Abstract
Purpose
Telomerase activity is one of the hallmarks of cancer and is a highly relevant therapeutic target. The effects of a novel human telomerase antagonist, imetelstat, on primary human glioblastoma (GBM) tumor-initiating cells were investigated in vitro and in vivo.
Experimental design
Tumor-initiating cells were isolated from primary GBM tumors and expanded as neurospheres in vitro. The GBM tumor-initiating cells were treated with imetelstat and examined for the effects on telomerase activity levels, telomere length, proliferation, clonogenicity and differentiation. Subsequently, mouse orthotopic and subcutaneous xenografts were used to assess the in vivo efficacy of imetelstat.
Results
Imetelstat treatment produced a dose-dependent inhibition of telomerase (IC50 0.45μM). Long-term imetelstat treatment led to progressive telomere shortening, reduced rates of proliferation and eventually cell death in GBM tumor-initiating cells. Imetelstat in combination with radiation and temozolomide had a dramatic effect on cell survival and activated the DNA damage response pathway. Imetelstat is able to cross the blood brain barrier in orthotopic GBM xenograft tumors. Fluorescently labeled GBM tumor cells isolated from orthotopic tumors, following systemic administration of imetelstat (30 mg/kg q3d) showed ∼70% inhibition of telomerase activity. Chronic systemic treatment on the same dose schedule produced a marked decrease in the rate of xenograft subcutaneous tumor growth.
Conclusion
This pre-clinical study supports the feasibility of testing imetelstat in the treatment of GBM patients, alone or in combination with standard therapies.
Keywords: neurospheres, telomeres, temozolomide, radiation, orthotopic
Introduction
Malignant gliomas are highly invasive and neurologically destructive tumors, and are considered among the deadliest types of human cancer (1). In glioblastoma multiforme (GBM) the prognosis remains poor with two-year survival of less than 5% (2). Improvements in surgery, radiation and chemotherapy have seen modest gains in long-term survival, measured in months. With the current standard of care, which includes maximal surgical resection (when possible), localized radiation (50-60Gy) and chemotherapy (temozolomide), the median survival is only 14.6 months (3).
Recent genomic studies have unveiled a highly complex picture of chromosomal amplifications and deletions, genetic mutations and epigenetic modifications which underscore the malignant behavior of GBM (4-6). Despite these significant advances in our basic understanding of GBM, the overall response rates to molecular targeted therapies have been disappointing (7), with perhaps the only exception being anti-angiogenesis inhibitors (8). In addition to identifying novel therapeutic targets for a malignancy with a highly disorganized genome, there is also recent evidence showing that rare populations of GBM cells possess an inexhaustible ability to self-renew and proliferate (9-11). Operationally defined as cancer stem cells (or tumor-initiating cells), such cells may be especially resistant to conventional chemotherapies and ionizing radiation (12). There is considerable controversy over whether a single cell marker (such as CD133, Prominin 1) can prospectively identify the tumor-initiating population in every tumor (13, 14). However, there is general agreement that GBM tumors cells which can be propagated in vitro as non-adherent neurospheres and produce intracranial tumors retain the genotype and phenotype of the patient's original tumor (15, 16). As such, effective therapies for GBM may benefit from targeting specifically these cells to achieve a more durable tumor response.
Re-activation of telomerase activity is perhaps the single most consistent feature of the cancer phenotype, representing an almost obligate requirement for tumor growth, and therefore represents a potentially effective cancer therapeutic target (17). Normal brain tissues do not express telomerase activity (18-21), with the exception of a small population of neural stem cells which may persist in the adult human brain (22). Several other reports have established a correlation between telomerase activity and histological grade of gliomas (23, 24). Over the past decade, an extensive body of basic research into the mechanisms of telomere regulation has lead to the identification of telomerase inhibitors (25) which may provide an effective, almost universal, cancer therapeutic strategy.
Imetelstat (GRN163L, Geron Corporation, Menlo Park CA), is a short chain oligonucleotide with high affinity and specificity for the template region of the RNA component of telomerase (hTR or hTERC). Imetelstat inhibits telomerase activity and has shown a highly favorable pharmacokinetic and minimal side-effect profile in early Phase I clinical trials. The chemical makeup of this oligonucleotide (thio-phosphoramidate) confers high resistance to nuclease digestion in blood and tissues, and due to its 5′ lipid chain (palmitoyl); the molecule has excellent cellular and tissue penetration and retention properties (26). Importantly, imetelstat is a competitive telomerase antagonist (not antisense that targets mRNA), and its mechanism of action is competitive with telomere binding leading to inhibition of telomerase and progressively telomere shortening.
Here we report that imetelstat produces a dose-dependent, reversible inhibition of telomerase activity in primary GBM tumor-initiating cells. Long-term exposure of primary GBM neurosphere cultures to imetelstat produced telomere attrition, growth arrest and eventually cell death. When administered intraperitoneal at clinically relevant doses, imetelstat was able to cross the blood brain barrier and block telomerase activity of orthotopic human GBM xenograft tumors in nude mice. In addition, it produced a marked decrease in the rate of subcutaneous xenograft tumor growth. Taken together our results strongly support the feasibility of using of imetelstat in the treatment of GBM patients.
Materials and Methods
Isolation of GBM tumor-initiating cells
Tumor samples were obtained from consenting patients at the University of Texas Southwestern Medical Center (Dallas, TX) with the approval of the Institutional Review Board. Tumor tissues were dissociated, then cultured at clonal density in serum-free defined media and/or labeled with a CD133 antibody (293C3-PE, Miltenyi Biotec, Auburn CA) for subsequent sorting with a FACS Calibur apparatus (BD Biosciences, San Jose, CA). The GBM primary cells were maintained as non-adherent neurospheres in a humidified atmosphere (5% CO2, 37 °C) in a chemically defined serum-free Dulbecco's modified Eagle's medium/F-12 medium (Cellgro, Manassas VA), consisting of human recombinant EGF (20 ng/ml; Sigma), bFGF (20 ng/ml; Upstate), B27 supplement (1×; Invitrogen, Carlsbad, CA), Insulin-Transferrin-Selenium-X (1×; Invitrogen, Carlsbad, CA), Penicillin-Streptomycin (100 units/ml, 100 μg/ml; HyClone, Logan, UT).
Estimation of telomerase activity and telomere lengths
The Telomeric Repeat Amplification Protocol (TRAP) was used to measure the telomerase activity (TRAPeze kit, Chemicon, Temecula CA) according to manufacturer's instructions. The telomerase products (6-bp ladder) and the 36-bp internal control (ITAS) bands were quantified using the AlphaImager 2000 software (Alpha Innotech Corporation, San Leandro CA). Relative telomerase activity (RTA) was calculated as the intensity ratio of the TRAP ladder to that of the ITAS band (relative intensity of each sample was normalized to that of the positive control).
Total DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen Sciences, MD). One microgram of total DNA was used for the Terminal Restriction Fragment (TRF) assay as previously described (27). The gel was exposed to a Phosphor screen overnight and analyzed using the Typhoon Trio Variable Mode Imager (Amersham Biosciences, Piscataway NJ).
Imetelstat treatment and proliferation assays
Neurospheres were passaged every 7 days to ensure that their size did not exceed the limit of diffusion (<150μm) and/or to refresh growth factor supplemented media. Imetelstat and mismatch control oligonucleotide (5′-palm-TAGGTGTAAGCAA-NH2-3′) were provided by Geron Corporation (Menlo Park, CA). For the viability assay of cells treated with imetelstat for extended periods of time, the Live/Dead kit (Invitrogen, Carlsbad, CA) was used according to the manufacturer's recommendations.
The proliferation assays were performed in 96-well tissue culture plates (BD Falcon, Bedford, MA) using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide; Sigma-Aldrich, St. Louis MO]. The MTT formazan produced in wells was solubilized with isopropanol 0.04 N HCl. Absorbance was measured on a Bio-Rad 680 Microplate Reader (Bio-Rad, Herkules CA) at a test wavelength of 570 nm and a reference wavelength of 630 nm. All proliferation experiments were performed in triplicate.
Mice xenografts experiments
In order to avoid contamination with host mouse cells and to non-invasively monitor intracranial tumor growth, we stably expressed mCherry red fluorescent protein and fire-fly luciferase (a gift from Dr. Tomoyuki Mashimo) in the GBM cells. 1 × 105 GBM tumor cells were stereotactically injected into the right caudate of 6-week-old nude mice. For the imetelstat experiments, each animal received the same total dose, but using a different schedule: (a) 30 mg/kg every 3 days; (b) 30 mg/kg every 2 days and (c) 30 mg/kg each day. The mice which were clinically symptomatic for an intracranial mass were sacrificed by an overdose of anesthetic. The tumor was excised, dissociated, washed and mCherry positive, live cells were gated from controls and imetelstat treatment groups on a Becton-Dickinson FACS Calibur (BD Biosciences, San Jose, CA). For the subcutaneous xenografts, up to one million GBM tumor cells were injected into both flanks of 6- to 8-week-old NOD-SCID mouse. The mice with subcutaneous tumors were separated in two cohorts (3 mice each), vehicle and imetelstat. For both cohorts, imetelstat treatment was initiated when tumor volume reached (3-4mm3).
Orthotopic tumor histopathology and in situ hybridization
Mice brains were fixed in 4% paraformaldehyde for 12 hours then cut in 5μm sections for haematoxylin and eosin (H&E) staining. Chromogenic in situ hybridization (CISH) for the EGFR gene was performed using a commercial probe and detection kit (SP-T Light 84-1300; Zymed, Carlsbad, CA). The primary antibodies and their dilutions were as follows: Nestin (1:1,000; Abcam, Cambridge, MA), Clusterin (B5) (1:800; Santa Cruz Biotechnology, Santa Cruz, CA), Ki-67 (prediluted; Ventana, Tucson, AZ), GFAP (1:400; PP 040; Biocare Medical, Concord, Ca) and EGFR (prediluted; PharmDx; Dako, Carpinteria, CA). All immunostains were performed in a Benchmark XT stainer (Ventana, Tucson, AZ) using the CC1 pretreatment solution (95°C for 32 min; Ventana, Tucson, AZ) and XT-UltraView Universal DAB detection system (Ventana, Tucson, AZ). The sections were counterstained lightly with hematoxylin.
Bioluminescent imaging (BLI)
BLI of mice was performed using the IVIS Lumina System (Xenogen Corp., Alameda CA) coupled to the LivingImage data acquisition software (Xenogen Corp.). D-luciferin (450 mg/kg in PBS in a total volume of 250μl; Biosynthesis, Naperville, IL) was administered subcutaneous in the neck region, images were acquired between 10 and 20 minutes post-luciferin administration and peak luminescent signal was recorded. The BLI signals emanating from the tumors were quantified by measuring photon flux within a region of interest using the LivingImage software package.
Results
Characterization of primary GBM tumor-initiating cells
In order to avoid the shortcomings associated with a single strategy of tumor-initiating cells isolation from primary tumors, we used three most common methods of enrichment: neurosphere formation, sorting based on the CD133 marker and serial transplantation in vivo. Next, we tested the ability of these individual GBM neurospheres cells to produce intracranial tumors in nude mice. Orthotopic tumors were established by injecting 5 × 105 GBM tumor cells into the right striatum and the mice were monitored clinically and by MR imaging. Focal neurological deficits consistent with an expanding intracranial mass were confirmed by MRI scans (T1+contrast / T2) (Figure 1). Routine histological analysis of the orthotopic tumors showed pathonemonic GBM features, including high MiB1 index (Ki67+), nuclear atypia, diffuse infiltration and modest angiogenesis (Figure 1). One of the populations of neurospheres (initially enriched for CD133+ cells by FACS) was chosen for all the subsequent studies, based on proliferation, tumor-initiating capacity and ability to induce orthotopic xenografts similar if not identical to the human disease. To demonstrate that these GBM tumor-initiating cells were capable of multilineage differentiation, tumor cells were exposed to 2% fetal calf serum for one week, as reported previously (9, 11). Quantitative RT-PCR results showed a marked down-regulation of neuronal (MAP2, NeuroD) and up-regulation of mature astrocyte (Clusterin, GFAP) genes, while down-regulating CD133 (Supplemental Figure 1A). The results confirmed that the GBM tumor-initiating cells maintained under neurosphere culture conditions expressed a neural stem cell-like phenotype and are capable of differentiation.
Figure 1. Isolation and characterization of primary GBM tumor-initiating cells.
Fresh primary GBM samples were dissociated and propagated as non-adherent neurospheres in serum-free defined media. Orthotopic injection with dissociated neurosphere cells (5 × 104/mouse) produced a neurologically symptomatic, T1-contrast enhancing tumor mass following a latency of 8-10 weeks. Histopathological features of the tumor were consistent with high-grade glioma, including GFAP positivity, diffuse infiltration, high MiB-1 index (Ki67 positive), and areas of neovascularization. Immunohistochemistry analysis confirmed EGFR over-expression. Chromagen-based in-situ hybridization (CISH) indicated increased EGFR copy number in the orthotopic tumors. Orthotopic tumors also showed a heterogeneous mix of nestin-positive tumor cells (neural stem/progenitor marker) intermingled with clusterin-positive cells (astrocyte marker).
GBM tumor-initiating cells treated with imetelstat show a dose-dependent and reversible inhibition of telomerase activity
Having confirmed that the primary GBM cell line possessed tumor-initiating cells properties, we proceeded to test the telomerase antagonist imetelstat. Telomerase expression levels in the GBM tumor-initiating cells was similar to the HeLa cells used as positive controls (Figure 2A). By contrast, normal adult human brain tissue, isolated from temporal lobe resections, had no detectable telomerase activity (data not shown).
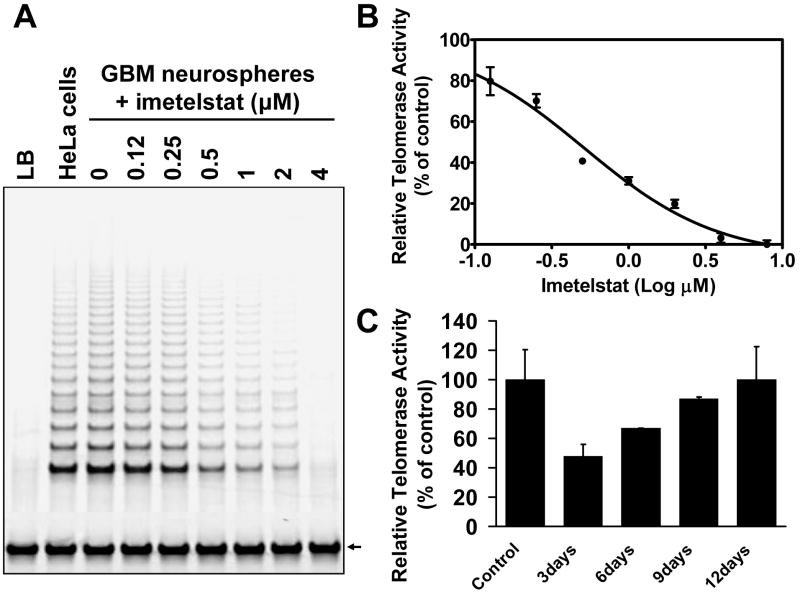
Figure 2. Imetelstat inhibits telomerase activity in GBM tumor-initiating cells in a dose-dependent manner which is reversed upon drug removal.
A. TRAP gel of the GBM neurospheres treated with various doses of imetelstat; B. IC50 of telomerase activity inhibition is 0.45μM for this specific GBM tumor-initiating line; C. TRAP activity recovers to normal levels after imetelstat removal from the media. The cells were treated with imetelstat for 72 hours then cultured in drug-free media and samples were collected for TRAP every three days. Equal numbers of cells were used for the TRAP assay. HeLa cells lysate was used as a positive control; the bars represent average data from three independent TRAP assays. The internal telomerase amplification starndard (ITAS) used for quantitation is indicated by an arrow.
Imetelstat inhibits telomerase activity of GBM tumor-initiating cells in vitro in a dose-dependent fashion (Figure 2A and 2B). At a dose of 0.45μM, imetelstat produced 50% inhibition of telomerase activity and ∼100% inhibition at 4μM (Figure 2B). In contrast, the imetelstat mismatch control oligonucleotide had no effect on the levels of telomerase activity even at doses of up to 4μM (data not shown). The inhibition of telomerase activity produced by imetelstat was found to be entirely reversible following drug removal from the culture media over a period of 12 days (Figure 2C).
Prolonged telomerase inhibition leads to telomere shortening, progressive growth arrest and eventual cell death
To test the effects of imetelstat on cell proliferation, GBM tumor-initiating cells were passaged in the presence of 2μM imetelstat. Over the first 4 weeks (∼4 population doublings) imetelstat treated and imetelstat-mismatch cultures showed no significant difference in proliferation, but a significant decrease in their clonogenic ability (Figure 3A). Thereafter, imetelstat-exposed cells showed a progressive decrease in the rate of proliferation, paralleled by a decrease in their capacity to form neurospheres when plated at low density (Figure 3B). After approximately 20 population doublings (24 weeks continuous imetelstat exposure), dissociated single tumor cells produced very small (<50μm) neurospheres which were ragged in appearance and largely positive for ethidium homodimer-1, indicating disrupted membrane integrity associated with ensuing cell death (see Figure 3B). In contrast, all three replicates of control cultures continued to proliferate normally.
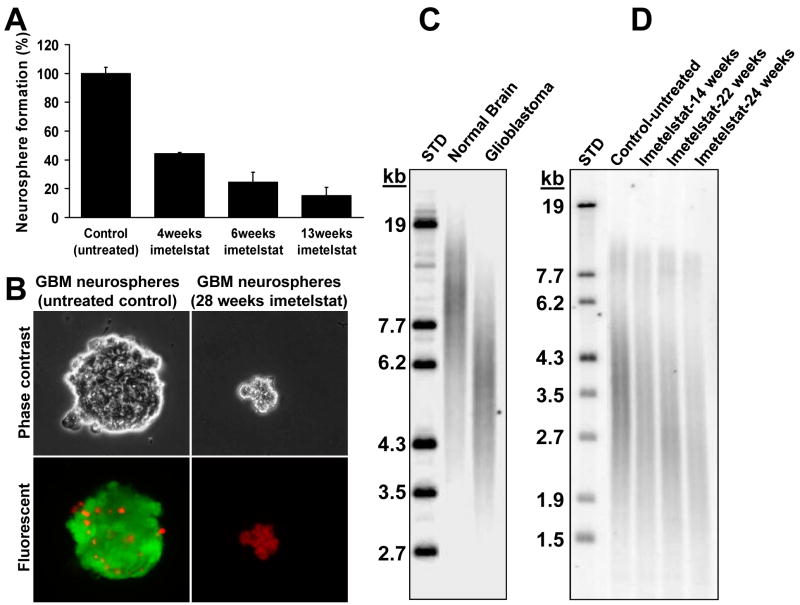
Figure 3. Long-term treatment with imetelstat in vitro leads to decrease clonogenic capacity, telomere shortening and cell death.
A. Clonogenic neurosphere formation assay of GBM tumor-initiating cells exposed to imetelstat. A total of 500 cells were plated on 10cm dishes, fresh media was exchanged after 5 days and the neurospheres were counted after 10-14 days; B. Live/dead assay on the long term-treated cells versus the untreated controls. Green cells - Calcein AM (live cells), red cells - ethidium homodimer (necrotic cells); C. TRF gel shows that GBM tumor cells have short telomeres (average about 6kb) compared to normal brain (∼10-12kb); D. TRF analysis of another primary glioma that has even shorter initial average telomere length (∼3.5-4kb) indicates progressive telomere shortening in GBM tumor-initiating cells treated in vitro 2×/week with 2μM imetelstat.
The experimental data confirmed that the telomere lengths of GBM tumors are shorter than normal brain telomeres (Figure 3C). The TRF (telomere length) blot indicated that GBM tumor-initiating cells also have short telomeres (∼3.5kb), with two distinct subpopulations (Figure 3D). The two distinct populations of telomeres (short and long) represent variations of telomere lengths in the same cell. This was confirmed by isolating several clones and each clone had a similar TRF pattern indicating that one or a few telomeres are longer in these cells (data not shown) and with imetelstat treatment all telomeres show progressive shortening. The average telomere length of long-term imetelstat treated GBM tumor-initiating cells showed a marked decline from ∼3.5 kilobases to <2.0 kilobases (Figure 3D), while oligonucleotide mismatch-treated cultures showed no decline after 28 weeks of continuous treatment (data not shown). These results suggest that telomerase inhibition of rapidly expanding populations of GBM tumor cells exposed to imetelstat caused progressive telomere shortening leading to cell cycle arrest and cell death.
Because telomere shortening and/or loss of telomerase activity in neuronal progenitors has been reported to promote neuronal differentiation (28, 29), we tested whether prolonged imetelstat exposure could trigger the differentiation of GBM tumor-initiating cells and as a result, lead to cell cycle arrest/cell death. Systematic and quantitative analysis of multiple lineage specific markers, including markers for stem/progenitor cells (CD133, Nestin), neuronal lineage (Tuj1, Map2, NeuroD) and astrocyte markers (GFAP, Clusterin) showed no evidence of differentiation (Supplemental Figure 1B) as a result of imetelstat treatment.
Addition of imetelstat to radiation and temozolomide increases therapeutic efficacy in vitro
Because the current standard of care for GBM patients (post resection) is ionizing radiation and temozolomide, we tested whether imetelstat had any additive or synergistic effects when combined with these therapy regimens. GBM tumor-initiating cells which had been treated with imetelstat for 16 weeks showed a marked decrease in the rate of proliferation, due to progressive telomere shortening. The effect of imetelstat on these cells was further enhanced by treatment with temozolomide and irradiation (Figure 4A) suggesting that addition of imetelstat to the standard of care may have some added therapeutic benefits.
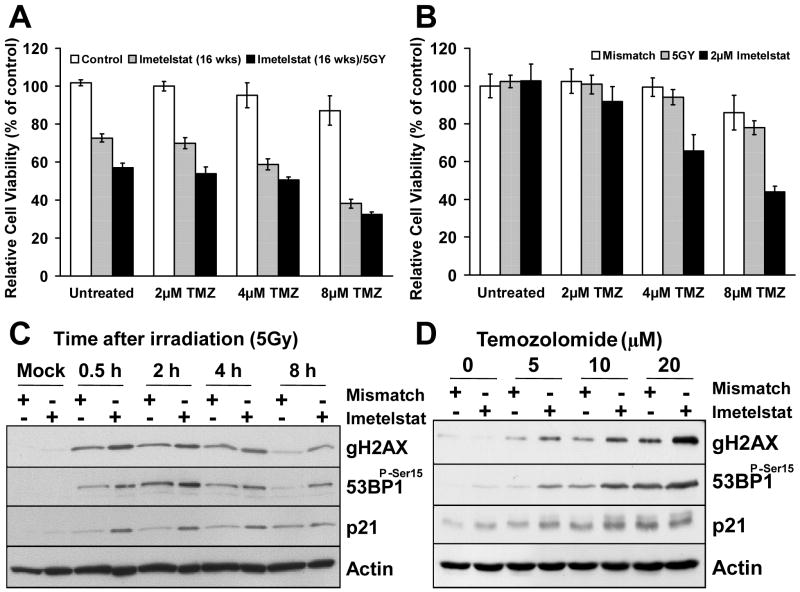
Figure 4. Radiation therapy and temozolomide are more efficient on GBM tumor-initiating cells when used in combination with imetelstat.
A. Cell survival assay (MTT) performed on cells treated with the telomerase inhibitor drug (16 weeks), TMZ and irradiation (5Gy). The data was normalized to the control, oligonucleotide mismatch-treated cells; B. Addition of TMZ to tumor cells pretreated with imetelstat for 72 hours produced a marked impact on tumor cell viability (P<0.001), as indicated by the MTT assay. Treatment with TMZ alone or in combination with 5Gy irradiation produced only a small decrease in tumor cell proliferation. C. Western blot of GBM tumor-initiating cells pre-treated with imetelstat (72hrs) followed by IR (5Gy) indicated a effect on DNA double strand breaks as measured by increased γH2AX (Ser139) and 53BP1 phosphorylation; D. Western blot of GBM tumor-initiating cells treated with a combination of imetelstat and TMZ (5, 10 and 20μM) showed increased activation of γH2AX and 53BP1 phosphorylation compared to the mismatch controls.
Short-term treatment (72h) with imetelstat in combination with temozolomide also led to significant cytotoxicity in GBM tumor-initiating cells, but 5Gy irradiation had little impact on cells survival or proliferation (Figure 4B). The magnitude and rate of DNA double-strand breaks repair was quantitatively assessed by western blot analysis of γH2AX and 53BP1 phosphorylation. GBM tumor-initiating cells irradiated with a total dose of 5Gy in the presence and absence of imetelstat showed peak γH2AX and 53BP1 phosphorylation at 30-120 minutes and near complete recovery by 8 hours (Figure 4C). The levels of these proteins were significantly higher in the cells treated with imetelstat compared to the mismatch controls. Similarly, treatment with TMZ over a dose-range of 5, 10 and 20μM also showed marked activation of γH2AX and 53BP1 phosphorylation in the imetelstat-treated samples (Figure 4D).
Imetelstat penetrates the blood-brain barrier (BBB) and inhibits telomerase activity in a GBM orthotopic glioblastoma model
To accurately estimate telomerase activity in orthotopic GBM tumor cells following systemic treatment with imetelstat, we used tumor cells which were transduced with a fire-fly luciferase reporter as well as monomeric cherry (Figure 5A and 5B). We first established that in our orthotopic mouse tumors (Supplemental Figure 2A and 2B) the microvasculature in regions of infiltrating tumor cells continued to express tight junction associated proteins ZO-1 and occludin (Supplemental Figure 2C and 2D), which are critical for maintaining the BBB (30). Double labeling with CD31 and smooth muscle antigen (SMA) was used as an indicator that capillaries surrounded by infiltrating tumor cells retained normal pericyte coverage (Supplemental Figure 2E). We also established that tumor-associated capillaries maintain normal astrocyte-foot process coverage by detecting a normal pattern of aquaporin-4 (AQP4) immunofluorescence, which is exclusively localized to the astrocytic processes (Supplemental Figure 2C and 2F). These results strongly indicate that the histopathology of these orthotopic GBM tumor cells was a near phenocopy of the clinical disease in humans, showing how diffusely infiltrating tumor cells can co-opt normal brain microcirculation which severely limits penetration of most chemotherapeutic approaches.
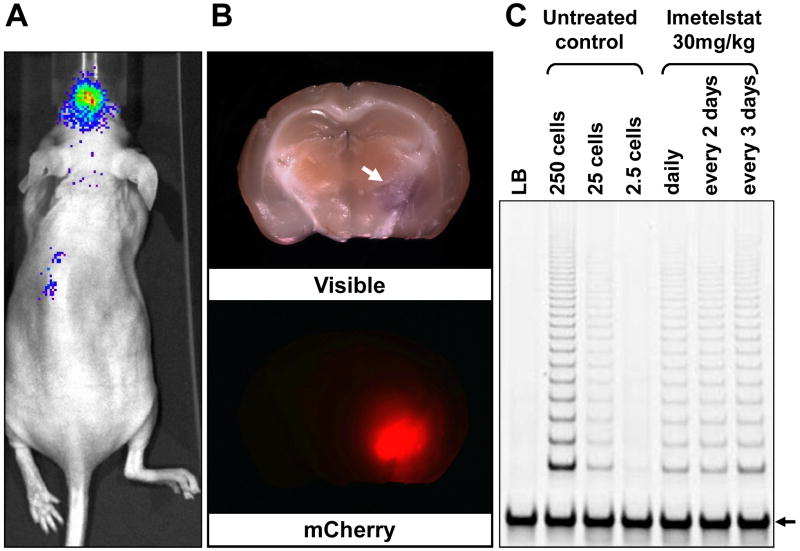
Figure 5. Imetelstat penetrates the blood-brain barrier and inhibits telomerase activity in orthotopic xenograft tumors.
A. Bioluminescent imaging of mCherry/Luc-labeled GBM tumor-initiating cells implanted orthotopically in the brain of nude mice; B. Macroscopic pathology of the mouse brain with the orthotopic tumor indicated by a white arrow. Fluorescent image of the mouse brain shows strong mCherry expression in tumor cells; C. TRAP assay of cells isolated by FACS from intracranial tumors of mice treated with imetelstat. Cell lysates corresponding to 250 cells were used for the imetelstat-treated samples. The internal telomerase amplification standard (ITAS) control is indicated by an arrow.
The intracranial bioluminescence signal was used to monitor the overall orthotopic tumor size. At the end of each treatment schedule the mice were sacrificed and mCherry positive cells were isolated. At approximately 50% of the maximum tolerated intracranial tumor size (based on pilot studies), the mice were treated with 30mg/kg imetelstat by an intraperitoneal injection using three different dosing schedules (see Materials and Methods). Telomerase activity was inhibited by 60-70% within 3-5 days, and no significant differences were observed between these three different dosing schedules (Figure 5C). These results compare favorably with subcutaneous xenografts of GBM tumor cells treated with the same total dose of imetelstat which showed similar telomerase inhibition (data not shown). The high levels of telomerase inhibition indicate that imetelstat can penetrate the BBB and induce significant inhibition of telomerase activity in brain tumor xenografts.
In vivo glioblastoma model shows inhibition of tumor growth in response to imetelstat treatment
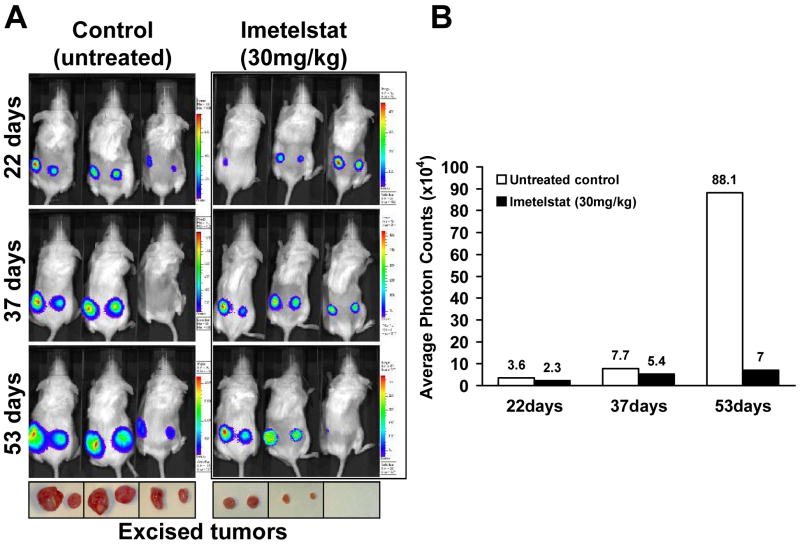
To test the effect of imetelstat on tumor growth in vivo, we established subcutaneous tumors in mice. Imetelstat led to a significant decrease in tumor size compared to the vehicle only treated group (Figure 6A). Based on the BLI data, there was more than a ten-fold difference in the average signal intensity between the imetelstat-treated animal group and the control group at the end of the 53 days of treatment (Figure 6B). At the termination of the experiment (based on tumor volume and animal care policies), the tumors were excised and measured (by caliper) in their greatest length and width (Figure 6A, bottom). The average tumor volume in the treated animals was more than ten-fold lower compared to the control animals (data not shown). The growth rate of GBM subcutaneous tumors in the imetelstat cohort was significantly lower compared to vehicle (Figure 6B). Taken together, these results suggest that imetelstat could be highly effective in reducing GBM tumor growth.
Figure 6. Imetelstat treatment inhibits tumor growth in mice with subcutaneous tumors.
A. Bioluminescent imaging of the control and treatment group: Mice were injected intraperitoneally with imetelstat twice a week (30 mg/kg) after the tumors became detectable. The tumors were excised at the end of treatment and are displayed below the corresponding animals; B. Chart representing the average photon counts from treated and untreated animals.
Discussion
Telomerase activity is a strong indicator of cellular malignancy (17), and in GBM tumors, high levels of telomerase expression correlate with tumor progression and poor prognosis (19-21, 31). Here we report that a novel human telomerase antagonist, imetelstat, is a potent inhibitor of telomerase activity in primary human GBM tumor-initiating cells. The ability to target these crucial subpopulations of cells that evade conventional and targeted therapies could be a significant step in developing effective strategies for GBM treatment.
For this study we used primary GBM tumor-initiating cells which produce orthotopic tumors with accurate GBM histopathology. The isolated tumor-initiating cells were also cultured as non-adherent neurospheres, conditions which maintain their stem-like properties (11, 13, 32). Imetelstat produced a dose-dependent, reversible inhibition of telomerase activity over a wide dose range that persists for several days, raising the possibility that the pharmacokinetics may be well suited for the clinical setting. The negative impact of imetelstat on proliferation was not evident until after ∼15-20 population doublings, when progressive telomere attrition leads to the induction of DNA damage signaling, end-to-end fusions and genetic instability, processes that can lead to apoptotic cell death.
Because tumor cells share the same telomere elongation machinery with normal proliferative stem-like cells, one major concern associated with the use of a telomerase inhibitors is the potential decline of regenerative capacity in normal stem cells. Such an adverse possibility is of particular concern for the organs with high rates of cellular turnover and especially relevant in elderly patients. Because telomere shortening occurs in most human tissues during aging and is accelerated in response to chronic diseases (33), it is important to determine not only the telomere length in tumor-initiating stem cells but also in normal stem cells. The results of our present studies clearly show that the average telomere lengths of GBM tumor cells are approximately three times shorter compared to normal human brain cells (∼10 vs. 3.5 kb). In principle, assuming equivalent rates of proliferation, this difference offers an ample therapeutic window to cause malignant tumor cells to undergo critical telomere shortening while telomeres in the normal stem cell compartment remain of adequate length. Moreover, following removal of imetelstat, telomerase activity rapidly recovers to normal levels which suggest that potential adverse telomere shortening in the normal stem cells may be reversible. Clearly, this is a significant advantage over conventional chemotherapies which often produce irreversible damage to all proliferative cells including stem cells of renewal tissues.
Recent studies have shown that combination of temozolomide (TMZ) and ionizing radiation (IR) have some therapeutic benefits. We explored whether addition of imetelstat would increase the therapeutic potential of these agents and demonstrate that GBM neurospheres cells treated with imetelstat for long periods of time are more sensitive to IR and TMZ (Figure 5A). While the additive effects seen with TMZ or IR and long-term imetelstat treated cultures were consistent with previous data on irradiated breast cancer cells (34), the effects of TMZ on short-term imetelstat-treated cells were surprising because this combination led to increased toxicity in the absence of telomere shortening (Figure 4B). This result appears to be specific for TMZ because the cells irradiated with a dose of 5Gy do not show significant cytotoxicity compared to the un-irradiated controls, but we cannot exclude the possibility that GBM cells subjected to other IR regimens may exhibit a similar response. One explanation for this mechanism of drug synergy may be that IR and TMZ induced telomeric DNA breaks which could not be repaired in the absence of telomerase, leading to an increased DNA damage response (Fig 5B and C). Another explanation is that treatment with imetelstat may activate an elevated autophagy response in GBM cells. It is documented that TMZ triggers the GBM cell death by autophagy (35, 36) and some reports suggest that GBM tumor-initiating cells are resistant to the TMZ-induced autophagy due to the down-regulation of critical proteins (37). Interestingly, the gene encoding one of these autophagy proteins (APG5) was found to be significantly up-regulated in myeloma cells treated with imetelstat (38). Moreover, it was shown that a conditionally replicating hTERT adenovirus can induce autophagic cell death in malignant glioma cells (39). Taken together, these data suggest that the telomere length-independent effects of imetelstat along with its primary effect on telomere elongation may be uniquely effective against GBM tumor-initiating cells.
One of the major challenges in brain tumor therapy remains the difficulty of delivering drugs across the blood-brain barrier (BBB). Building on previous biodistribution studies (40), our hypothesis was that imetelstat could penetrate the BBB and efficiently inhibit telomerase in orthotopic GBM tumors. Despite strong evidence of tight junctions, our data clearly shows for the first time that intraperitoneal-introduced imetelstat was able to penetrate the BBB in sufficient intra-parenchymal concentration to block ∼70% of telomerase activity in human GBM orthotopic xenograft cells.
The in vitro data indicated that GBM tumor-initiating cell must undergo at least 15-20 population doublings before significant telomere attrition may occur. Because in the orthotopic model 5 × 104 cells were injected into the brain, the total cell number following 20 population doublings would be 5 × 1010 cells, which would exceed the 1-2 mm3 maximum tumor mass an adult mouse cranium can accommodate without brainstem compression, herniation and death. For this reason, we used subcutaneous xenografts to investigate the in vivo effect of imetelstat on the tumor growth rate and size, because this model can accommodate much larger tumor volumes (2 cm3) without significant morbidity. Furthermore, imetelstat penetration data shows that telomerase inhibition of GBM tumors cells is very similar for the subcutaneous and intracranial tumors, therefore the subcutaneous xenograft model is more than adequate for a therapeutic proof of concept. Drug treatment was initiated once the subcutaneous tumors were clearly visible (∼1 mm) and the results show significant differences between the imetelstat- and vehicle-treated mice (Figure 6). Regular monitoring of tumor size by bioluminescence initially showed little difference between the treated and control cohorts, but as the tumor masses approached 25% of maximal tolerate size (500 mm3), imetelstat-treated tumors were showing a significantly slower tumor growth, presumably due to a subset of tumor cells having undergone the required number of population doublings for critical telomere shortening. Most GBM patients undergo aggressive de-bulking resection (unless contraindicated by tumor location or other co-morbidities) which will ensure that there is sufficient space to permit tumor growth and erosion of telomeres to critical levels that trigger cellular quiescence and/or cell death. We predict an even greater therapeutic efficacy and perhaps a durable response when imetelstat is combined with radiation and temozolomide, which currently provide only a partial response. Taken together, the in vitro and in vivo data is encouraging for pursuing testing of imetelstat in GBM patients
Statement of Translational Relevance
Glioblastoma is one of the most lethal human cancers and the chemotherapeutic options are still limited by the reduced capacity of drugs to penetrate the blood-brain barrier. Cancer relapse is believed to be caused by small populations of tumor-initiating cells which can escape conventional therapies. Imetelstat is a novel telomerase inhibitor that inhibits telomerase and induces telomere shortening in glioblastoma tumor-initiating cells in addition to the bulk tumor mass. This pre-clinical study demonstrates that imetelstat is a highly efficient and specific agent, both in vitro and in mouse orthotopic primary glioma xenografts. Imetelstat not only penetrates the blood brain barrier but also shows increased efficacy in combination with ionizing radiation and temozolomide, the current standard of care for glioblastoma. The experimental data supports the future implementation of imetelstat in clinical studies for glioblastoma.
Supplementary Material
Acknowledgments
We thank Geron Corporation (Menlo Park, CA) for providing the imetelstat drug used in this study. Supported in part by CA70907 and CA127297
Footnotes
C.O.M. and S.K.C. contributed equally to this research; R.M. B and J.W.S are co-senior contributors
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. The New England journal of medicine. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Buckner JC. Factors influencing survival in high-grade gliomas. Seminars in oncology. 2003;30:10–4. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Mulholland PJ, Fiegler H, Mazzanti C, et al. Cell cycle. Vol. 5. Georgetown, Tex: 2006. Genomic profiling identifies discrete deletions associated with translocations in glioblastoma multiforme; pp. 783–91. [DOI] [PubMed] [Google Scholar]
- 5.Marko NF, Toms SA, Barnett GH, Weil R. Genomic expression patterns distinguish long-term from short-term glioblastoma survivors: a preliminary feasibility study. Genomics. 2008;91:395–406. doi: 10.1016/j.ygeno.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 6.CancerGenomeAtlas. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes & development. 2007;21:2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 8.Jouanneau E. Angiogenesis and gliomas: current issues and development of surrogate markers. Neurosurgery. 2008;62:31–50. doi: 10.1227/01.NEU.0000311060.65002.4E. discussion -2. [DOI] [PubMed] [Google Scholar]
- 9.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–5. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 10.Dirks PB. Brain tumour stem cells: the undercurrents of human brain cancer and their relationship to neural stem cells. Philosophical transactions of the Royal Society of London. 2008;363:139–52. doi: 10.1098/rstb.2006.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 12.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 13.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer research. 2003;63:5821–8. [PubMed] [Google Scholar]
- 14.Wang J, Sakariassen PO, Tsinkalovsky O, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–8. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Tunici P, Bissola L, Lualdi E, et al. Genetic alterations and in vivo tumorigenicity of neurospheres derived from an adult glioblastoma. Molecular cancer. 2004;3:25. doi: 10.1186/1476-4598-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shay JW, Wright WE. Telomerase: a target for cancer therapeutics. Cancer cell. 2002;2:257–65. doi: 10.1016/s1535-6108(02)00159-9. [DOI] [PubMed] [Google Scholar]
- 18.DeMasters BK, Markham N, Lillehei KO, Shroyer KR. Differential telomerase expression in human primary intracranial tumors. American journal of clinical pathology. 1997;107:548–54. doi: 10.1093/ajcp/107.5.548. [DOI] [PubMed] [Google Scholar]
- 19.Le S, Zhu JJ, Anthony DC, Greider CW, Black PM. Telomerase activity in human gliomas. Neurosurgery. 1998;42:1120–4. doi: 10.1097/00006123-199805000-00099. discussion 4-5. [DOI] [PubMed] [Google Scholar]
- 20.Huang F, Kanno H, Yamamoto I, Lin Y, Kubota Y. Correlation of clinical features and telomerase activity in human gliomas. Journal of neuro-oncology. 1999;43:137–42. doi: 10.1023/a:1006258817785. [DOI] [PubMed] [Google Scholar]
- 21.Harada K, Kurisu K, Tahara H, Tahara E, Ide T, Tahara E. Telomerase activity in primary and secondary glioblastomas multiforme as a novel molecular tumor marker. Journal of neurosurgery. 2000;93:618–25. doi: 10.3171/jns.2000.93.4.0618. [DOI] [PubMed] [Google Scholar]
- 22.Ostenfeld T, Caldwell MA, Prowse KR, Linskens MH, Jauniaux E, Svendsen CN. Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Experimental neurology. 2000;164:215–26. doi: 10.1006/exnr.2000.7427. [DOI] [PubMed] [Google Scholar]
- 23.Langford LA, Piatyszek MA, Xu R, Schold SC, Jr, Shay JW. Telomerase activity in human brain tumours. Lancet. 1995;346:1267–8. doi: 10.1016/s0140-6736(95)91865-5. [DOI] [PubMed] [Google Scholar]
- 24.Falchetti ML, Larocca LM, Pallini R. Telomerase in brain tumors. Childs Nerv Syst. 2002;18:112–7. doi: 10.1007/s00381-002-0562-7. [DOI] [PubMed] [Google Scholar]
- 25.Harley CB. Telomerase and cancer therapeutics. Nature reviews. 2008;8:167–79. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 26.Herbert BS, Gellert GC, Hochreiter A, et al. Lipid modification of GRN163, an N3′-->P5′ thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene. 2005;24:5262–8. doi: 10.1038/sj.onc.1208760. [DOI] [PubMed] [Google Scholar]
- 27.Gellert GC, Dikmen ZG, Wright WE, Gryaznov S, Shay JW. Effects of a novel telomerase inhibitor, GRN163L, in human breast cancer. Breast cancer research and treatment. 2006;96:73–81. doi: 10.1007/s10549-005-9043-5. [DOI] [PubMed] [Google Scholar]
- 28.Kruk PA, Balajee AS, Rao KS, Bohr VA. Telomere reduction and telomerase inactivation during neuronal cell differentiation. Biochemical and biophysical research communications. 1996;224:487–92. doi: 10.1006/bbrc.1996.1054. [DOI] [PubMed] [Google Scholar]
- 29.Richardson RM, Nguyen B, Holt SE, Broaddus WC, Fillmore HL. Ectopic telomerase expression inhibits neuronal differentiation of NT2 neural progenitor cells. Neuroscience letters. 2007;421:168–72. doi: 10.1016/j.neulet.2007.03.079. [DOI] [PubMed] [Google Scholar]
- 30.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascular pharmacology. 2002;38:323–37. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 31.Chong EY, Lam PY, Poon WS, Ng HK. Telomerase expression in gliomas including the nonastrocytic tumors. Human pathology. 1998;29:599–603. doi: 10.1016/s0046-8177(98)80009-9. [DOI] [PubMed] [Google Scholar]
- 32.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer research. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 33.Aubert G, Lansdorp PM. Telomeres and aging. Physiological reviews. 2008;88:557–79. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 34.Gomez-Millan J, Goldblatt EM, Gryaznov SM, Mendonca MS, Herbert BS. Specific telomere dysfunction induced by GRN163L increases radiation sensitivity in breast cancer cells. International journal of radiation oncology, biology, physics. 2007;67:897–905. doi: 10.1016/j.ijrobp.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 35.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell death and differentiation. 2004;11:448–57. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 36.Ulasov IV, Sonabend AM, Nandi S, Khramtsov A, Han Y, Lesniak MS. Combination of adenoviral virotherapy and temozolomide chemotherapy eradicates malignant glioma through autophagic and apoptotic cell death in vivo. Br J Cancer. 2009;100:1154–64. doi: 10.1038/sj.bjc.6604969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu J, Liu ZG, Liu XM, et al. Glioblastoma stem cells resistant to temozolomide-induced autophagy. Chin Med J (Engl) 2009;122:1255–9. [PubMed] [Google Scholar]
- 38.Shammas MA, Koley H, Bertheau RC, et al. Telomerase inhibitor GRN163L inhibits myeloma cell growth in vitro and in vivo. Leukemia. 2008;22:1410–8. doi: 10.1038/leu.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito H, Aoki H, Kuhnel F, et al. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J Natl Cancer Inst. 2006;98:625–36. doi: 10.1093/jnci/djj161. [DOI] [PubMed] [Google Scholar]
- 40.Dikmen ZG, Gellert GC, Jackson S, et al. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer research. 2005;65:7866–73. doi: 10.1158/0008-5472.CAN-05-1215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.