane anesthesia (for details see Fidler et al., 2006). Briefly, the stomach was externalized through a side incision and the IG end of the catheter (Dow Corning silastic tubing, ID 0.76 mm × OD 1.65 mm with a 2–3 mm knob of larger silastic tubing, ID 1.57 mm × OD 3.18 mm, slipped over the end and fastened with Dow Corning Medical Adhesive A) was inserted into the stomach through a puncture and secured with a purse string suture and sutures through a piece of polypropylene mesh (Davol Inc., Cranston, RI) threaded onto the catheter approximately 1 cm from the IG end. The stomach was returned to the cavity and the incision through the muscle and peritoneum was sutured. The catheter was threaded subcutaneously to a small incision on the back and attached to the backmount (bent blunt 20 ga. Needle with aluminum hub cemented to a 2.5 cm2 piece of polypropylene mesh with cranioplastic cement (300CCP and 300CCL, Plastics One, Roanoke, VA). The backmount was sutured to the muscle and all skin incisions were sutured. The rats were allowed 7±1 days to recover from surgery prior to the start of the experiment. Beginning the day after surgery, the rats were infused daily with 2 ml of sterile water to ensure catheter patency.
Habituation
During the habituation phase, the rats were placed in the chambers and attached to the leash, but no infusions were given in the chambers. Daily manual infusions of 2 ml sterile water continued when the rats were removed from the chambers for daily chamber maintenance. Rats had free access to food (Rodent Diet 5001) and to two bottles of saccharin (0.2 % w/v). The right and left saccharin bottles were available during alternate 30-min periods. The tube that was inserted first alternated daily over the 3 days of habituation and was counterbalanced within and across groups. This bottle alternation was intended to ensure that all of the animals encountered both fluid bottles and to reduce side preferences. On the first day of habituation, the sipper tubes were inserted all the way into the chamber and on the next 2 days they were gradually pulled back so that on the third day the tubes were flush with the wall of the chamber. The tubes remained in this position for the rest of the experiment. Rats were removed from the chambers for approximately 1 h per day and during this time chambers were cleaned, food replenished and fluid bottles refilled.
Passive-Infusion
Procedural details during this phase differed across experiments and are described separately below.
No-Choice Self-Infusion Phase
The timing of the start of this phase (relative to the start of the final passive infusion) differed between groups; those details are described in the Methods section for individual experiments. The purpose of this phase was to insure that all rats were exposed to the ethanol self-infusion contingency before introducing the choice procedure. Rats had access to food and to one drinking tube, labeled S+. For each rat, the position of the S+ tube (right or left) was its preferred side as determined by its consumption and licks during the habituation and passive infusion phases. Flavor of the S+ solution—grape or cherry Kool-Aid (0.05% w/v in 0.2% w/v saccharin)—was counterbalanced within and across groups. Licks on the S+ tube were paired with ethanol infusions on an FR10 schedule. Every 10th lick produced a 4-s infusion of 20% v/v ethanol (at a rate of 1.26 ml/min) up to a limit of 1.5 g/kg/30 min. Given the range of body weights across all three experiments (270–499 g), the ethanol dose administered per infusion ranged from .027 to .049 g/kg. The number of 4-s infusions necessary to produce the 1.5 g/kg limit was determined individually for each rat (range: 31–56). The Kool-Aid solution remained available even if the limit was reached but no further ethanol infusions were delivered. This limit was imposed in order to reduce the likelihood that large ethanol bouts would induce conditioned taste aversion to the paired flavor. It should be noted that this procedure allowed for partial extinction of the lick-ethanol contingency. Because rats were already on a partial reinforcement schedule (FR10), it is difficult to know how this type of partial extinction might have affected performance. Data analysis showed that most S+ licks occurred during 5-min samples in which ethanol reinforcers were delivered (group means across all studies ranged between 82–94%), indicating a relatively high overall rate of reinforcement.
Rats were removed from the chambers once per day for assessment of intoxication/withdrawal and so that they could be weighed and manually infused with water and STAT™ (if necessary). Chambers were cleaned, food replenished and the Kool-Aid bottles and pump reservoirs refilled at this time.
Choice Self-infusion Phase
During the choice phase, each rat had access to food and to two Kool-Aid solutions, grape and cherry. The S+ flavor and position remained the same as in the No-Choice phase. The other flavor (grape or cherry) and the other position (right or left) were assigned to the S−. Licks on the S− tube were paired with water infusions on an FR10 schedule. The total number of water infusions possible during a 30 min period was the same as the number of ethanol infusions. As in the previous phase, rats were removed from the chamber once per day in order to clean and replenish the chambers.
Dependent Variables
The volume of fluid (mls) consumed from each tube was recorded daily during all phases. Licks were counted in consecutive 5-min periods for all days. Total ethanol (g/kg) infused was calculated daily during the passive and self-infusion phases. During the self-infusion phases (no-choice and choice) ethanol intake per bout was also calculated. A bout was defined as one or more consecutive 5 min intervals during which ethanol was infused. A bout was considered to have ended when no ethanol was infused during the next 5 min period.
Intoxication was assessed on a 7-point scale (Fidler et al., 2006; Majchrowicz, 1975). Ratings ranged from 0, no overt signs of intoxication to 6, absence of eyeblink and all reflexes. Animals received a composite withdrawal score which was the sum of their scores on scales for limb flexion, tail stiffness, body posture, agitation/activity, startle, and tremor (Chester et al., 2002; Fidler et al., 2006; Roberts et al., 1996). Additional details on these scales can be found elsewhere (Fidler et al., 2006).
Experiment 1
In our initial attempt to replicate the Deutsch and Cannis (1980) protocol, we passively infused rats with ethanol over a 72-h period before initiating ethanol self-infusion (Exp. 1, Fidler et al., 2006). Although this exposure schedule might nominally be described as “continuous”, details of the procedure indicate that it was actually intermittent because two criteria were used to limit exposure in order to avoid severe impairment or overdosing. First, the ethanol pump was turned off whenever the rat’s body temperature (measured via an implanted telemetry device) dropped below a preset criterion. Second, as an additional safeguard, the pump was turned off whenever the total g/kg infused during a 6-h time block exceeded a predetermined limit. Thus, rats received periods of ethanol infusion alternating with periods of no infusion in unique patterns that were determined by each rat’s sensitivity to ethanol’s effect on core temperature. As a further protection against overdosing and to allow occasional periods of recovery, we also inserted a 6-h ethanol-free period each day in later experiments beginning on day 2 of passive exposure (Exps. 2–3, Fidler et al., 2006). This 6-h interval, which exceeded the average duration of the ethanol-free periods imposed by the temperature criterion, added to the “intermittency” of our passive exposure procedure.
In an effort to simplify our passive phase protocol in a way that would facilitate manipulation of passive phase parameters (and to make the procedure generally more accessible to other laboratories), we changed our strategy for passive ethanol exposure in the current studies. Instead of using a “continuous” ethanol infusion that was interrupted when body temperatures dropped below a criterion, we scheduled a series of three slow ethanol infusions each day separated by relatively long intervals of time (5.67 h between the onset of each infusion). The unit dose for each infusion was increased across days, unless rats were severely impaired when intoxication ratings were taken after the third daily infusion. Each day also included a relatively long ethanol-free recovery period after completion of the third infusion (~10 – 12 h). Thus, our standard procedure for IG ethanol exposure was more similar to the intermittent vapor exposure procedure than to the continuous vapor exposure reported in previous studies (Lopez & Becker, 2005; O’Dell et al., 2004). In Experiment 1, we examined the effect of lengthening the duration of the ethanol-free period between each series of passive ethanol infusions. More specifically, we compared IG ethanol self-infusion by rats that had previously received passive ethanol infusions on 5 consecutive days (Massed Group) to rats that had also received 5 days of passive infusion, but with an ethanol-free day after each day of infusions (Spaced Group). We hypothesized that when the time between periods of passive infusion was extended, Spaced Group rats would experience a more intense withdrawal syndrome (greater number of withdrawal symptoms over a longer duration) and that this might lead to greater ethanol self-infusion.
Procedure
Passive-Infusion Phase
Groups were matched on bodyweight at the end of the Habituation phase. All of the rats had continuous access to two water bottles and food in the chambers throughout this phase. During this and subsequent phases, rats that did not maintain their body weight were given supplemental infusions of 25% v/v STAT™ in sterile water. Additional water was always infused after STAT™ in order to keep the catheters clear.
All animals received 5 days with passive ethanol infusions and 5 days in the chamber without infusions, but the temporal arrangement of these days differed. The Spaced group received infusions of 10% (v/v) ethanol on days 1, 3, 5, 7, and 9 of the passive infusion phase and the Massed group received ethanol infusions on days 6–10 of the passive infusion phase (see Table 1). On the first day of infusions (for each group), rats received three infusions (2.5 g/kg, 10% v/v ethanol, 0.19 ml/min), with the first infusion beginning 5 min after the start of the session, and the second and third starting 340 min after the start of the previous infusion. Dose was manipulated by varying the duration of infusion individually for each animal. About 6 h after onset of the last infusion for the day, rats were removed from the chambers and placed on a grid surface where they were rated for intoxication. The ethanol dose per infusion for the next day of passive infusions was increased, decreased or held constant based on the intoxication ratings. Guided by previous experience (Fidler et al., 2006), our general goal was to maintain intoxication ratings between 2 and 3 on the 7-point scale. The unit dose per infusion for the next day was generally increased by 0.5 g/kg/infusion for intoxication ratings < 2 (except on the 3rd passive day when dose was only increased for ratings < 1), decreased by 0.5 g/kg/infusion for intoxication ratings ≥ 3 or held constant for intoxication ratings of 2. For rats that were severely impaired (intoxication ratings ≥ 4 at the time of withdrawal assessment) the first infusion of the day was omitted to allow greater recovery time. For rats with intoxication ratings of 5 or 6, the first infusion was omitted and the dose per infusion was decreased by 0.5 g/kg/infusion. The omitted infusion was restored on subsequent days if the intoxication rating was < 3.
Table 1.
Timing of passive infusion days for each group in each experiment
| Days in the Passive Infusion Phase | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp. 1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Massed (n = 15) | P1 | P2 | P3 | P4 | P5 | |||||
| Spaced (n = 15) | P1 | P2 | P3 | P4 | P5 | |||||
| Exp. 2 | — | — | — | — | 1 | 2 | 3 | 4 | 5 | 6 |
| Immediate (n = 11) | — | — | — | — | P1 | P2 | P3 | P4 | P5 | |
| Delay (n = 11) | — | — | — | — | P1 | P2 | P3 | P4 | P5 | |
| Exp. 3 | — | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Master (n = 7) | — | P1 | P2 | P3 | NCC1 | P4 | NCC2 | P5 | NCC3 | P6 |
| Yoked (n = 6) | — | P1 | P2 | P3 | NCC1 | P4 | NCC2 | P5 | NCC3 | P6 |
Note: P1-6 indicates the days on which passive infusions were delivered to each group. Since the total number of days in the passive infusion phase differed across experiments the days with passive infusions have been shifted to emphasize the between-experiment comparison with respect to the delivery of the final passive infusions relative to the start of the self-infusion phase. The Spaced group (Exp. 1) had the same interval between the last passive infusion and the start of the no-choice phase as the Delay group (Exp. 2). Similarly, the Massed group (Exp. 1), Immediate group (Exp. 2), and Master and Yoked groups (Exp. 3) had the same interval between the last passive infusion and the start of the no-choice phase. “—” indicates days that were not included in Experiments 2 and 3. On days with no symbols or letters, rats remained in the chambers with food and water available but no infusions were delivered. In Experiment 3, NCC1-3 refers to days that were No Choice self-infusion days for the Master group. Ethanol intake of individual rats in the Yoked group was Yoked to that of the individual rats in the Master group on these days. Thus, the NCC days were another variant of passive infusion days for the Yoked group.
Six h after the intoxication ratings (12 h after onset of the last ethanol infusion), rats were removed from the chambers and placed on a grid surface and rated for withdrawal (and/or residual intoxication). At this time, rats were also weighed and infused with STAT™ (as necessary) and water. The chambers were cleaned, food replenished and water bottles and pump reservoirs refilled. For the Spaced group, there were no ethanol infusions the next day but the rats were removed from the chambers at the usual times, giving additional withdrawal scores 30 and 36 h after onset of the last ethanol infusion. Rats in the Massed group were also removed from the chambers twice per day and placed on the grid surface, even on the 5 days before they received any ethanol infusions.
No-Choice Self-Infusion Phase
The no-choice self-infusion phase started 36 h after onset of the final passive infusion for the Spaced group and 12 h after onset of the final passive infusion for the Massed group.
Experiment 2
Because the Massed and Spaced groups in Experiment 1 differed both in the interval between passive infusion days and in the delay between the final passive infusion and the start of self-infusion, it is possible that either (or both) of these differences could have been responsible for observed group differences in ethanol self-infusion. To address this issue, Experiment 2 examined the effect of the time interval between the final passive infusion and the start of the self-infusion phase in two groups that initially received the same treatment (similar to the Massed Group procedure) during passive exposure. For the Immediate group, 12 h elapsed between onset of the final passive infusion and onset of the self-infusion phase, similar to the Massed Group treatment in Experiment 1. For the Delay group, the interval was 36 h, which was similar to the treatment for the Spaced Group in Experiment 1. A difference between the Immediate and Delay groups in ethanol self-infusion would suggest that differences between the Massed and Spaced groups in Experiment 1 were due, at least in part, to the different time interval between the passive and self-infusion phases.
Procedure
Passive Infusion Phase
The procedure for the Delay and Immediate groups in this experiment was generally similar to that of the Massed group in Experiment 1, except that there were fewer (or no) days without infusions at the beginning of the passive infusion phase (see Table 1). Each group received 5 consecutive days of passive infusions with the same timing and dosing criteria as in Experiment 1. For the Delay group, the first day of ethanol infusions was the first day of the 6-day passive infusion phase, which meant that the final ethanol infusion began 36 h before onset of the self-infusion phase. For the Immediate group, the first day of ethanol infusions was on the second day of the passive infusion phase, which meant that the final ethanol infusion began 12 h before onset of the self-infusion phase.
Self-Infusion Phase
No-choice and choice self-infusion phases were conducted as in Experiment 1.
Experiment 3
Previous experiments involving chronic forced exposure have suggested that giving animals the opportunity to self-administer ethanol between periods of forced exposure may further enhance ethanol self-administration (e.g., Deutsch & Walton, 1977; Finn et al., 2007; Roberts et al., 2000). In Experiment 3, we tested whether rats given the opportunity to self-infuse ethanol (on No Choice Control or NCC days) between periods of passive infusions (Master Group) would increase subsequent choice self-infusion relative to rats whose ethanol exposure was passively yoked to that of the Master Group on NCC days (Yoked Group). We hypothesized that the Master Group, which had the opportunity to control its ethanol intake on NCC days, would subsequently have higher ethanol self-infusion than the Yoked Group.
Method
Procedure
Passive Infusion Phase
Rats were matched at the end of the Habituation phase so that individual rats in each pair (Master and Yoked) were of similar size. The first 3 days of passive infusions were conducted as in the previous experiments (three infusions per day with ethanol dose starting at 2.5 g/kg/infusion). Following the third day of passive infusions, no-choice control (NCC) days were alternated with additional days of passive infusions (see Table 1). On each NCC day rats had access to one Kool-Aid solution presented on the preferred side, as assessed by licks and consumption during Habituation and the first 3 days of the Passive Infusion phase. The flavor of the solution, grape or cherry, was counterbalanced. For rats in the Master group, licks were paired with infusions of 20% v/v ethanol on an FR10 schedule so that every 10th lick produced a 4-s infusion (at 1.3 ml/min). Each rat in the Yoked group was paired with a rat in the Master group. Each time the Master completed the FR for an infusion, both the Master and the Yoked animal were infused. Licks made by the Yoked animals did not result in infusions. Thus, for the Master group, this was a no-choice self-infusion day like those at the beginning of self-infusion in Experiments 1–2. However, for the Yoked group, these days were additional passive infusion days, albeit with a different schedule, rate of infusion and drinking fluid. At the end of each NCC day, the rats were removed from the chambers and their level of intoxication/withdrawal was assessed. At this time they were also weighed and manually infused with STAT™ (if necessary) and water. Chambers were cleaned and food and fluids replenished. This phase continued until there had been 3 NCC days and 6 passive infusion days (see Table 1). Unit ethanol doses on later passive infusion days were based on intoxication ratings from the previous passive infusion days as in earlier experiments.
No-Choice and Choice Self-Infusion Phases
This phase started 12 h after onset of the final passive infusion for rats in both groups. For rats in the Master group, this phase was identical to the NCC days. For rats in the Yoked group, this phase was the first time that they had control over their ethanol intake. These no-choice days were conducted like those in Experiments 1 and 2. The choice phase was conducted as in Experiments 1–2, except that it was only 4 days in duration.
Reversal Phase
After four choice days, the contingencies were reversed for rats that were not showing a preference for the ethanol-paired flavor (as indexed by S+ preference ratios for licks and consumption). The flavor/position that had been paired with water infusions was now paired with ethanol infusions and the flavor/position that had been paired with ethanol infusions was now paired with water infusions. During the dark part of the light cycle, 20 μl blood samples were taken from the tip of the tail 30 min after the start of bouts in which rats infused 1.5 g/kg/30 min. The blood sample was added to 50 μl of chilled 5% ZnSO4 and stored on ice. Next, 50 μl of 0.3 M Ba(OH)2 and 300 μl of distilled water were added to each sample. The samples were vortexed and then centrifuged at 12,000 rpm for 5 min. The supernatant was removed and analyzed by gas chromatography (Rustay & Crabbe, 2004).
Results
Subject Attrition
Experiment 1
Data for two rats were removed from the experiment. One rat from the Massed Group died during the passive infusion phase due to an apparent overdose. One rat from the Spaced group was euthanized during the Self-infusion phase due to excessive weight loss. Fifteen rats in each group completed the experiment.
Experiment 2
One rat died following surgery. One rat was removed from the study due to a clogged catheter. Two rats died from an apparent overdose and six rats were removed from the study following an experimenter error that affected their access to ethanol. All data for these animals were removed from the experiment. Eleven animals in each group completed the experiment.
Experiment 3
Two rats in the Yoked group were removed from the experiment as a result of mechanical problems with their chambers. One rat in the Master group was removed from the experiment due to health problems (following the second NCC day). The rat that had been Yoked to the sick Master was Yoked to a surviving Master for the final NCC day. Seven animals in the Master group and six animals in the Yoked group completed the experiment.
Daily Ethanol Intake
Passive Infusion Phase
Mean daily ethanol dose (g/kg ± SEM) passively infused or self-infused during each experiment is shown in Figures 1–3. To facilitate the between-group comparison of ethanol dose during the passive phase, only the five (Experiments 1–2) or six (Experiment 3) days on which passive ethanol infusions were delivered are shown for each group. Ethanol intake was analyzed separately for each phase in each experiment using mixed ANOVAs with Group as a between-subjects factor and Day as a within-subjects factor. Separate analyses were performed to compare intake on the last day of each phase to the first day of the next.
Figure 1.
Mean (± SEM) ethanol dose (g/kg/day) for rats in the Spaced (circles) and Massed (squares) groups during the ethanol exposure days of the passive infusion phase (P1–P5, open symbols), the 2 no-choice days of self-infusion (NC1-2, hatched symbols) and the 8 days of choice (CH1-8, filled symbols) in Experiment 1. Rats in the Spaced group (n=15) received passive infusions of 10% ethanol on alternate days (5 ethanol exposures, days without infusions not shown) prior to the self-infusion phases. Rats in the Massed group (n=15) had equivalent exposure to the chamber but received passive infusions of 10% ethanol on 5 consecutive days prior to the self-infusion phases. During the no-choice phase, rats in both groups had access to a Kool-Aid solution and licks were paired with infusions of 20% ethanol. On choice days, a second Kool-Aid solution was also available. Licks on the second solution were paired with infusions of water. * significant simple main effect of group on the days indicated (ps < .002). ** significant main effect of group in the choice phase (p < .002).
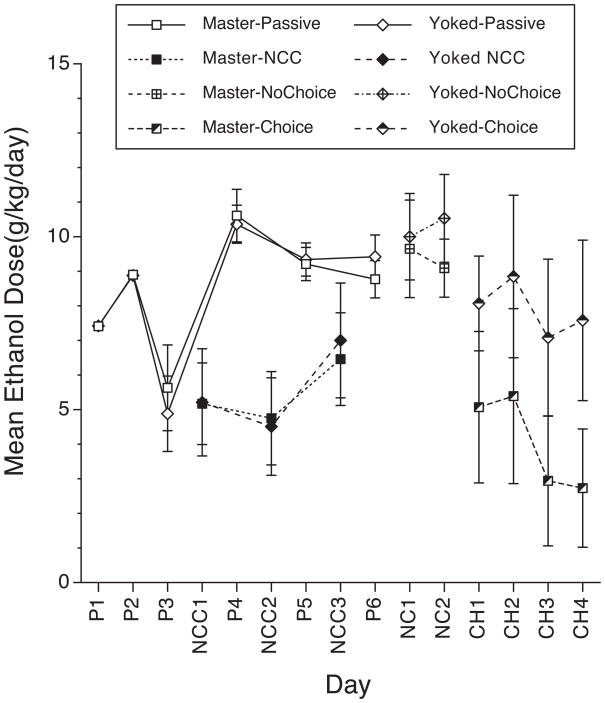
Figure 3.
Mean (± SEM) ethanol dose (g/kg/day) for rats in the Master (n=7, squares) and Yoked (n=6, diamonds) Groups in Experiment 3 during the passive infusion (P1–P6, open symbols), no-choice control (NCC1-3, closed symbols), no-choice (NC1-2, hatched symbols), and choice (CH1-4, top filled symbols). Groups differed only in their treatment during the no-choice control days during which Masters controlled the ethanol dose for themselves and the Yoked rats. For the Masters, these were regular no-choice days with one fluid available and licks on this fluid produced infusions of 20% ethanol on an FR10 schedule. Each rat in the Yoked group received the same infusions as a rat in the Master group but the infusions were non-contingent. During No-Choice days, rats in both groups had access to a Kool-Aid solution and licks were paired with infusions of 20% ethanol. On choice days, a second Kool-Aid solution was also available. Licks on the second solution were paired with infusions of water on an FR10 schedule.
In Experiment 1, the passively infused ethanol dose was very similar for the 2 groups over the first 2 infusion days. On later passive infusion days, the groups diverged with the Spaced Group receiving more ethanol than the Massed group. The mean cumulative dose (±SEM) infused across all 5 days was 45.7±0.5 g/kg for the Spaced Group and 38.2±0.9 g/kg for the Massed Group. The Group × Passive Day ANOVA revealed significant main effects of Group [F(1,28) = 52.5, p < .0001] and Passive Day [F(4,112) = 11.9, p < .0001] as well as a significant interaction [F(4,112) = 14.1, p < .0001]. Simple effects analyses revealed a significant main effect of Group during the last 3 infusion days [P3: F(1,28) = 12.6, p < .002; P4: F(1,28) = 16.1, p < .0001; P5: F(1,28) = 40.1, p < .0001]. In each case, the Spaced Group received more ethanol than the Massed Group. Because the experimenter determined the dose infused each day based on daily intoxication ratings, the higher doses received by Spaced Group rats indicates that they were less sensitive to ethanol-induced impairment, perhaps because they had more recovery time between infusion days than the Massed group.
In Experiment 2, the passively infused ethanol dose also diverged across days, with the Delay Group (42.2±0.4 g/kg) receiving a slightly higher cumulative dose than the Immediate Group (39.0±1.3). A Group × Passive Day ANOVA revealed significant main effects of Group [F(1,20) = 5.9, p < .03] and Passive Day [F(4,80) = 5.9, p < .0001] and a significant interaction [F(4,80) = 4.5, p < .003]. Simple effects analyses revealed significant main effects of Group during the second, fourth and fifth infusion days [P2: F(1,22) = 8.3, p < .01; P4: F(1,22) = 8.1, p < .01; P5: F(1,22) = 6.6, p < .02]. Although these group effects might be attributed to the fact that the Immediate Group had one more habituation day than the Delay group (see Table 1), there is no reason to expect that this small procedural difference would significantly affect the subsequent level of intoxication produced by ethanol (which determined the ethanol dose infused on the next day). Because both groups were treated identically after ethanol infusions began, the group differences are more likely due to sampling error than to a treatment effect.
In Experiment 3, both groups received similar mean cumulative ethanol doses over the 6 passive infusion days (Master: 50.1±1.7 g/kg; Yoked: 50.4±0.8 g/kg) and over the 3 NCC days (Master: 16.4±2.8 g/kg; Yoked: 16.7±3.8 g/kg). Both groups received a lower dose than was intended on the third infusion day (P3) due to an experimenter error (four rats in each group received only one ethanol infusion instead of three). A Group × Passive Day ANOVA including all 6 passive infusion days revealed only a significant main effect of day [F(5,55) = 15.4, p < .0001]. Separate analysis of ethanol intake on the 3 no-choice control days revealed no significant main effects or interactions. A paired t-test (considering just the ethanol intakes for the Master group) revealed no difference between intake on the third day of passive infusion (P3) and the first no-choice control day (NCC1). Ethanol intake on the three NCC days (by the Masters) did not differ.
No-Choice Self-Infusion Phase
In each experiment, the ethanol dose self-infused during the no-choice days was as high or higher than the dose that had been passively infused on the last day of passive infusions. Group mean intakes on No-Choice days ranged from 8.9 to 10.3 g/kg (Figures 1–3). A Group × Day ANOVA comparing ethanol intake on the last day of passive exposure with the first day of no-choice for Experiment 1 revealed significant main effects of group [F(1,28) = 10.9, p < .004] and day [F(1,28) = 7.7, p < .02], as well as a significant interaction [F(1,28) = 5.0, p < .04]. Further examination of the interaction by simple effects analyses revealed no significant change between days in the Spaced Group, but a significant increase across days for the Massed group [F(1,14) = 8.2, p < .02]. Similar analyses for Experiments 2 and 3 revealed no significant change in ethanol intake across days and no significant group differences.
Group × No-Choice Day ANOVAs for each experiment yielded only a marginal main effect of day in Experiment 2 [F(1,20) = 4.1, p < .06], reflecting a slightly higher ethanol intake on the second no-choice day than on the first. There were no between-group differences in the amount of ethanol self-infused on No-Choice days in any experiment.
Choice phase
Ethanol intake on choice days was generally lower than intake on no-choice days in all three experiments (Figures 1–3). This observation was supported by significant main effects of Day for each experiment in separate Group × Day ANOVAs that compared intakes on the second no-choice day to those on the first choice day [Exp. 1: F(1,28) = 13.6, p = .001; Exp. 2: F(1,20) = 19.5, p < .0001; Exp. 3: F(1,11) = 5.6, p < .04].
As can be seen in Figure 1, the Massed Group (6.4±1.0 g/kg/d) consistently self-infused more ethanol on Choice days than the Spaced Group (2.2±0.6 g/kg/d) in Experiment 1. This observation was supported by a Group × Choice Day ANOVA that revealed significant main effects of Group [F(1,28) = 13.2, p < .002] and Day [F(7,196) = 2.8, p <.01], but no interaction. Consistent with the group difference in daily ethanol intake was the finding that the Massed Group had a higher proportion of rats (15/15) with at least 1 day of ethanol intake > 5 g/kg than did the Spaced group (9/15). A z-test (Bruning and Kintz, 1968) confirmed that these proportions were significantly different [z = 5.5, p < .00001]. Similarly, significantly more rats in the Massed Group (11/15) than in the Spaced Group (4/15) had at least one day with ethanol intake > 10 g/kg [z = 4.1, p < .00001].
In Experiments 2 and 3, self-infused ethanol intakes during the choice phase were generally similar to those of the Massed Group in Experiment 1, with overall group mean intakes ranging from 4.1 to 7.9 g/kg/d (see Figures 2 and 3). Moreover, there were no group differences in either experiment. This conclusion was supported by separate Group × Choice Day ANOVAs for each experiment that revealed only a significant main effect of Day in Experiment 2 [F(7,140) = 2.2, p < .04], reflecting a general decrease in intake over days. The main effect of Group and interaction were not significant for either experiment. In Experiment 2, there was no difference between groups in the proportion of animals with at least one day’s ethanol intake > 5 g/kg (9/11 Delay and 8/11 Immediate rats) or > 10 g/kg (4/11 Delay and 6/11 Immediate rats). However, in Experiment 3, a higher proportion of rats in the Yoked group (5/6 rats) than in the Master group (3/7) self-infused more than 5 g/kg on at least 1 choice day [z = 2.3, p < .03]. There was no difference in the proportion of rats self-infusing > 10 g/kg on at least 1 choice day (3/7 Masters and 3/6 Yoked rats).
Figure 2.
Mean (± SEM) ethanol dose (g/kg/day) for rats in the Delay (n=11, diamonds) and Immediate (n=11, squares) Groups during the passive infusion phase (P1–P5, open symbols), the 2 no-choice days of self-infusion (NC1-2, hatched symbols) and the 8 days of choice (CH1-8, filled symbols) in Experiment 2. Groups had equivalent exposure to the chamber and both groups received passive infusions of 10% ethanol on 5 consecutive days. Rats in the Delay group received their last passive infusion beginning 36 h before the start of the no-choice phase (same interval as the Spaced group in Experiment 1) whereas rats in the Immediate group received their last passive infusion beginning 12 h before the start of the no-choice phase (same interval as the Massed group in Experiment 1). During the no-choice phase, rats in both groups had access to one Kool-Aid solution and licks were paired with infusions of 20% ethanol. On choice days, a second Kool-Aid solution was also available. Licks on the second solution were paired with infusions of water. * significant simple main effect of group on the days indicated (ps < .02).
Group intakes on the single “reversal” Choice day at the end of Experiment 3 were relatively high, but did not differ between the Master (9.7±0.7 g/kg) and Yoked (9.5±1.6 g/kg) groups.
Intoxication
Group mean intoxication scores were generally lowest on the 1st day of passive exposure (P1) and peaked on the 3rd or 4th day of exposure (Table 2). Decreases in intoxication scores on the final day of passive exposure most likely reflected reductions in the doses administered by the experimenter on those days (P5–P6, Figures 1–3). Despite the significant group difference in passively infused ethanol dose in Experiment 1 (see Figure 1 and above), there were no differences between the Spaced (2.5±0.1) and Massed Groups (2.2±.01) in the mean intoxication rating on passive infusion days. Group × Passive Day ANOVAs conducted separately for each experiment revealed significant main effects of day for each experiment [Exp. 1: F(4,112) = 18.9, p < .0001; Exp. 2: F(4,80) = 10.6, p < .0001; Exp. 3: F(5,55) = 5.6, p < .0001], but no effects of Group or interaction in Experiments 1 or 3. There was a significant interaction in Experiment 2 [F(4,80) = 6.6, p < .001], reflecting a group difference in the pattern of intoxication across days. Simple effect analyses indicated that the Immediate Group was more intoxicated than the Delay group on the 2nd and 3rd days with passive infusions [P2: F(1,20) = 6.0, p < .03; P3: F(1,20) = 9.9, p < .006], whereas the Delay group was more intoxicated than the Immediate group on the last day of passive infusions [F(1,20) = 8.0, p < .02]. As suggested earlier, because both groups were treated identically after passive ethanol infusions began, these group differences are likely due to sampling error
Table 2.
Mean (± SEM) intoxication ratings for all groups in Experiments 1–3.
| Experiment 1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| Day | Massed | Spaced | Immediate | Delay | Master | Yoked |
| P1 | 0.73±0.21 | 0.60±0.19 | 0.73±0.38 | 0.36±0.20 | 0.00±0.00 | 0.17±0.17 |
| P2 | 2.67±0.30 | 1.53±0.41 | 2.00±0.43 | 0.82±0.23 | 1.57±0.57 | 1.50±0.67 |
| P3 | 3.87±0.41 | 3.60±0.38 | 3.73±0.54 | 1.73±0.33 | 1.57±0.75 | 0.33±0.33 |
| NCC1 | 0.00±0.00 | 0.00±0.00 | ||||
| P4 | 3.40±0.46 | 2.87±0.36 | 3.09±0.41 | 3.18±0.52 | 2.71±0.97 | 3.50±0.92 |
| NCC2 | 0.57±0.57 | 0.33±0.33 | ||||
| P5 | 1.80±0.44 | 2.53±0.34 | 1.18±0.66 | 3.55±0.51 | 1.43±0.84 | 1.83±0.65 |
| NCC3 | 0.00±0.00 | 0.33±0.33 | ||||
| P6 | 0.29±0.29 | 0.67±0.42 | ||||
| NC1 | 0.93±0.33 | 0.20±0.20 | 0.00±0.00 | 0.09±0.09 | 0.14±0.14 | 0.67±0.42 |
| NC2 | 0.47±0.27 | 0.20±0.20 | 0.00±0.00 | 0.00±0.00 | 0.29±0.29 | 0.33±0.33 |
| CH1 | 0.47±0.27 | 0.27±0.27 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| CH2 | 0.13±0.09 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.17±0.17 |
Note: On days in which passive infusions were delivered (P1-6) intoxication ratings were made 6 h after the start of the last infusion. On self-infusion days, No-Choice Control (NCC1-3), No-Choice (NC1-2) and Choice (CH1-2), intoxication ratings were made at the end of the 23-h session (in the mid-afternoon). No signs of intoxication were observed after CH2. Days NCC1-3 and P6 were only included in Experiment 3.
Withdrawal
The group means (±SEM) for each of the time-points at which withdrawal was assessed are shown in Table 3. Levels of withdrawal were generally low for assessments at 12 h after onset of the last daily passive infusion (mean scores ≤ 1.2), but tended to increase when assessments were made 30–36 h after the last passive infusion (Spaced Group in Experiment 1, Delay Group in Experiment 2). Analysis of withdrawal scores at 12 h using separate Group × Day ANOVAs for each experiment revealed a main effect of Group only in Experiment 1 [F(1,28) = 6.5, p < .02], reflecting stronger withdrawal in the Massed Group (0.6±0.2) than in the Spaced Group (0.2±0.1). Comparison of the final withdrawal score for the Massed and Spaced groups (12 and 36 h after the final infusion on P5, respectively) showed no group difference in withdrawal scores at the onset of self-infusion. Group × Day ANOVAs of the 12 h withdrawal scores yielded no significant Group effects or interactions for Experiments 2 or 3, although there was a significant main effect of day in Experiment 2 [F(4,80) = 2.6, p < .05]. Comparison of the final withdrawal score for the Immediate and Delay groups (12 and 36 h after the final infusion on P5, respectively) showed no group difference in withdrawal ratings at the onset of self-infusion. However, the mean withdrawal rating in the Delay Group at 30 h (4.2±0.9) was significantly higher than that of the Immediate Group at 12 h (1.2±0.6) [F(1,20) = 8.6, p < .01].
Table 3.
Mean (±SEM) withdrawal ratings for each group in Experiments 1–3.
| Experiment 1 | Experiment 2 | Experiment 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Massed | Spaced | Immediate | Delay | Master | Yoked | |||||
| Day | 12 h | 12 h | 30 h | 36 h | 12 h | 12 h | 30 h | 36 h | 12 h | 12 h |
| P1 | 0.4±0.2 | 0.3±0.1 | 0.5±0.2 | 0.2±0.1 | 0.3±0.1 | 0.1±0.1 | 0.0±0.0 | 0.0±0.0 | ||
| P2 | 0.3±0.1 | 0.1±0.1 | 0.4±0.2 | 0.1±0.1 | 0.1±0.1 | 0.1±0.1 | 0.0±0.0 | 0.0±0.0 | ||
| P3 | 0.1±0.1 | 0.1±0.1 | 1.9±0.6 | 1.2±0.5 | 0.2±0.1 | 0.3±0.2 | 0.7±0.6 | 1.2±1.0 | ||
| NCC1 | 0.4±0.4 | 0.0±0.0 | ||||||||
| P4 | 1.1±0.5 | 0.1±0.1 | 1.1±0.3 | 0.7±0.3 | 0.8±0.5 | 0.2±0.1 | 0.1±0.1 | 0.2±0.2 | ||
| NCC2 | 0.6±0.6 | 0.5±0.5 | ||||||||
| P5 | 1.1±0.5 | 0.2±0.1 | 1.3±0.3 | 0.7±0.3 | 1.2±0.6 | 0.6±0.4 | 4.2±0.9 | 2.6±0.6 | 0.1±0.1 | 0.2±0.2 |
| NCC3 | 1.6±1.0 | 0.2±0.2 | ||||||||
| P6 | 0.3±0.3 | 0.7±0.4 | ||||||||
Note: Times are relative to the start of the final passive infusion (except for NCC days in Experiment 3 when withdrawal ratings were made at the usual time of day regardless of when the last ethanol infusion (self-administered or yoked was delivered).
Ethanol Bouts per Day
The mean numbers of ethanol bouts per day (#) are shown in Table 4 for all groups and all self-infusion days in Experiments 1–3. Separate Group × Day ANOVAs were used to analyze the data from each phase for each experiment. The analysis of ethanol bouts per day yielded significant main effects of Group during the No-Choice or Choice phases only in Experiment 1 [NC: F(1,28) = 8.1, p < .009; CH: F(1,28) = 9.3, p < .006]. The Spaced group (12.1±0.7) had more ethanol bouts than the Massed group (9.1±0.8) on No Choice days. However, the Massed Group (6.6±1.3) had more ethanol bouts than the Spaced Group (2.4±0.7) on Choice days.
Table 4.
The Mean (±SEM) number of S+ bouts and the mean (±SEM) bout size (g/kg/bout) per day for all groups in Experiment 1–3.
| Exp. 1 | Exp. 2 | Exp. 3 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Massed Group | Spaced Group | Immediate Group | Delay Group | Master Group | Yoked Group | |||||||||||||
| Day | # | Size | n | # | Size | n | # | Size | n | # | Size | n | # | Size | n | # | Size | n |
| NCC1 | 7.1±1.6 | 0.8±0.1 | 6 | |||||||||||||||
| NCC2 | 5.1±1.4 | 0.9±0.1 | 6 | |||||||||||||||
| NCC3 | 5.9±1.2 | 1.0±0.2 | 7 | |||||||||||||||
| NC1 | 9.3±0.9 | 1.0±0.1 | 14 | 12.7±0.7 | 0.8±0.1 | 15 | 8.2±1.4 | 1.1±0.1 | 9 | 10.8±1.2 | 0.9±0.1 | 11 | 8.1±1.6 | 1.3±0.1 | 7 | 8.5±1.4 | 1.2±0.1 | 6 |
| NC2 | 8.9±0.9 | 1.1±0.1 | 15 | 11.5±0.9 | 0.9±0.0 | 15 | 8.6±1.3 | 1.2±0.1 | 10 | 12.6±1.9 | 0.9±0.1 | 11 | 8.7±0.8 | 1.1±0.1 | 7 | 10.3±1.8 | 1.1±0.1 | 6 |
| CH1 | 8.0±1.0 | 1.0±0.1 | 15 | 5.6±1.2 | 0.9±0.1 | 14 | 4.9±1.4 | 1.1±0.2 | 9 | 7.1±1.9 | 1.0±0.1 | 10 | 4.6±1.9 | 0.9±0.2 | 5 | 6.8±1.1 | 1.2±0.1 | 6 |
| CH2 | 5.7±1.1 | 1.0±0.1 | 13 | 3.1±1.1 | 0.6±0.2 | 10 | 6.2±1.8 | 0.8±0.2 | 9 | 5.8±1.9 | 0.7±0.1 | 9 | 4.4±2.0 | 1.0±0.3 | 4 | 10.0±2.6 | 0.8±0.2 | 6 |
| CH3 | 6.5±1.2 | 0.9±0.1 | 14 | 2.6±1.0 | 0.5±0.2 | 9 | 6.8±1.7 | 0.8±0.1 | 10 | 6.8±1.8 | 0.7±0.1 | 9 | 2.6±1.3 | 0.9±0.4 | 3 | 9.8±3.6 | 0.7±0.2 | 6 |
| CH4 | 5.9±1.2 | 1.0±0.1 | 14 | 2.0±0.9 | 0.6±0.2 | 9 | 9.2±2.5 | 0.8±0.1 | 9 | 4.3±1.6 | 0.6±0.1 | 8 | 2.3±1.1 | 0.9±0.3 | 4 | 9.2±3.3 | 0.8±0.2 | 6 |
| CH5 | 7.1±1.7 | 1.0±0.1 | 15 | 2.1±0.7 | 0.8±0.2 | 8 | 7.2±2.2 | 0.7±0.2 | 11 | 5.2±1.8 | 0.9±0.1 | 8 | ||||||
| CH6 | 6.9±1.6 | 1.0±0.1 | 13 | 2.5±0.7 | 0.7±0.2 | 10 | 5.2±1.7 | 0.9±0.2 | 8 | 3.6±1.6 | 0.5±0.2 | 8 | ||||||
| CH7 | 7.1±1.7 | 0.9±0.1 | 14 | 2.2±0.7 | 0.7±0.1 | 10 | 6.8±1.8 | 0.8±0.2 | 10 | 5.2±2.1 | 0.8±0.2 | 7 | ||||||
| CH8 | 6.9±1.5 | 0.9±0.1 | 13 | 2.6±0.9 | 0.7±0.2 | 10 | 5.6±1.2 | 0.7±0.2 | 10 | 4.1±1.8 | 0.6±0.2 | 9 | ||||||
| REV1 | 9.1±1.6 | 1.2±0.1 | 7 | 9.2±2.1 | 1.2±0.1 | 6 | ||||||||||||
Ethanol Intake per Bout
Mean bout sizes (g/kg/bout) are also listed for all groups on all days in Table 4. These means ranged from 0.5 to 1.3 g/kg/bout. Because rats that had no bouts on a particular day were excluded from calculation of that day’s mean, different numbers of animals contributed to each daily mean (final n’s are listed in the rightmost column for each group in Table 4). Separate Group × Days ANOVAs applied to the data from each experiment (using only rats that had ethanol bouts on all days) yielded no significant main effects or interactions, indicating that ethanol bout size was generally similar in all groups and remained relatively constant across days. Thus, the difference in total daily ethanol intake between the Massed and Spaced groups during the Choice phase in Experiment 1 was better explained by the larger number of bouts in the Massed Group rather than by differences in bout size.
Consistent with previous findings in rats given chronic passive ethanol exposure (Fidler et al., 2006), we also found that a substantial proportion of ethanol intake during the choice phase could be attributed to relatively large drinking bouts. Averaged across groups in Experiments 1–3, 70%, 66% and 70% of the total ethanol dose self-administered during the choice phase was infused in bouts > 1.0 g/kg. Although one-way ANOVAs on the percentage (arc-sin transformed) of total dose consumed in bouts > 1.0 g/kg showed no significant group effect in any experiment, there was a trend for the percentage to be greater in the Massed Group (80.8% ± 4.0) than in the Spaced Group (59.8% ± 9.5) in Experiment 1 [F(1,28) = 3.88, p = .06].
Blood Ethanol Concentration
Blood ethanol concentrations measured 30 min after the start of a bout in which rats met the experimenter-imposed limit of 1.5 g/kg/30 min ranged from 0.32–2.24 mg/ml, with an average of 1.18 mg/ml over 17 samples (Experiment 3, reversal day). This range of values presumably reflects differences in cumulative ethanol intake in the hours prior to the blood draw, the temporal duration of the infusion bout in which the limit was reached, the amount of food in the stomach at the time of the bout and individual differences in ethanol pharmacokinetics. Mean ethanol intakes during the reversal day were 9.7 ± 0.7 and 9.5 ± 1.6 g/kg for the Master and Yoked Groups, respectively. In an additional set of animals that infused 26% v/v ethanol with a 2.0 g/kg/30 min limit (data not shown), average BEC was 1.93 mg/ml.
S+ and S− Licks and Consumption
Mean (± SEM) licks on the S+ and S− Kool-Aid solutions are reported for each experiment in Table 5. Separate Group × Day ANOVAs on the lick data for the No-Choice days yielded no significant effects in any experiment, consistent with the lack of group differences in ethanol intakes (g/kg) during this phase. During the Choice phase, rats generally made more licks on the S− tube than on the S+ tube, an observation that was supported by a significant main effect of Fluid when separate Group × Fluid × Day ANOVAs were applied to data from Experiments 1 [F(1,28) = 16.0, p < .0001] and 2 [F(1, 20) = 11.6, p < .004], but not Experiment 3. The main effects of Day [Exp. 1: F(7, 196) = 3.1, p < .005; Exp. 2: F(7, 140) = 2.5, p < .02] and the Fluid × Day interactions [Exp. 1: F(7,196) = 3.1, p < .005; Exp. 2: F(7,140) = 2.6, p < .02] were also significant in the first two experiments. The only significant effect involving Group in any experiment was the Group × Fluid interaction in Experiment 1 [F(1,28) = 6.2, p < .02]. Between-group follow-up analyses indicated that the Massed group made more S+ licks than the Spaced group [F(1,28) = 13.6, p < .002], but the groups did not differ in S− licks. Within-group follow-up analyses showed that the Spaced group made significantly fewer licks on the S+ than on the S− [F(1,14) = 25.2, p < .0001], but there was no difference in the number of licks directed toward the S+ and S− for the Massed group. The only instance in which S+ licks substantially exceeded S− licks was on the reversal day in Experiment 3. Group × Fluid ANOVA confirmed the significant main effect of Fluid [F(1,11) = 19.2, p < .001], but showed no effect of Group or interaction.
Table 5.
Mean (±SEM) licks (× 1000) on the ethanol-paired (S+) and unpaired (S−) Kool-Aid tubes during the Self-Infusion phases by all groups in Experiments 1–3.
| Experiment 1 | Experiment 2 | Experiment 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Massed | Spaced | Immediate | Delay | Master | Yoked | |||||||
| Day | S+ | S− | S+ | S− | S+ | S− | S+ | S− | S+ | S− | S+ | S− |
| NCC1 | 2.1±0.4 | |||||||||||
| NCC2 | 3.1±1.1 | |||||||||||
| NCC3 | 2.3±0.5 | |||||||||||
| NC1 | 4.4±0.6 | 3.9±0.2 | 4.6±0.8 | 3.5±0.3 | 3.8±0.5 | 4.6±0.7 | ||||||
| NC2 | 3.7±0.4 | 3.5±0.2 | 4.4±0.7 | 3.5±0.5 | 3.5±0.5 | 4.6±0.4 | ||||||
| CH1 | 3.1±0.6 | 2.8±0.8 | 2.1±0.5 | 4.4±1.4 | 2.6±0.7 | 7.4±2.7 | 2.6±0.6 | 5.8±2.1 | 1.8±0.8 | 3.7±1.4 | 3.8±0.6 | 4.1±1.2 |
| CH2 | 2.6±0.5 | 8.5±3.7 | 0.8±0.3 | 7.3±1.2 | 2.4±0.7 | 9.7±3.8 | 2.2±0.7 | 5.0±1.5 | 2.2±1.1 | 4.2±1.5 | 3.3±0.9 | 3.8±1.4 |
| CH3 | 3.0±0.7 | 4.4±1.6 | 0.6±0.3 | 9.0±2.2 | 2.6±0.7 | 5.9±2.1 | 2.3±0.7 | 4.3±1.0 | 1.3±0.8 | 6.2±1.2 | 2.9±0.9 | 4.8±1.9 |
| CH4 | 2.7±0.6 | 5.2±1.5 | 0.7±0.4 | 8.8±1.9 | 2.9±0.7 | 7.2±2.0 | 1.6±0.7 | 9.3±2.4 | 1.3±0.8 | 6.6±1.6 | 3.2±1.0 | 3.9±1.2 |
| CH5 | 3.2±0.6 | 4.6±1.1 | 0.6±0.2 | 11.0±2.1 | 2.3±0.6 | 7.3±1.6 | 2.0±0.7 | 7.4±2.3 | ||||
| CH6 | 3.3±0.7 | 5.1±1.4 | 0.8±0.3 | 8.6±1.6 | 2.4±0.7 | 8.6±1.6 | 1.1±0.5 | 9.4±2.6 | ||||
| CH7 | 3.3±0.8 | 5.7±1.9 | 0.6±0.3 | 9.1±1.8 | 2.6±0.7 | 6.8±1.8 | 1.8±0.8 | 8.0±1.8 | ||||
| CH8 | 3.9±1.1 | 6.0±2.0 | 0.7±0.3 | 8.4±1.4 | 2.4±0.8 | 7.7±2.0 | 1.1±0.7 | 11.8±3.6 | ||||
| REV1 | 4.8±0.4 | 0.9±0.8 | 5.4±0.9 | 3.1±1.0 | ||||||||
Identical ANOVAs applied to the consumption data (not shown) yielded outcomes similar to those described for the lick data and are not reported here.
Lick Preference Ratios
Mean (± SEM) S+ preference ratios, calculated as S+ licks/total S+ and S− licks, are shown in Table 6. Consistent with patterns in the licks data, most of the mean preference ratios were below 0.5, indicating a relative aversion for S+. In Experiment 1, however, the Massed group (0.52±0.09) was relatively indifferent to the two flavors, whereas the Spaced Group (0.14±0.03) showed a strong aversion for S+ [main effect of Group: F(1,28) = 14.8, p < .001]. The only other significant effect to emerge from separate Group × Day ANOVAs applied to the choice phase preference data for each experiment was an effect of Day in Experiments 1 [F(7,196) = 3.9, p < .001] and 2 [F(7,140) = 2.2, p < .05]. However, analysis of the reversal day preference ratios yielded a marginally significant effect of Group in Experiment 3 [F(1,11) = 4.8, p = .05], reflecting the higher preference ratio in Master rats (0.90±0.08) compared to Yoked rats (0.65±0.07).
Table 6.
Mean S+ preference ratios (± SEM) during the self-infusion choice days in each experiment. The preference ratio is S+ Licks/((S+ Licks) + (S− Licks))
| Experiment 1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| Day | Massed | Spaced | Immediate | Delay | Master | Yoked |
| CH1 | 0.60±0.09 | 0.45±0.11 | 0.40±0.10 | 0.51±0.13 | 0.44±0.19 | 0.53±0.12 |
| CH2 | 0.53±0.11 | 0.12±0.07 | 0.40±0.12 | 0.40±0.12 | 0.42±0.19 | 0.53±0.16 |
| CH3 | 0.56±0.11 | 0.10±0.05 | 0.53±0.12 | 0.41±0.13 | 0.22±0.15 | 0.50±0.18 |
| CH4 | 0.46±0.11 | 0.10±0.06 | 0.43±0.12 | 0.24±0.11 | 0.18±0.14 | 0.47±0.15 |
| CH5 | 0.49±0.11 | 0.08±0.03 | 0.37±0.13 | 0.34±0.12 | ||
| CH6 | 0.49±0.11 | 0.11±0.04 | 0.32±0.12 | 0.21±0.11 | ||
| CH7 | 0.50±0.12 | 0.07±0.03 | 0.44±0.13 | 0.28±0.13 | ||
| CH8 | 0.50±0.12 | 0.10±0.04 | 0.41±0.14 | 0.19±0.11 | ||
| REV1 | 0.90±0.08 | 0.65±0.07 | ||||
Discussion
Contrary to our prediction, Experiment 1 showed that increasing the duration of the ethanol-free interval between periods of passive IG ethanol exposure from 10–12 h (Massed Group) to 34–36 h (Spaced Group) significantly reduced the amount of ethanol self-infused during subsequent choice days (Figure 1). Thus, even though they were exposed to significantly more ethanol during the passive phase, the Spaced Group self-infused less ethanol than the Massed Group during the choice phase. Moreover, Spaced Group rats developed a significant aversion for the S+ flavor whereas Massed Group rats did not. Examination of drinking patterns showed that the group difference in self-infused ethanol could be explained by a difference in the daily number of ethanol bouts rather than by a difference in ethanol bout size. Experiment 2, which failed to show an effect of the time delay (12 vs. 36 h) between the last passive ethanol exposure and onset of self-infusion (Figure 2), suggested that the greater self-infusion by Massed Group rats in Experiment 1was not due to use of the shorter time delay for that group. Finally, Experiment 3 showed that inserting no-choice self-infusion days between the last few passive exposure days did not affect subsequent self-infusion (Figure 3).
With the exception of the Spaced Group, the daily amounts of ethanol self-infused by rats that received our new passive phase dosing regimen (group means ranging from 4.1 to 7.9 g/kg/d) were as high or higher than those shown by rats that were passively exposed to ethanol using the temperature-feedback protocol described in our earlier studies (group means ranging from 3.8 to 4.9 g/kg/d, Experiments 1–3; Fidler et al., 2006). It is notable that all of these passive infusion paradigms were intermittent at least to some extent. Groups exposed to the Massed procedure in the three experiments reported here received three passive ethanol infusions per day (with infusions starting 340 min apart) followed by an ethanol-free period of at least 10 h. In contrast to the high intakes produced by exposure to the Massed procedure, the low daily amount of ethanol self-administered by the Spaced Group during the choice phase (2.2 g/kg/d) was quite similar to the levels previously observed in four groups of control rats that were infused with water or nothing during the passive phase (group means ranging from 1.0 to 2.2 g/kg/d, Control Experiment; Fidler et al., 2006). The Spaced Group was also similar to previous control groups in showing fewer ethanol bouts per day than the Massed Group. Although such cross-experiment comparisons must be interpreted cautiously, our findings in the Spaced Group suggest that lengthening the time delay between each series of passive infusions to 36 h largely eliminated the enhancing effect of passive ethanol exposure on later IG self-infusion. It is not known whether similar increases in the “off” time in an intermittent ethanol vapor exposure protocol (e.g., 16 hours on/8 hours off, Lopez & Becker, 2005) would reduce or eliminate the enhancing effect of chronic vapor exposure on ethanol drinking or operant oral self-administration. However, this outcome seems likely in light of earlier data showing that extending the time interval between consecutive periods of ethanol vapor exposure to 24 h eliminated the dependence enhancing effects of repeated vapor exposure seen at shorter time intervals (Goldstein, 1974). As it stands, the ethanol exposure induced by an ethanol vapor protocol is quite different from that induced in our passive infusion protocol. As far as we are aware, no studies have been done where ethanol vapor is delivered in brief exposures that would mimic the IG procedure reported here.
Examination of the intoxication and withdrawal scores suggests at least two possible interpretations of the self-infusion difference between the Massed and Spaced groups. One possibility, suggested by the finding that the groups had similar intoxication scores despite the higher level of passive ethanol exposure in the Spaced Group, is that the Spaced Group developed greater tolerance to ethanol-induced impairment than the Massed Group (i.e., a higher ethanol dose was needed to produce the target level of impairment in the Spaced Group). This interpretation might explain the lower self-infusion in the Spaced Group if one assumed that tolerance to ethanol’s impairing effect was accompanied by near complete tolerance to its reinforcing effect. However, given the paucity of data showing development of tolerance to ethanol reinforcement or data showing that more widely spaced ethanol exposures enhance ethanol tolerance, this interpretation is not compelling. It seems more likely that the greater sensitivity of the Massed Group to the impairing effects of ethanol was due to their shorter period of recovery after each set of passive ethanol infusions. That is, the impairing effects of ethanol may have combined additively with the general fatigue produced by each series of three closely spaced ethanol infusions in the Massed Group.
Our withdrawal data offer stronger support for an alternative interpretation of the Massed-Spaced self-infusion difference based on differences in development of ethanol dependence (with degree of dependence inferred from the magnitude of the withdrawal response). More specifically, the Massed Group showed significantly higher withdrawal scores than the Spaced Group 12 h after onset of the last daily infusion (Table 3). Moreover, rats exposed to a Massed procedure in Experiment 2 (Delay Group) showed much stronger withdrawal 30–36 h after the last passive infusion than Spaced Group rats tested at the same long post-infusion delays in Experiment 1. Both observations support the general conclusion that the Massed procedure induced a greater degree of dependence than the Spaced procedure. Thus, the higher level of choice ethanol self-infusion produced by the Massed procedure might be explained by the opportunity for greater negative reinforcement by ethanol (i.e., greater alleviation of aversive effects of ethanol withdrawal). Given the temporal pattern of withdrawal over time after the Massed procedure, it is possible that the ability of passive exposure to enhance subsequent self-infusion depends on having access to ethanol during the time period between 24–48 h after the last passive infusion when withdrawal appeared to reach its peak. However, previous research has shown that chronic ethanol vapor exposure can later enhance operant oral self-administration, even when the first opportunity to self-administer is delayed for 2 weeks after the end of vapor exposure (e.g., Roberts et al., 2000). Future research must address whether passive IG ethanol exposure using our Massed procedure has effects on IG self-infusion that persist beyond the 12–36 h delay intervals used here.
Although the foregoing analysis suggests that the Massed procedure produced greater dependence than the Spaced procedure, it is important to note that the magnitude of withdrawal, per se, immediately or 6 h before onset of self-infusion was not a reliable predictor of ethanol intake. For example, the Massed and Spaced groups showed similar withdrawal ratings immediately before self-infusion, but showed a large difference in ethanol intake (Experiment 1). Conversely, the Immediate and Delay groups showed similar ethanol intakes, despite showing a large difference in withdrawal ratings 6 h before self-infusion (Experiment 2). Thus, although our withdrawal ratings were generally useful for ranking groups on strength of dependence, ratings made shortly before self-infusion were not useful for predicting differences in ethanol intake. One problem with comparing group ratings made just before self-infusion is that those ratings reflected different phases in the time course of withdrawal for each group. That is, the Spaced group was likely past its peak whereas the Massed group had not yet reached its peak. In fact, as shown in Table 3, the Delay-group withdrawal data (Exp. 2) suggest that rats exposed to a Massed procedure showed peak withdrawal 30 h after onset of the final passive infusion (mean score = 4.2), whereas rats exposed to the Spaced procedure (Exp. 1) showed much weaker withdrawal at that same time point (mean score = 1.3). Thus, one can speculate that the neurobiological dysregulation associated with dependence was probably greater for the Massed group than for the Spaced group during self-infusion, thereby providing a stronger basis for negative reinforcement.
The fact that Spaced group rats received more ethanol per day than Massed group rats during the last three days of the passive phase is a potential confound for interpretation of the self-infusion difference. That is, the group difference in ethanol self-infusion might be attributed to this difference in cumulative ethanol exposure rather than to differences in passive infusion “spacing” or ethanol dependence. However, this explanation seems unlikely because the expected effects of higher ethanol exposure, i.e., stronger tolerance and dependence in the Spaced group, do not readily explain the finding that the Spaced group self-infused less ethanol (see above). Our passive phase dosing procedure, which linked changes in the daily infusion dose for each rat to its own level of intoxication, was specifically designed to match groups for intoxication and to avoid overdosing. Had we attempted to match groups for dose received by increasing the dose given to Massed group rats to the level received by Spaced group rats, we would most likely have induced substantial subject attrition due to lethal overdoses in the Massed group. Alternatively, we might have reduced the daily doses given to Spaced group rats to the levels received by Massed group rats. However, in that case, interpretation of the group difference in self-infusion would be confounded by a difference in the levels of intoxication achieved during passive exposure. Moreover, there is no reason for believing that a reduction in cumulative passive ethanol exposure in the Spaced group (which would be expected to reduce tolerance and dependence) would increase later self-infusion to the levels observed in the Massed group. Because the Massed procedure produced much stronger withdrawal than the Spaced procedure, our interpretation based on the level of dependence produced by each procedure seems more plausible.
Analysis of the S+ and S− lick data and S+ preference ratios in Experiment 1 showed that Spaced Group rats developed a strong aversion for the S+ flavor whereas Massed Group rats consumed both flavors equally. Although the groups in later studies—all of which can be viewed as receiving variations of the Massed procedure—tended to show a mild aversion for S+ on choice days, their relative intakes of S+ were still generally higher than those seen in the Spaced Group or in control groups in our previous self-infusion studies (Fidler et al., 2006). The foregoing observations raise the possibility that the Massed procedure produced greater ethanol self-infusion than the Spaced procedure because it produced greater tolerance to aversive drug effects that would otherwise condition a taste aversion to S+. The general trend toward a decrease in S+ preference ratios over days is also consistent with the suggestion that repeated pairings of the S+ flavor with IG ethanol gradually produced conditioned taste aversion.
The absence of an absolute preference for S+ over S− in our Massed IG groups contrasts with the finding of greater operant responding for oral ethanol than for water in ethanol vapor inhalation studies with rats (e.g., Funk & Koob, 2007). Although this difference might suggest that IG self-infusion of ethanol is inherently more aversive than oral self-administration of ethanol, there is no direct evidence to support this suggestion. Moreover, our previous study showed that IG infusion (of water) by itself does not produce a conditioned aversion to a paired flavor relative to a flavor that is not paired with infusion. One important difference between previous IG and vapor inhalation studies is that the IG studies have involved continuous (i.e., 23 h/day) access to self-infused ethanol in rats with no prior self-administration experience, whereas the vapor inhalation studies have typically involved limited access (e.g., 0.5–12 h/day) in rats with substantial self-administration experience under limited-access conditions. It seems possible that initial training to “binge” on ethanol during limited-access sessions coupled with the use of relatively short post-exposure test sessions increased the likelihood that the vapor inhalation studies would show an absolute preference for ethanol compared to procedures like ours in which ethanol is available continuously.
In Experiment 3, we found that rats given opportunities to self-infuse ethanol on alternate days during the last few days of passive exposure (Master Group) did not alter later self-infusion compared to rats that were passively exposed to the same ethanol doses on those days (Yoked Group). We had initially hypothesized that this early experience with control over ethanol intake might result in higher self-infusion by the Masters compared to their Yoked controls, but we saw no significant differences in daily intake. In fact, there was a trend in the opposite direction, supported by the finding that a significantly higher proportion of Yoked rats (83%) than Master rats (43%) self-infused more than 5 g/kg on at least one choice day. Although rats in both groups initially received 3 consecutive days of passive ethanol exposure (massed procedure), it is possible that the interpolated opportunities to self-infuse ethanol actually interfered with further development of dependence in Master rats compared to Yoked rats, thereby offsetting any advantage provided by the earlier opportunity to self-infuse. This suggestion is supported by previous research showing that yoked exposures to ethanol (Weise-Kelly & Siegel, 2001) or cocaine (Dworkin et al., 1995) produce greater impairment than self-administered drug, and the hypothesis that development of (non-associative) tolerance and dependence may be enhanced in animals that experience greater drug-induced “functional impairment” (Kalant et al., 1971).
The daily ethanol intakes achieved in the present studies (group means ranging from 4.1 to 7.9 g/kg/d) are notable because they generally exceeded the mean daily intakes typically reported in home cage 2-bottle choice drinking procedures for various inbred rat strains as well as the genetically heterogeneous N:NIH strain (Li & Lumeng, 1984). Moreover, our daily intakes were similar to the daily intakes previously reported during home-cage 2-bottle choice drinking or IG self-infusion by male rats selectively bred for high ethanol intake/preference (P rats; Murphy et al., 1988; Stewart & Li, 1997; Waller et al., 1984). Our daily intakes were also in the same range as those reported in a recent study that examined choice IG self-infusion procedure in Sprague-Dawley rats (5 g/kg/d using 24% v/v ethanol, Ackroff & Sclafani, 2001). Although the terminal choice phase of that study was procedurally similar to our choice phase, the rationales, training procedures and interpretations for these two studies are quite different. Whereas those investigators were primarily interested in the ability of ethanol infusions to induce conditioned preference to a paired flavor solution, we were primarily interested in the impact of chronic ethanol exposure on ethanol self-infusion in a choice procedure. Moreover, in contrast to the relatively short period of passive exposure used in our studies (5 d), their training phase was quite lengthy (50+ d), included a period of training under food restriction (19 d) and involved extensive exposure to an ethanol concentration (6%) and daily doses that were much lower than those used in our studies. Finally, whereas our data suggest that the enhancement of ethanol intake was likely related to induction of dependence, their outcome seems better explained by ethanol’s nutritive value rather than by its pharmacological effects.
Overall, our studies add to a growing literature that shows that chronic ethanol exposure—in this case, via passive IG infusions of ethanol—can enhance subsequent self-administration of ethanol. Importantly, our data provide new information on a critical variable, namely, the periodicity of chronic ethanol exposure. Previous research using the ethanol vapor exposure procedure has shown that continuous vapor exposure is less effective than intermittent exposure for enhancing later self-administration when intermittency is defined as alternating periods of ethanol vs. no-ethanol within each 24-h period across several consecutive days of exposure (Lopez & Becker, 2005; O’Dell et al., 2004). Consistent with our previous studies using the IG model (Fidler et al., 2006), the present studies also showed elevated self-administration when consecutive days of passive exposure contained alternating periods of ethanol and no-ethanol (Massed procedure). Our novel finding is that the enhancing effect of passive ethanol exposure was substantially diminished when the “off” time between periods of ethanol intoxication was extended from 12 to 36 h (Spaced Group). Based on our withdrawal data, this outcome may be best explained by proposing that the longer recovery between consecutive periods of intoxication interfered with development of dependence. When considered together with the vapor exposure data, our data suggest that the optimal schedule for passive ethanol exposure may be one that involves an ethanol-free interval of intermediate-duration (8–12 h) between periods of intoxication across consecutive days, presumably because very short (i.e., nearly continuous ethanol exposure) or very long ethanol-free intervals interfere with development of dependence.
Acknowledgments
This research was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism, NIH (U01-AA13479-INIA Project). Data from Experiment 1 were presented in a poster at the Research Society on Alcohol meeting in 2004. A special thanks is extended to Matthew Powers for his assistance in data checking.
References
- Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric infusion of ethanol in rats. Pharmacol Biochem Behav. 2001;68(2):327–38. doi: 10.1016/s0091-3057(00)00467-6. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28(12):1829–38. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Begleiter H. Ethanol consumption subsequent to physical dependence. Adv Exp Med Biol. 1975;59:373–8. doi: 10.1007/978-1-4757-0632-1_26. [DOI] [PubMed] [Google Scholar]
- Bruning J, Kintz B. Computational Handbook of Statistics. Scott Foresman and Company; Glenview, IL: 1968. [Google Scholar]
- Chester JA, Price CS, Froehlich JC. Inverse genetic association between alcohol preference and severity of alcohol withdrawal in two sets of rat lines selected for the same phenotype. Alcohol Clin Exp Res. 2002;26(1):19–27. [PubMed] [Google Scholar]
- Deutsch JA, Cannis JT. Rapid induction of voluntary alcohol choice in rats. Behav Neural Biol. 1980;30(3):292–8. doi: 10.1016/s0163-1047(80)91174-7. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Hardy WT. Ethanol tolerance in the rat measured by the untasted intake of alcohol. Behavioral biology. 1976;17(3):379–89. doi: 10.1016/s0091-6773(76)90727-6. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Koopmans HS. Preference enhancement for alcohol by passive exposure. Science. 1973;179(79):1242–3. doi: 10.1126/science.179.4079.1242. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Walton NY. Diazepam maintenance of alcohol preference during alcohol withdrawal. Science. 1977;198(4314):307–9. doi: 10.1126/science.561997. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Walton NY. A rat alcoholism model in a free choice situation. Behavioral biology. 1977;19(3):349–60. doi: 10.1016/s0091-6773(77)91712-6. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the Extended Amygdala in C57BL/6J Mice Do Not Block the Intermittent Ethanol Vapor-Induced Increase in Ethanol Consumption. Alcohol Clin Exp Res. 2008;32(2):197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl) 1995;117(3):262–6. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Clews TW, Cunningham CL. Reestablishing an intragastric ethanol self-infusion model in rats. Alcohol Clin Exp Res. 2006;30(3):414–28. doi: 10.1111/j.1530-0277.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31(6):939–49. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–8. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26(44):11324–32. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61(1):78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Lumeng L, Koob GF. Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol Clin Exp Res. 2008;32(9):1688–96. doi: 10.1111/j.1530-0277.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. Rates of onset and decay of alcohol physical dependence in mice. J Pharmacol Exp Ther. 1974;190(2):377–83. [PubMed] [Google Scholar]
- Hardy WT, Deutsch JA. Preference to ethanol in dependent rats. Behavioral biology. 1977;20(4):482–92. doi: 10.1016/s0091-6773(77)91098-7. [DOI] [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE, Gibbins RJ. Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacol Rev. 1971;23(3):135–91. [PubMed] [Google Scholar]
- Le Magnen J, Marfaing-Jallat P. Further study of induced behavioral dependence on ethanol in rats. Alcohol. 1984;1(4):269–73. doi: 10.1016/0741-8329(84)90048-x. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L. Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcohol Clin Exp Res. 1984;8(5):485–6. doi: 10.1111/j.1530-0277.1984.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181(4):688–96. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacology (Berl) 1975;43(3):245–54. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Marfaing-Jallat P, Le Magnen J. Induction of high voluntary ethanol intake in dependent rats. Pharmacol Biochem Behav. 1982;17(4):609–12. doi: 10.1016/0091-3057(82)90331-8. [DOI] [PubMed] [Google Scholar]
- Marfaing-Jallat P, Le Magnen J. Relationship between initial sensitivity to ethanol and the high alcohol intake in dependent rats. Pharmacol Biochem Behav. 1985;22(1):19–23. doi: 10.1016/0091-3057(85)90479-4. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Waller MB, Gatto GJ, McBride WJ, Lumeng L, Li TK. Effects of fluoxetine on the intragastric self-administration of ethanol in the alcohol preferring P line of rats. Alcohol. 1988;5(4):283–6. doi: 10.1016/0741-8329(88)90066-3. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28(11):1676–82. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20(7):1289–98. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22(6):581–94. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Crabbe JC. Genetic analysis of rapid tolerance to ethanol’s incoordinating effects in mice: inbred strains and artificial selection. Behav Genet. 2004;34(4):441–51. doi: 10.1023/B:BEGE.0000023649.60539.dd. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Hyytiä P, Heinrichs SC, Koob GF. Effects of chronic ethanol exposure on oral self-administration of ethanol or saccharin by Wistar rats. Alcohol Clin Exp Res. 1996;20(1):164–71. doi: 10.1111/j.1530-0277.1996.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Li TK. The neurobiology of alcoholism in genetically selected rat models. Alcohol health and research world. 1997;21(2):169–76. [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26(10):1494–501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31(1):11–8. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33(3):643–52. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42(2):91–7. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Gatto GJ, Lumeng L, Li TK. Intragastric self-infusion of ethanol by ethanol-preferring and - nonpreferring lines of rats. Science. 1984;225(4657):78–80. doi: 10.1126/science.6539502. [DOI] [PubMed] [Google Scholar]
- Weise-Kelly L, Siegel S. Self-administration cues as signals: drug self-administration and tolerance. Journal of experimental psychology Animal behavior processes. 2001;27(2):125–36. [PubMed] [Google Scholar]