Abstract
Echinacea is a top-selling herbal remedy that purportedly acts as an immunostimulant. However, the specific immunomodulatory effects of Echinacea remain to be elucidated. We focused on defining the effects of Echinacea purpurea extracts in dendritic cells (DCs), which generate innate and adaptive immune responses. We hypothesized that E. purpurea extracts would enhance murine bone marrow-derived DC (BMDC) activation leading to increased immune responses. The fate and function of DCs from C57Bl/6 mice was evaluated following 48 h exposure to E. purpurea root and leaf extracts. Flow cytometry revealed that the polysaccharide-rich root extract increased the expression of MHC class II, CD86, and CD54 surface biomarkers whereas the alkylamide-rich leaf extract inhibited expression of these molecules. Production of IL-6 and TNF-α increased in a concentration-dependent manner with exposure to the root, but not leaf, extract. In contrast, the leaf but not root extract inhibited the enzymatic activity of cyclooxygenase-2. While both extracts decreased the uptake of ovalbumin by BMDCs, the leaf but not root extract inhibited the antigen-specific activation of naïve CD4+ T cells from OT II/Thy1.1 mice. Collectively, these results suggest that E. purpurea can be immunostimulatory, immunosuppressive, and/or anti-inflammatory depending on the portion of the plant and extraction method.
Keywords: Echinacea purpurea, dendritic cells, antigen presenting cells, inflammation, immunity
I. Introduction
Echinacea has become one of the most popular herbal supplements (Ernst et al., 2002) used to alleviate colds, sore throats, coughs, and other respiratory infections (Gruenwald et al., 1998). Three of the nine species of Echinacea have medicinal applications: Echinacea angustifolia, Echinacea pallida, and Echinacea purpurea, which is the most commonly consumed species in the U.S. (Borchers et al., 2000). Though the active phytochemicals in this plant vary due to age of plant, portion of plant, growth conditions, geographical location, and extraction method (Perry et al., 2001), the most common constituents present in Echinacea include alkylamides, caffeic acid derivatives, polysaccharides, and lipoproteins (Bauer et al., 1998). Polysaccharides are typically present at highest concentration in aqueous or fresh pressed juice extracts while alkylamides are more likely to be major constituents in ethanolic extracts. Furthermore, it has recently been shown that bacterial lipoproteins may also be responsible for the observed stimulatory activity of Echinacea in macrophages (Pugh et al., 2008). Such lipoproteins can be present in plants without contamination from external bacteria due to the presence of endophytes.
Several studies have described the effects of E. purpurea on the immune system. In 1997, Burger et al. revealed the immunostimulatory effect of unpurified fresh pressed E. purpurea juice on human peripheral blood macrophages in vitro. At low concentrations, E. purpurea induced the secretion of IL-1, IL-6, IL-10, and TNF-α. Goel et al. (2002) conducted a study addressing the phagocytic activity of alveolar macrophages. It was determined that E. purpurea extract fed to mice enhanced phagocytosis in these cells. Most recently, Sasagawa et al. (2006) examined the effects of ethanolic extracts obtained from aerial portions of E. purpurea on stimulated Jurkat T cells. They found that low concentrations of the extract suppressed the ability of activated T cells to produce IL-2, a key cytokine involved in the early phase of T cell activation. The public generally considers this herb safe and effective as suggested by the dramatic increase in Echinacea use; however, additional studies are needed to further define the immunomodulatory effects of Echinacea.
Dendritic cells (DCs) are professional antigen presenting cells (APCs) that act at the interface of the innate and adaptive branches of the immune system. After encountering and capturing antigen in the periphery, immature DCs migrate to lymphoid organs and undergo maturation. In these tissues, DCs present antigens to antigen-specific T cells and generate adaptive immunity or induce tolerance (Banchereau et al., 2000). During stages of DC differentiation (immature, mature, activated), the ability to uptake antigens and key accessory molecules varies. As DCs become activated, they are less able to internalize antigens. Thus, immature DCs have the greatest ability to uptake antigens. Furthermore, during maturation and activation, DCs change their expression of accessory molecules. For example, MHC class I and MHC class II expression is increased along with accessory molecules such as CD86, CD80, CD54, and CD40. Cytokines are also released during the maturation and activation of DCs. These proteins can bind to receptors on immune cells to induce inflammatory responses.
Currently, only two studies have been published that address the effects of E. purpurea on DCs (Wang et al., 2006; Wang et al. 2008). These studies primarily address the genomic changes that occur in human DCs following treatment with various E. purpurea preparations. The purpose of this study was to investigate the effects of extracts derived from different portions of E. purpurea on DCs derived from C57Bl/6 mice. We hypothesized that E. purpurea extracts enhance DC activation, ultimately leading to increased innate and adaptive immune responses. To test this hypothesis, various aspects of DC fate and function were examined by a series of in vitro experiments.
2. Materials & Methods
2.1. Mice
C57Bl/6 and OT II/Thy1.1 mice aged 6-12 weeks were bred and maintained in the animal research facilities at the University of Montana. Male mice were used as cell donors for all experiments as our Echinacea extracts initially affected BMDCs from male mice and to our knowledge no information exists to suggest that gender differences exist in the function of BMDCs generated from mice. Cells from OT II/Thy1.1 mice were utilized since they possess a transgenic T cell receptor that specifically recognizes a defined ovalbumin peptide (OVA323-339), making them OVA-specific and extremely useful for studying T cell activation when activated by OVA-laden antigen presenting cells (Barnden et al., 1998). Mice were housed under specific pathogen-free conditions and maintained on 12 h dark/light cycles. Standard laboratory food and water were provided ad libitum. All protocols for the use of animals were approved by the University of Montana Institutional Animal Care and Use Committee and adhered to the current NIH guidelines for animal usage.
2.2. Bone marrow-derived dendritic cells (BMDCs)
BMDCs were prepared as previously described (Grauer et al., 2002) with modifications. Briefly, to collect bone marrow cells, the femur and tibia of mice were flushed using a 27-gauge needle with complete media (cRPMI) comprised of RPMI (GibcoBRL, Grand Island, NY) supplemented with 10% FBS (Hyclone, Logan, UT), 50 μM mercaptoethanol, 20 mM HEPES, 10 mM sodium pyruvate, and 50 μg/ml gentamicin (GibcoBRL, Grand Island, NY). Cells were subjected to a density gradient using Lympholyte®-M reagent (Cedarlane Laboratories Limited, Ontario, Canada) to enrich hematopoietic cells and then washed with cRPMI. Flt3-ligand-derived BMDCs were prepared as previously described (Brasel et al., 2000 and Brawand et al., 2002) with modifications. Briefly, the bone marrow cells were grown in 10 ml conditioned media and 300 ng/ml Flt3-ligand (Perpro Tech, Rocky Hill, NJ) to a final concentration of 1×106 cells/ml in T75 flasks. Conditioned media containing IL-6 was obtained by culturing red blood cell-depleted splenocytes for 10 days in cRPMI, collecting the supernatant, and storing it at -20°C for future use. Conditioned media and growth factor were removed and replenished on day 5. Non-adherent cells, representative of immature DCs, were harvested after 10 days and evaluated for viability and expression of CD11c, the murine DC lineage-specific marker. Cells were ≥ 95% viable as determined by Trypan blue exclusion and were ≥ 90% CD11c+ as determined by flow cytometry.
2.3. Extracts and chemicals
Extracts were prepared at HerbPharm, Inc. (Williams, OR) from 2-year-old cultivated E. purpurea aerial and root portions as described by Sasagawa et al. (2006). Ethanolic extraction (75% ethanol) of the aerial portions (stems, leaves, and flowers) yielded an alkylamide-rich fraction while aqueous extraction of the roots yielded a polysaccharide-rich fraction. Extracts were analyzed via high performance liquid chromatography/electropsray ionization mass spectrometry (HLPC/ESI-MS-MS) as previously described by Cech et al. (2006). Extracts were also analyzed for polysaccharides (V-Labs Inc., Covington, LA). The sugars are reported as alditol acetates and compared to standard sugar solutions. Quantification of the major constituents of each extract is shown in Table 1. Both leaf and root extracts tested negative for endotoxin contamination by the Limulus Amebocyte Lystate (LAL) gel assay (Cambrex Corporation, East Rutherford, NJ) at the limit of detection (<0.06 EU/ml).
Table 1.
Quantification of constituents present in leaf and root extracts of E. purpurea.
| A. Concentration of alkylamides and caffeic acid derivativesa | ||
|---|---|---|
| Constituent | Leaf Extract (mM) |
Root Extract (mM) |
| Dodeca-2E,4E,8Z,10E/Z tetraenoic acid isobutylamide |
1.3 ± 0.099 | 0.027 ± 0.010 |
| Caftaric acid | 1.1 ± 0.15 | 0.83 ± 0.15 |
| Chlorogenic acid | 0.040 ± 0.011 | Not Detected |
| Cichoric acid | 1.4 ± 0.22 | 0.067± 0.022 |
| B. Relative neutral sugar concentrationb | ||
|---|---|---|
| Constituent |
Leaf Extract (%) |
Root Extract (%) |
| Glucitol acetate | 69.4 | 73.5 |
| Mannitol acetate | 11.5 | 18.7 |
| Arabinitol acetate | 5.3 | 3.7 |
| Rhamnitol acetate | 4.4 | 3.4 |
| Galactitol acetate | 7.2 | 2.2 |
| Xylitol acetate | 2.2 | 2.0 |
Aqueous root and ethanolic leaf extracts were analyzed for alkylamides and caffeic acid derivatives via high performance liquid chromatography/electropsray ionization mass spectrometry (HLPC/ESI-MS-MS) with a solvent gradient consisting of aqueous acetic acid and acetonitrile.
Extracts were also analyzed for polysaccharides. The sugars are reported as alditol acetates and compared to standard sugar solutions.
2.4. Cell activation and treatment
BMDCs (1.0×106) were cultured in 1 ml cRPMI in 6-well plates and treated with 0.5% ethanol or E. purpurea root (150 or 450 μg/ml) or leaf extracts (50 or 150 μg/ml) at a final ethanol concentration of 0.5%. After 48 h, supernatants were collected and frozen at -20°C for future evaluation of cytokine production, and at this time cells (≥90% viable as determined by Trypan blue exclusion) were harvested for phenotypic analysis by flow cytometry. Previous studies in our laboratory investigated the cytotoxicity of both root and leaf extracts at concentrations ranging from 50 μg/ml to 500 μg/ml. We found that the root extract was not cytotoxic at any concentrations tested while cells treated with higher concentrations of the leaf extract were slightly less viable (data not shown). Therefore, we utilized lower concentrations for both extracts in the studies described.
2.5. Flow cytometry
Expression of accessory molecules on BMDCs was determined by flow cytometry as previously described (Shepherd et al., 2001). Briefly, BMDCs were harvested and washed with PAB (1% bovine serum albumin and 0.1% sodium azide in PBS). To eliminate non-specific staining, cells were blocked with 30 μg purified rat and/or hamster IgG (Jackson ImmunoResearch, West Grove, PA) for 10 min. Optimal concentrations of fluorochrome-conjugated monoclonal antibodies (mAbs) were used to stain cells for an additional 10 min. Antibodies used in these experiments included CD11c-APC (HL3), MHC class II-FITC (2G9), CD86-PE (GL-1), CD54-PB (YN1/1.7.4), and their corresponding isotype controls, all of which were purchased from BD Biosciences (San Jose, CA). One to five hundred thousand events were collected and analyzed using a BD FACSAria flow cytometer and FACSDiva software (BD Biosciences, San Jose, CA).
2.6. Cytokine assays
Supernatants from cultured cells were examined for levels of the pro-inflammatory cytokines, TNF-α and IL-6, and the anti-inflammatory cytokine, IL-10, via enzyme-linked immunosorbent assay (ELISA). Samples were analyzed per the manufacturer's instructions using mouse cytokine-specific BD OptEIA ELISA kits (BD Pharmingen, San Diego, CA). Nitric oxide (NO) levels in supernatants were measured with the Greiss Reagent System (Promega, Madison, WI).
2.7. Cyclooxygenase (COX) enzyme activity
E. purpurea leaf and root extracts were analyzed for COX-1 and COX-2 enzyme activity per the manufacturer's instructions using the COX inhibitor screening assay (Cayman Chemical Company, Ann Arbor, MI).
2.8. Antigen uptake with ovalbumin (OVA) in BMDCs
BMDCs were cultured at 1.0×106 cells per well with 1 ml cRPMI in 6-well plates for 48 h. The cells were treated with 0.5% ethanol, 150 μg/ml E. purpurea leaf extract, or 150 μg/ml E. purpurea root extract. Cells were then washed with cRPMI to remove excess Echinacea extracts prior to the addition of 10 μg/ml OVA-FITC for 2 h. Cells were harvested and analyzed by flow cytometry or fluorometry. For flow cytometry analysis, cells were harvested and washed with PAB. One hundred thousand cells were analyzed using a BD FACSAria flow cytometer and FACSDiva software. For fluorometry analysis, cells were centrifuged at 1500 rpm for 5 min using a Shandon Cytospin 3 Cytocentrifuge. Slides were air-dried, coverslipped, and examined using a Nikon E-800 fluorescent microscope at 100× magnification with a Nuance multi-spectral imaging system (version 1.6.2.368 Alpha).
2.9. Clonal expansion of OVA-specific T cells
The clonal expansion of OVA-specific OT II T cells was performed as previously described by Lyons and Parish (1994) with modifications. Briefly, BMDCs were pre-treated with 150 μg/ml leaf or root extract for 48 h, washed to remove extracts, and treated with 100 μg/ml OVA323-339 peptide overnight. T cells were isolated from spleens of OT II T cell receptor transgenic mice and purified using CD4-PE antibodies (BD Pharmingen, San Diego, CA) and anti-PE beads (Miltenyi Biotec, San Diego, CA). The purified T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE, Molecular Probes, Inc., Eugene, OR). BMDCs were added at various ratios (1:1, 1:2.5, 1:10 DC:T cell ratio) to CFSE-labeled OT II T cells. After 4 days, clonal expansion of OVA-specific T cells was determined by FACS analysis.
2.10. Statistical analysis
All statistical analyses were performed using GraphPad Prism 4.0a for Macintosh (GraphPad Software, San Diego, CA). Data sets with multiple comparisons were evaluated by one-way analysis of variance (ANOVA) with Dunnett's test. Values of p < 0.05 were determined to be significant.
3. Results
3.1. E. purpurea extracts alter the expression of key accessory molecules on BMDCs
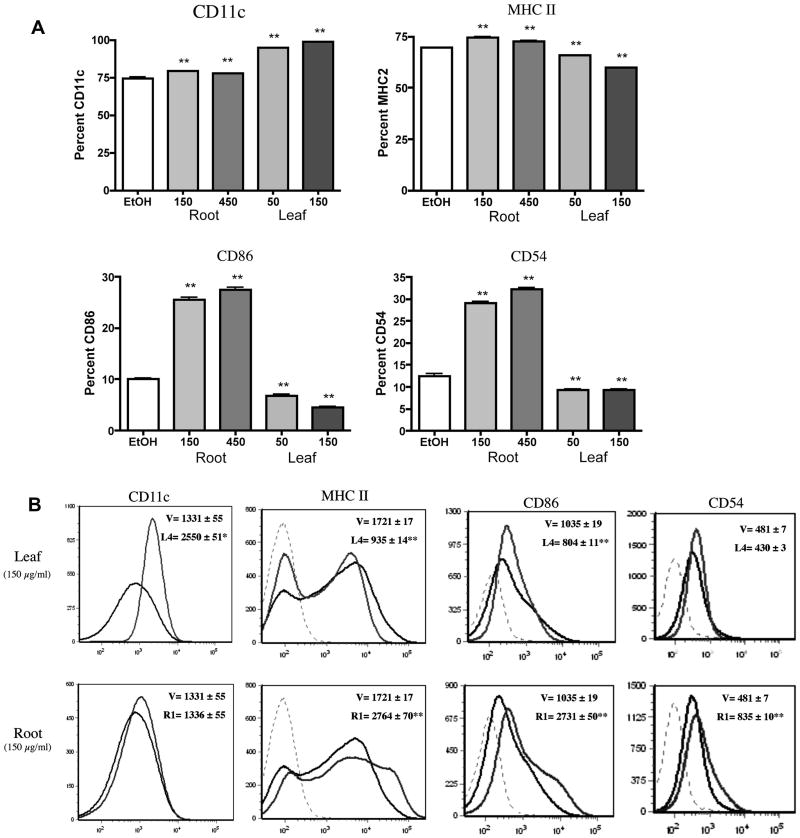
To evaluate the immunomodulatory potential of E. purpurea root and leaf extracts, murine BMDCs were phenotypically examined by flow cytometry for the key accessory molecules CD11c, MHC class II, CD86, and CD54. Immature murine BMDCs typically display lower levels of these markers while mature BMDCs increase their expression following activation. Changes in expression of the accessory molecules were observed in cells treated with the root extract. At both 150 μg/ml and 450 μg/ml, the root extract slightly increased the frequency of CD11c+ BMDCs from 75% in control-treated cells to 81% and 78%, respectively (Fig. 1A). However, the relative expression (MFI) of CD11c was not affected relative to the ethanol controls (Fig. 1B). The root extract increased the frequency of BMDCs that expressed MHC class II from 68% in control-treated cells to 75% and 72%, respectively (Fig. 1A) as well as augmented its relative expression by nearly 60 percent (Fig. 1B). A concentration-dependent increase in the frequency of BMDCs expressing CD86 (10% in control-treated cells to 25% and 27%), or CD54 (12% in control-treated cells to 30% and 32%) was observed at 150 μg/ml and 450 μg/ml of the root extract, respectively (Fig. 1A). Additionally, the relative expression of these markers nearly doubled when compared to the vehicle controls (Fig. 1B). The root extract did not affect cell viability (data not shown).
Figure 1.
BMDC expression of accessory molecules is differentially affected by E. purpurea leaf and root extracts. Flt3-ligand-derived BMDCs (106) were treated with extracts or the ethanol (0.5%) controls for 48 h at the designated concentrations. The percentage positive (A) and Mean Channel Fluorescence (B) profiles were determined by FACS analysis. Results are shown as mean ± SEM (n=6) and are representative of two separate experiments. (B) Histograms represent BMDCs treated with the ethanol vehicle (black line) or E. purpurea leaf extracts (gray). Dashed lines indicate isotype controls. L4 corresponds to our leaf extract while R1 corresponds to our root extract. *p<0.05 and **p<0.01 compared to control.
Treatment of BMDCs with E. purpurea leaf extract resulted in contrasting effects on most of the accessory molecules. Similar to cells treated with the root extract, an increased frequency of CD11c+ cells from 75% in the control to 94% and 100% was observed with the leaf extract at both concentrations tested, 50 μg/ml and 150 μg/ml, respectively (Fig. 1A). Moreover, the relative expression of CD11c nearly doubled (Fig. 1B). Contrary to the effects of the root extract, the frequency of BMDCs expressing MHC class II, CD86, and CD54 was decreased in leaf-treated cells (Fig. 1A). At both 50 μg/ml and 150 μg/ml, the leaf extract decreased the frequency of MHC class II+ BMDCs from 68% in control-treated cells to 63% and 60%, respectively. A decreased frequency of BMDCs expressing CD86 (10% in control-treated cells to 6% and 4%) and CD54 (12% in controls to 10% and 10%) was observed at 50 μg/ml and 150 μg/ml, respectively. Furthermore, the relative expression of MHC class II and CD86, but not CD54, was also decreased following treatment of the BMDCs with the leaf extract. As with the root extract, the leaf extract was not cytotoxic to BMDCs (data not shown).
3.2. Root but not leaf extracts of E. purpurea induce the secretion of TNF-α and IL-6
When stimulated, BMDCs can secrete various cytokines including pro-inflammatory cytokines, such as TNF-α and IL-6, and anti-inflammatory cytokines, such as IL-10. Additionally, nitric oxide (NO) is released as an important inflammatory mediator upon activation. Production of the pro-inflammatory cytokines, TNF-α and IL-6, was measured in the supernatants from BMDCs treated with Echinacea root or leaf extracts. The root extract induced the secretion of both cytokines to approximately 100 pg/ml and 350 pg/ml at 150 μg/ml and 450 μg/ml, respectively (Fig. 2). BMDCs exposed to leaf extract did not secrete either cytokine (Fig. 2). Additionally, the root and leaf extracts did not induce the secretion of either IL-10 or NO by the BMDCs (data not shown).
Figure 2.
BMDCs secrete TNF-α and IL-6 following exposure to root but not leaf extracts. Flt3-ligand-derived BMDCs were treated as described in Fig. 2. Supernatants were collected and analyzed by ELISA. Results are mean ± SEM (n=6) and are representative of two separate experiments. **p<0.01 compared to control.
3.3. E. purpurea leaf but not root extracts selectively attenuate COX activity
E. purpurea leaf and root extracts were examined for their potential to affect COX-1 and COX-2 enzyme activity. COX-1 is the constitutively expressed isoform that protects the gastrointestinal mucosal lining and maintains both kidney and platelet function whereas COX-2 is the inducible isoform that increases expression during inflammation. Neither the leaf nor the root extract inhibited COX-1 activity (Table 2). The root extract also failed to significantly inhibit COX-2 activity. Conversely, the percent inhibition of COX-2 activity increased in a concentration-dependent manner up to 85% with 8 μg/ml leaf extract.
Table 2.
Cyclooxygenase-2 (COX-2) enzyme activity is inhibited by the E. purpurea leaf extract.
| COX-2 | LEAF | μg/ml | 2 | 4 | 8 |
| % Inhibition | 28 ± 2* | 63 ± 1* | 85 ± 1* | ||
| ROOT | μg/ml | 0.1 | 1 | 10 | |
| % Inhibition | 6 ± 4 | 1 ± 1 | 2 ± 1 | ||
| COX-1 | LEAF | μg/ml | 2 | 4 | 8 |
| % Inhibition | 7 ± 1 | 5 ± 3 | 7 ± 4 | ||
| ROOT | μg/ml | 0.1 | 1 | 10 | |
| % Inhibition | ND | ND | ND |
The enzymatic activity of purified COX-1 and COX-2 was determined in the presence of varying concentrations of E. purpurea leaf or root extracts by the Cayman COX inhibitor screening assay per the manufacturer's instructions. Results are mean ± SEM (n=3).
p<0.05 compared to control. ND = not detected.
3.4. Antigen uptake by BMDCs is decreased following exposure to E. purpurea extracts
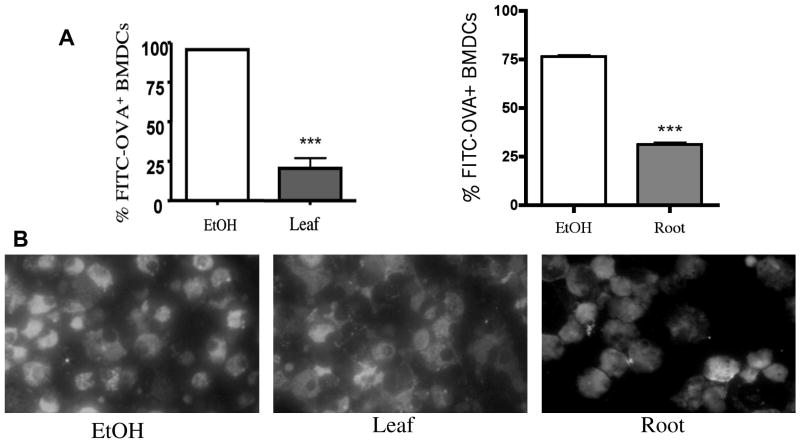
The ability to uptake antigen is a critical function of DCs. Immature DCs are specialized to uptake antigen, which initiates antigen processing and presentation. Because of the inhibitory effects of E. purpurea leaf extract on activation marker expression and soluble mediator production, antigen uptake was examined in BMDCs treated with the leaf extract. As shown in Figure 3A, the frequency of BMDCs that engulfed OVA-FITC was decreased by 75% after exposure to E. purpurea leaf extracts when compared to the ethanol control as determined by flow cytometry. Unexpectedly, the cells exposed to root extract also decreased antigen uptake by 45%. Fluorescent microscopy qualitatively confirmed the decreased antigen-uptake by flow cytometry (Fig. 3B).
Figure 3.
Phagocytosis of FITC-OVA by BMDCs is decreased following exposure to E. purpurea extracts. BMDCs were treated with ethanol (0.5%) or extracts (150 μg/ml) for 48 h. Samples were washed and FITC-OVA added for 2 h. Cells were then harvested and analyzed by flow cytometry (A) or fluorescence microscopy (B). Results are mean ± SEM (n=4) and are representative of two separate experiments. ***p<0.001 compared to control.
3.5. E. purpurea leaf extracts inhibit antigen-specific interactions between BMDCs and CD4+ T cells
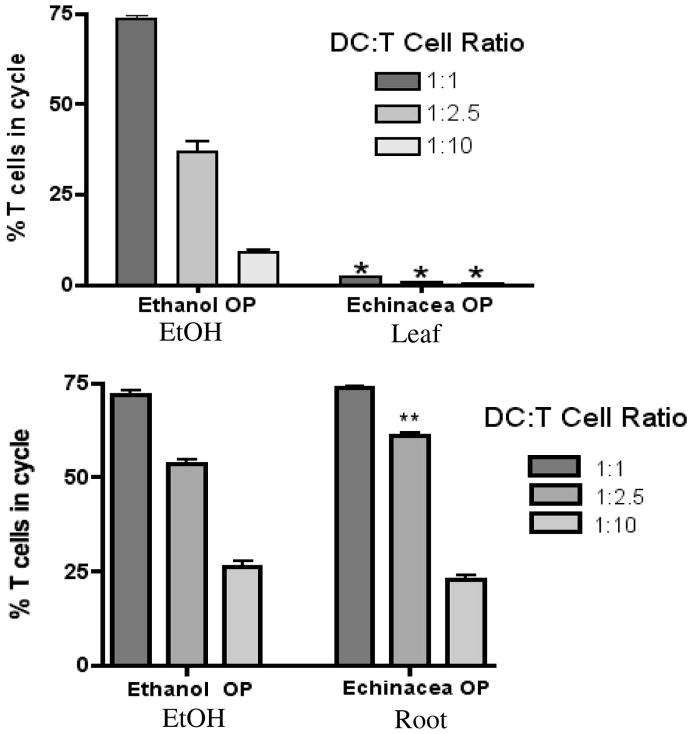
In order to initiate a T cell-mediated immune response, DCs must successfully present antigen to T cells. To determine the functional ability of E. purpurea extract-treated BMDCs to simulate the antigen specific activation of naïve CD4+ T cells, we added BMDCs pretreated with leaf or root extract and OVA peptide to CFSE-labeled T cells at various ratios. Examination of OVA-specific T cell clonal expansion via FACS analysis revealed that leaf extracts decreased the percentage of T cells in cycle to under 2% at all ratios tested while the root extract modestly increased clonal expansion of CD4+ OT II T cells by 8% at the DC:T cell ratio of 1:2.5 (Fig. 4).
Figure 4.
OVA-loaded BMDCs treated with E. purpurea extracts modulate clonal expansion of CD4+ OT II T cells. BMDCs were pre-treated with leaf or root extract (150 μg/ml) for 48 h prior to overnight addition of OVA323-229 peptide. BMDCs were added at various ratios to CFSE-labeled OT II T cells. After 4 days, clonal expansion of OVA-specific T cells was determined by FACS analysis. Results are mean ± SEM (n=4). **p<0.005 or ***p<0.0001 compared to control.
4. Discussion
Due to its popularity as an herbal supplement, many researchers have attempted to define the immunomodulatory mechanisms of Echinacea. E. purpurea has been shown to have immunostimulatory effects on monocytes, macrophages, natural killer cells, and T cells in vitro (Goldrosen and Straus, 2004). To date, only two studies have attempted to elucidate the immunomodulatory effects of E. purpurea on DCs. In 2006, Wang et al. revealed that their E. purpurea altered immune-related genes in human DCs. Additionally, the potential cellular signaling events were investigated via genomic and proteomic analyses following DC exposure to their butanol extracts (Wang et al., 2008). Since DCs play an important role in both innate and adaptive immunity, it is essential investigate the effects of E. purpurea on these cells. In this study, we set out to define the fate and function of murine DCs following exposure to E. purpurea aqueous root extracts and ethanolic leaf extracts.
DCs undergo several changes during maturation including morphological changes (Trombetta and Mellman, 2005), loss of endocytic and phagocytic receptors, chemokine secretion (Martín-Fontecha et al., 2004; Penna et al., 2002; Piqueras et al., 2006; Tang and Cyster, 1999; Yoneyana et al., 2002), upregulation of costimulatory molecules (Caux et al., 1994), translocation of MHC class II biomarkers to the cell surface (Cella et al., 1997; Pierre et al., 1997), and cytokine secretion (Heufler et al., 1996). Ultimately these changes prepare DCs to successfully interact with T cells in the context of antigen. Activation of the antigen-specific T cell results in clonal expansion and differentiation, leading to adaptive immunity. Our study addresses key stages in DC maturation following exposure to E. purpurea extracts.
We first examined the expression of MHC class II and various costimulatory molecules on the cell surface following treatment with E. purpurea leaf and root extracts. These cell surface molecules are expressed on DCs and serve as ligands for receptors on naïve T cells. Differential expression of key accessory molecules was observed for the aqueous, polysaccharide-rich root extract and ethanolic, alkylamide-rich leaf extract. E. purpurea root extracts increased the expression of molecules involved in antigen presentation (MHC class II) and costimulation (CD86 and CD54) while the leaf extracts decreased their expression. These results suggest a stimulatory role by the root extract and inhibitory role by the leaf extract. Similar effects were observed by Wang et al. (2006) as they investigated the immunomodulatory effects of E. purpurea extracts on human monocyte-derived DCs. In their study, E. purpurea extracts were isolated from ethanolic extractions of the root, aerial portions, and the whole plant. When examining the effects of various E. purpurea extracts on DC maturation, they found that their root extract stimulated cells in a similar manner as LPS; however, the stem plus leaf extract inhibited DC maturation. DNA microarray was utilized to further investigate gene expression associated with treatment of the extracts. They found that CD86 expression was decreased when DCs were exposed to the stem plus leaf extract while CD54 expression was up regulated when DCs were exposed to root extracts. The results obtained with human DCs are consistent with our results obtained with murine DCs. If antigen presentation and costimulation are altered, the possibility exists that events downstream, such as T cell activation, could be affected as discussed in more detail below.
Activated DCs secrete cytokines, which are soluble mediators involved in inflammation and immunity. TNF-α and IL-6 are inflammatory cytokines commonly released after phagocytosis. Additionally, the COX-2 enzyme is induced as part of the inflammatory response. Inhibition of inflammatory cytokines and/or COX-2 enzyme activity could be effective strategies to inhibit inflammation. In our study, the polysaccharide-rich root extract increased secretion of IL-6 and TNF-α, while the leaf extract had no effect on the secretion of these cytokines. Moreover, the leaf, but not root, extract inhibited COX-2 enzyme activity. Since production of these inflammatory mediators was decreased, it is possible that this reflects a reduced capacity of the DCs to contribute to the inflammatory response after exposure to the leaf extract. Since the concentrations of leaf extract used in these studies did not affect cell viability, suppressed cytokine production was not due to cytotoxicity. As with the expression of accessory molecules, it appeared that the aqueous root extract possessed stimulatory effects while the alkylamide-rich leaf extract generated inhibitory effects. These results strongly suggest that the fate and function of DCs is differentially affected when exposed to various E. purpurea extracts. Sullivan et al. (2008) examined similar parameters when testing the effects of E. purpurea polysaccharides on macrophages in vitro. They found that polysaccharides isolated from E. purpurea stimulated the production of many cytokines including IL-6, TNF-α, IL-12, and NO. Consequently, it appears as though polysaccharides isolated from E. purpurea can generate the same response by two important populations of APCs. In future studies it may be interesting to investigate additional cytokines secreted by DCs, such as TGF-β, which can influence other cell populations.
DCs recognize pathogens via specific surface phagocytic receptors, which signal DCs to internalize and process antigens to become APCs. Activation of CD4+ T cells requires recognition of the MHC class II-antigen complex on the DC surface as well as the production of various costimulatory molecules and cytokines. Because our leaf extract decreased the production of key accessory molecules and cytokines, we investigated the ability of extract-treated DCs to internalize antigen. Antigen uptake was reduced following treatment of BMDCs with E. purpurea leaf extract. Although not tested in this study, it is possible that the leaf extract alters membrane fluidity in DCs such that pinocytosis is affected thereby altering antigen uptake. Collectively, the APC functions of DCs were hindered following exposure to the alkylamide-rich leaf extract, so it was necessary to examine how these effects might affect T cell activation. We found that the clonal expansion of T cells by antigen-loaded DCs was reduced following the treatment of the DCs with the leaf extract. In 2006, Sasagawa et al. showed that an alkylamide-rich aerial E. purpurea extract inhibited IL-2 production by Jurkat T cells in vitro. Since IL-2 is produced upon T cell activation and is also necessary for clonal expansion, this result strongly suggests that alkylamides might inhibit T cell mediated immunity by potentially affecting both DCs and CD4+ T cells. In addition to the effects of alkylamides, the polysaccharide-rich extract also influenced the interactions between DCs and T cells. Surprisingly, we found that even though the aqueous E. purpurea extract had an immunostimulatory effect on DCs with the aforementioned parameters, antigen uptake by DCs decreased following extract treatment. Although this extract differs from the leaf extract in phytochemical composition, it is possible that it also possesses an ability to alter pinocytosis via effects on the cell membrane. However, the interactions between DCs and T cells were not inhibited as root-treated DCs were able to activate T cells effectively and even modestly increase the percentage of T cells in cycle. These results suggest that there may be a threshold at which decreased antigen uptake by DCs negatively affects T cell activation.
The differential effects of alkylamides and polysaccharides present in the extracts isolated from E. purpurea may be due to differences in the mechanisms of action of the two compounds. We found that our leaf extract, primarily composed of cichoric acid and dodeca-2E, 4E, 8Z, 10E/Z tetraenoic acid isobutylamide, inhibited both innate and adaptive functions of DCs. It has been suggested that the lipophilic alkylamides isolated from Echinacea species are likely the active components responsible for the immunosuppressive effects of the plant (Bauer, 1997; Woelkart and Bauer, 2007). Furthermore, alkylamides can bind the cannabinoid receptor 2 (CB2), which is expressed by many immune cells (Bouaboula et al., 1993) including human myeloid DCs (Matias et al., 2002) and several murine DC subsets (Do et al., 2004; Maestroni et al., 2004). Woelkart et al. (2005) verified that alkylamides present in various Echinacea species, including E. purpurea, were agonists of CB2. In 2004, Gertsch et al. found that the immunomodulatory effects of Echinacea on human monocytes and macrophages were mediated through the CB2 receptor, which may have resulted in the modulation of TNF-α and various signal transduction pathways. Thus, it is possible that the effects we observed from the alkylamide-rich fraction could be mediated through the CB2 receptor in DCs. Conversely, we found that our aqueous, polysaccharide-rich root fraction, primarily composed of glucitol acetate and mannitol acetate, generally acted on DCs in a stimulatory manner. As previously mentioned, Sullivan et al. (2008) examined the effects of E. purpurea polysaccharides on macrophages. Their results suggested that the polysaccharides acted on macrophages in both TLR-4 dependent and TLR-4 independent pathways involving ERK, p38, and JNK, which ultimately led to the activation of the NF-κB transcription factor. Further evaluation of the effects of the extract-treated DCs on these possible signaling pathways is warranted.
It should be noted that the dietary supplement industry is loosely regulated so manufacturers are not required to list details of extraction methods for their products. Therefore, consumers do not necessarily have a way of determining the concentration of biologically active constituents in a particular supplement. Although the phytochemical profiles vary significantly different between batches from the same manufacturer as well as between manufacturers, investigators have attempted to clarify the pharmacokinetics of Echinacea extracts. In 2005, Matthias et al. revealed that following oral consumption of ethanolic E. purpurea liquid extracts, alkylamides appeared in plasma after 20 minutes and reached a maximum concentration of 336±131 ng/ml in human plasma, which demonstrates that alkylamides are bioavailable. Although the in vitro concentrations of plant material utilized in our studies are higher than the plasma concentrations identified in this study, the alkylamides constitute only a small fraction. Therefore, our concentrations are likely physiologically relevant but would need to be confirmed in vivo.
Taken together, our results suggest that alkylamides present in ethanolic extracts may suppress the fate and function of murine DCs while high levels of complex polysaccharides present in aqueous extracts may activate murine DCs. Thus, the potential for E. purpurea to modulate immune function may be linked to the portion of the plant and extraction method. In future studies it would be worthwhile to further explore the biochemical and molecular mechanisms of action of both extracts and investigate the effects of both the root and leaf extracts on immune function in vivo. In addition, follow-up studies on DC response to isolated Echinacea constituents, such as alkylamides and polysaccharides, are warranted.
Acknowledgments
This project was supported by grants from NSF-EPSCoR (EPS-0091995) and NCRR (P20RR17670). NCRR is a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. The authors thank the CEHS Molecular Histology & Fluorescent Imagery Core and the Fluorescence Cytometry Core at UM for their support. The authors also wish to thank Drs. Celine Beamer, Jerry Smith, and Scott Wetzel for their critical reviews of this manuscript.
Abbreviations
- APC
antigen presenting cell
- BMDC
bone marrow-derived dendritic cell
- CFSE
carboxyfluorescein succinimidyl ester
- COX
cyclooxygenase
- DC
dendritic cell
- ERK
extracellular regulated kinase
- FITC
fluorescein isothiocyanate
- JNK
c-Jun NH2 terminal kinase
- IL
interleukin
- MHC
major histocompatibility complex
- NF-κB
nuclear factor-κB
- OVA
ovalbumin
- TGF-β
transforming growth factor-β
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based and ß-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Bauer R. In: Phytomedicines of Europe. Lawson LD, Bauer R, editors. American Chemical Society; Washington, DC: 1998. p. 140. [Google Scholar]
- Bauer R. Chemistry, analysis and immunological investigations of Echinacea phytopharmaceuticals. In: Wagner H, editor. Immunomodulatory agents from plants. Basel, Boston, Berlin: Birkhäuserverlag; 1999. pp. 41–88. [Google Scholar]
- Borchers AT, Keen CL, Stern JS, Gershwin ME. Inflammation and Native American medicine: the role of botanicals. Am J Clin Nutr. 2000;72(2):339–347. doi: 10.1093/ajcn/72.2.339. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, Le Fur G, Cassellas P. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96(9):3029–3039. [PubMed] [Google Scholar]
- Brawand P, Fitzpatrick DR, Greenfield BW, Brasel K, Maliszewski CR, De Smedt T. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J Immunol. 2002;169(12):6711–6719. doi: 10.4049/jimmunol.169.12.6711. [DOI] [PubMed] [Google Scholar]
- Burger RA, Torres AR, Warren RP, Caldwell VD, Hughes BG. Echinacea-induced cytokine production by human macrophages. Int J Immunopharmacol. 1997;19(7):371–379. doi: 10.1016/s0192-0561(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180(4):1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech NB, Eleazer MS, Shoffner LT, Crosswhite MR, Davis AC, Mortenson AM. High performance liquid chromatography/electrospray ionization mass spectrometry for simultaneous analysis of alkamides and caffeic acid derivatives from Echinacea purpurea extracts. J Chromatogr A. 2006;1103:219–228. doi: 10.1016/j.chroma.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388(6644):782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Activation through cannabinoid receptors 1 and 2 on dendritic cells Triggers NF-κB-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol. 2004;173:2373–2382. doi: 10.4049/jimmunol.173.4.2373. [DOI] [PubMed] [Google Scholar]
- Ernst E. The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John's Wort, Ginseng, Echinacea, Saw Palmetto, and Kava. Ann Intern Med. 2002;136(1):42–53. doi: 10.7326/0003-4819-136-1-200201010-00010. [DOI] [PubMed] [Google Scholar]
- Goel V, Chang C, Slama JV, Barton R, Bauer R, Gahler R, Basu TK. Alkylamides of Echinacea purpurea stimulate alveolar macrophage function in normal rats. Int Immunopharmacol. 2002;2(2-3):381–387. doi: 10.1016/s1567-5769(01)00163-1. [DOI] [PubMed] [Google Scholar]
- Goldrosen MH, Straus SE. Complementary and alternative medicine: assessing the evidence for immunological benefits. Nat Rev Immunol. 2004;4(11):912–921. doi: 10.1038/nri1486. [DOI] [PubMed] [Google Scholar]
- Grauer O, Wohlleben G, Seubert S, Weishaupt A, Kämpgen E, Gold R. Analysis of maturation states of rat bone marrow-derived dendritic cells using an improved culture technique. Histochem Cell Biol. 2002;117(4):351–362. doi: 10.1007/s00418-002-0384-4. [DOI] [PubMed] [Google Scholar]
- Gruenwald J, Brendler T, Jaenicke C, editors. PDR for Herbal Medicines. Medical Economics Co.; Montvale, NJ: 1998. [Google Scholar]
- Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Enk A, Steinman RM, Romani N, Schuler Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26(3):659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171(1):131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ. The endogenous cannabinoid 2-arachinonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. FASEB J. 2004;18(15):1914–1916. doi: 10.1096/fj.04-2190fje. [DOI] [PubMed] [Google Scholar]
- Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5(12):1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- Matias I, Pochard P, Orlando P, Salzet M, Pestel J, Di Marzo V. Presence and regulation of the endocannabinoid system in human dendritic cells. Eur J Biochem. 2002;269(15):3771–3778. doi: 10.1046/j.1432-1033.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- Matthias A, Addison RS, Penman KG, Dickinson RG, Bone KM, Lehmann RP. Echinacea alkamide disposition and pharmacokinetics in humans after tablet ingestion. Life Sci. 2005;77(16):2018–2029. doi: 10.1016/j.lfs.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Matthias A, Banbury L, Bone KM, Leach DN, Lehmann RP. Echinacea alkylamides modulate induced immune responses in T-cells. Fitoterapia. 2008;79(1):53–58. doi: 10.1016/j.fitote.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Penna G, Vulcano M, Sozzani S, Adorini L. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells. Hum Immunol. 2002;63(12):1164–1171. doi: 10.1016/s0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- Perry NB, Burgess EJ, Glennie VL. Echinacea standardization: analytical methods for phenolic compounds and typical levels in medicinal species. J Agric Food Chem. 2001;49(4):1702–1706. doi: 10.1021/jf001331y. [DOI] [PubMed] [Google Scholar]
- Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinmann RM, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388(6644):787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006;107(7):2613–2618. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh ND, Tamta H, Balachandran P, Wu X, Howell J, Dayan FE, Pasco DS. The majority of in vitro macrophage activation exhibited by extracts of some immune enhancing botanicals is due to bacterial lipoproteins and polysaccharides. Int Immunopharmacol. 2008;8(7):1023–1032. doi: 10.1016/j.intimp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa M, Cech NB, Gray DE, Elmer GW, Wenner CA. Echinacea alkylamides inhibit interleukin-2 production by Jurkat T cells. Int Immunopharmacol. 2006;6(7):1214–1221. doi: 10.1016/j.intimp.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Shepherd DM, Steppan LB, Hedstrom OR, Kerkvliet NI. Anti-CD40 treatment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-exposed C57Bl/6 mice induces activation of antigen presenting cells yet fails to overcome TCDD-induced suppression of allograft immunity. Toxicol Appl Pharmacol. 2001;170(1):10–22. doi: 10.1006/taap.2000.9080. [DOI] [PubMed] [Google Scholar]
- Sullivan AM, Laba JG, Moore JA, Lee TD. Echinacea induced macrophage activation. Immunopharmacol Immunotoxicol. 2008;30(3):553–574. doi: 10.1080/08923970802135534. [DOI] [PubMed] [Google Scholar]
- Tang HL, Cyster JG. Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science. 1999;284:819–822. doi: 10.1126/science.284.5415.819. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- Wang CY, Chiao MT, Yen PJ, Huang WC, Hou CC, Chien SC, Yeh KC, Yang WC, Shyur LF, Yang NS. Modulatory effects of Echinacea purpurea extracts on human dendritic cells: A cell- and gene-based study. Genomics. 2006;88(6):801–808. doi: 10.1016/j.ygeno.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Wang CY, Staniforth V, Chiao MT, Hou CC, Wu HM, Yeh KC, Chen CH, Hwang PI, Wen TN, Shyur LF, Yang NS. Genomics and proteomics of immune modulatory effects of a butanol fraction of echinacea purpurea in human dendritic cells. BMC Genomics. 2008;9:479. doi: 10.1186/1471-2164-9-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelkart K, Xu W, Pei Y, Makriyannis A, Picone RP, Bauer R. The endocannabinoid system as a target for alkylamides from Echinacea angustifolia roots. Planta Med. 2005;71(8):701–705. doi: 10.1055/s-2005-871290. [DOI] [PubMed] [Google Scholar]
- Woelkart K, Bauer R. The role of alklamides as an active principle of Echinacea. Planta Med. 2007;73(7):615–623. doi: 10.1055/s-2007-981531. [DOI] [PubMed] [Google Scholar]
- Yoneyama H, Narumi S, Zhang Y, Murai M, Baggiolini M, Lanzavecci A, Ichida T, Asakura H, Matsushima K. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell1lymphocytes in secondary lymph nodes. J Exp Med. 2002;195(10):1257–1266. doi: 10.1084/jem.20011983. [DOI] [PMC free article] [PubMed] [Google Scholar]