Abstract
Background
Childhood adversity increases the risk of psychopathology, but the neurobiological mechanisms underlying this vulnerability are not well-understood. In animal models, early adversity is associated with dysfunction in basal ganglia regions involved in reward processing, but this relationship has not been established in humans.
Methods
Functional magnetic resonance imaging was used to examine basal ganglia responses to (a) cues signaling possible monetary rewards and losses, and (b) delivery of monetary gains and penalties, in 13 young adults who experienced maltreatment before age 14 and 31 non-maltreated controls.
Results
Relative to controls, individuals exposed to childhood adversity reported elevated symptoms of anhedonia and depression, rated reward cues less positively, and displayed a weaker response to reward cues in the left globus pallidus. There were no group differences in right hemisphere basal ganglia response to reward cues, or in basal ganglia response to loss cues, no-incentive cues, gains, or penalties.
Conclusions
Results indicate that childhood adversity in humans is associated with blunted subjective responses to reward-predicting cues as well as dysfunction in left basal ganglia regions implicated in reward-related learning and motivation. This dysfunction may serve as a diathesis that contributes to the multiple negative outcomes and psychopathologies associated with childhood adversity. The findings suggest that interventions that target motivation and goal-directed action may be useful for reducing the negative consequences of childhood adversity.
Keywords: Maltreatment, stress, anhedonia, reward, basal ganglia, fMRI
Childhood adversity, including abuse, neglect, and exposure to dysfunctional household environments (e.g., witnessing parental violence, living with substance abusing individuals) increases the risk for psychopathology and substance abuse (1-6), and can lead to dysregulated hypothalamic-pituitary-adrenal stress responses (7), neuropsychological impairments (8), and dysfunction in brain regions implicated in learning and memory (9). However, potential effects on brain reward circuitry in adulthood have gone unexplored, although there is evidence of altered reward processing in maltreated children (10). This is important because reward system dysfunction may underlie anhedonia (11), a core component of stress-related psychopathology (12). Although maltreatment is associated with anhedonia (13) and melancholia (14), neural mechanisms underlying these relationships remain unknown.
In experimental animals, chronic stressors can weaken preferences for sucrose solutions and conditioning for rewarded locations, delay approach to palatable foods, and increase thresholds for brain stimulation reward (15-19). These effects are hypothesized to reflect dysfunction in dopaminergic (DA) circuits that project to the basal ganglia (19), which are sensitive to early adversity (20). Importantly, in experiments using cue-outcome designs these DA circuits are more strongly associated with incentive motivational processes elicited by reward-predicting cues than with hedonic processes triggered by rewarding outcomes (21-23), suggesting that early adversity may preferentially affect responses to reward-predicting cues. Consistent with this assumption, early adversity in marmosets decreased motivation to obtain rewards without affecting consummatory behavior (17). Thus, we hypothesized that childhood adversity in humans could weaken basal ganglia responses to reward-predicting cues while leaving responses to actual rewards intact.
To test this hypothesis, we used fMRI and a monetary incentive delay (MID) task (22,23) to investigate reward processing in young adults exposed to maltreatment (24). This task was selected because it recruits basal ganglia activity across a variety of samples, including adolescents (25), young adults (22,23), and older adults (26). Trials began with cues signaling potential rewards, losses, or no-incentive. Next, participants pressed a button in response to a briefly presented target; they were instructed that rapid reaction times (RTs) increased their chances of receiving gains and avoiding penalties. Finally, feedback indicated whether money was gained or lost. Analyses focused on basal ganglia regions of interest (ROIs). Participants also rated cues and outcomes for arousal and valence.
We predicted that, relative to controls, maltreated participants would show slower RT on reward trials, rate reward cues as less positive, and show weaker basal ganglia responses to reward cues. Group differences in response to gains were not predicted, based on recent animal findings (17) and evidence that early adversity affects DA transmission (19) that is most strongly associated with reward anticipation (22,23,27).
Methods and Materials
Participants
Maltreated group
Maltreated participants were recruited from a study exploring relations between social risk factors and psychopathology in young adulthood (24). Recruitment was directed at 63 individuals: 18 could not be relocated, 21 were excluded (Supplementary Material), three could not be scheduled, and two declined. The remaining 19 underwent fMRI scanning; data from six individuals were excluded due to excessive head movement. The final sample consisted of 13 young adults (four males; Table 1). Eight have been studied since infancy (mean±SD age at enrollment=8.88±5.36 months), five have been studied since young adulthood (20.60±1.34 years).
Table 1.
Sociodemographic and Self-Reported Mood Data
| Maltreated Group | Control Group | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Statistic | p-value | |
| % Female | 69% | N/A | 45% | N/A | χ2(1)=2.14 | >.13 |
| Age | 24.58 | .88 | 37.08 | 13.77 | t(40)=4.87 | < .001 |
| Education | 12.92 | 2.22 | 15.28 | 1.65 | t(40)=3.84 | < .001 |
| % Caucasian | 77% | N/A | 76% | N/A | χ2(1)=.006 | > .94 |
| MASQ-GDD* | 22.77 | 12.90 | 14.89 | 2.97 | t(39)=3.10 | < .005 |
| MASQ-GDA* | 17.15 | 7.10 | 14.00 | 2.46 | t(39)=1.56 | > .13 |
| MASQ-AD* | 55.69 | 20.84 | 42.57 | 7.81 | t(39)=2.20 | < .047 |
| MASQ-AA* | 20.62 | 3.93 | 18.54 | 1.95 | t(39)=1.81 | > .09 |
| CES-D | 12.15 | 12.93 | N/A | N/A | N/A | N/A |
| BDI-II* | N/A | N/A | 2.25 | 2.46 | N/A | N/A |
Note. MASQ: Mood and Anxiety Symptom Questionnaire (35); GDD: General Distress Depressive symptoms; GDA: General Distress Anxious symptoms; AD: Anhedonic Depressive symptoms; AA: Anxious Arousal symptoms; CES-D: Center for Epidemiological Studies Depression Scale (36); BDI-II: Beck Depression Inventory (37); N/A: not applicable.
One control participant had missing values for the MASQ and BDI.
Participants were recruited based on evidence of emotional, physical, and/or sexual abuse during childhood that met state guidelines for maltreatment. Evidence of abuse was rated in the original study from multimodal assessments including the Adult Attachment Interview (28,29), the revised Conflict Tactics Scale (30), the Traumatic Stress Schedule (31), whether a report of concern for safety was substantiated by protective services before age seven, and whether major disruption of placement with the primary caretaker had occurred. Reliability of abuse ratings was high (ICC = .99, n = 37). Among the thirteen participants, twelve reported abuse before age 12 (emotional abuse n=1; physical or sexual abuse n=6; multiple types of abuse n=5), with one participant reporting abuse beginning at age 13 (sexual abuse) (see Supplementary Material for details).
Maltreated participants were right-handed (32), reported no history of medical or neurological conditions, and met fMRI safety criteria. Two maltreated participants reported using psychotropic medications in the weeks prior to scanning (citalopram, n=1; hydrocodone, n=1).
Controls
Maltreated participants were compared to community controls (n=31) who performed the same task for another study (33). Data from two controls were discarded due to excessive head movement. The final sample included 16 males (Table 1). Controls were righthanded (32), reported no history of neurological or medical conditions, no current or past psychopathology, no psychotropic medication use, and met fMRI safety criteria. Controls were older and more educated than maltreated participants, but gender and racial composition were similar (Table 1). Community controls were used because the study from which maltreated individuals were recruited did not yield an adequate number of non-maltreated individuals for comparison.
Procedure
Informed consent
Participants consented to an IRB-approved protocol and were debriefed after the study. Maltreated and control participants were compensated $100 and $80, respectively, for the fMRI session, and were given $20-$22 as “earnings” from the task.
Psychopathology assessments
Structured clinical interviews for DSM disorders (SCID-I: 34) were administered once to controls to rule out psychopathology. Two SCIDs were administered to maltreated participants. The first covered lifetime through young adulthood (age at first SCID: 20.10±1.43 years). The second was administered shortly before the experimental session (1.17±1.58 months before) and focused on the interval between young adulthood and the experimental session (interval between SCIDs: 4.48±1.47 years). Both groups completed the Mood and Anxiety Symptoms Questionnaire (MASQ: 35), which assesses anxious arousal (AA), anhedonic depression (AD), and general distress due to anxiety (GDA) or depression (GDD). Maltreated participants completed the Center for Epidemiological Studies Depression Scale (CES-D: 36); controls completed the Beck Depression Inventory (BDI-II: 37).
MID task
The MID task was based on previous studies (22) and identical to a prior version (23). There were five blocks of 24 trials. Trials began with one of three visual cues (1.5 s) signaling potential outcomes (reward: +$; loss: −$; no-incentive: 0$) (8 trials/cue per block). Following a jittered inter-stimulus interval (ISI: 3-7.5 s), a red square was presented for a variable duration. Participants responded to the square with a button press. Following a second ISI (4.4-8.9 s), visual feedback (1.5 s) indicated delivery of a gain (range: $1.96 to $2.34; mean: $2.15), penalty (range:−$1.81 to −$2.19; mean: −$2.00), or “no change”. Reward trials ended with gains or no change, loss trials ended with penalties or no change, and no-incentive trials ended in no change. An inter-trial-interval separated the trials (3-12 s).
To achieve a balanced design, half the reward and loss trials ended in gains and penalties, respectively. However, participants were told that rapid RTs increased their chances of receiving gains and avoiding penalties so that RT could be used to probe incentive motivation. After blocks two and four, participants rated cues and outcomes for arousal (1=low, 5=high) and valence (1=negative, 5=positive). Ratings data were not collected for two maltreated participants due to time constraints, and reward cue valence ratings were not collected from one control due to error.
MRI Acquisition
MRI data were acquired on a 1.5T Symphony/Sonata scanner (Siemens Medical Systems; Iselin, NJ), using tilted slice acquisition and z-shimming to minimize susceptibility artifacts (38). During structural imaging, a T1-weighted MPRAGE volume was acquired (TR/TE: 2730/3.39 ms; FOV: 256 mm; 1×1×1.33 mm voxels; 128 slices). During functional imaging, gradient echo T2*-weighted echoplanar images were acquired (TR/TE: 2500/35ms;FOV: 200 mm; 3.125×3.125×3 mm voxels; 35 slices; 222 volumes).
Data Analysis
Mood
Between-groups t-tests tested for differences on the MASQ.
RT and affective ratings
After removing outliers (RTs exceeding mean±3SD), RTs were entered into a Group × Cue × Block analysis of variance (ANOVA). Ratings were entered into Group × Cue or Group × Outcome ANOVAs. Significant differences were followed-up with t-tests. The Greenhouse-Geisser correction was used when sphericity was violated.
fMRI
Neuroimaging data were analyzed using FS-FAST (http://surfer.nmr.mgh.harvard.edu) and FreeSurfer (39). Pre-processing included motion and slice-time correction, removal of slow trends using a second order polynomial, intensity normalization, and spatial smoothing (6 mm FWHM Gaussian filter). Hemodynamic responses were modeled as a gamma function convolved with stimulus onsets. A temporal whitening filter estimated and corrected for autocorrelation in the noise. Participants with head movement greater than 3.75 mm or degrees were excluded (approximately the size of 1 functional voxel; control: n=2; maltreated: n=6). For remaining participants, motion parameters were included as nuisance regressors.
Four basal ganglia ROIs were defined by FreeSurfer’s sub-cortical segmentation algorithm: nucleus accumbens (NAcc), caudate, putamen, and globus pallidus (39,40; Supplementary Material). Average beta weights measuring the fit of the data to the model were extracted from each ROI for the cues and three outcomes (gains, penalties, no change feedback on no-incentive trials) and entered into Group × Cue (or Outcome) × Hemisphere × Structure (NAcc, caudate, putamen, pallidus) ANOVAs. Significant effects were followed up with ANOVAs and t-tests. Analysis-of-covariance (ANCOVA) was not used because groups differed on the potential covariates, age and education, violating a key assumption of ANCOVA (41; Supplementary Material).
Basal ganglia volumetry
Basal ganglia volumes were extracted from FreeSurfer, divided by intracerebral volume, multiplied by 100 to yield percent intracerebral volume scores, and entered into a Group × Hemisphere × Structure ANOVA.
Regression analyses including age and education
Group differences were followed-up with hierarchical regressions to determine if they remained after removing variance associated with age and education. Possible effects of age on findings were also investigated by comparing maltreated participants to a sub-sample of 13 age-matched controls (Supplementary Material); it was not possible to select a sub-sample of education-matched controls.
Results
Clinical Data
Seventy-seven percent of maltreated participants met DSM-IV criteria for an Axis I disorder at some time (Table 2). On the SCID proximal to scanning, one participant met criteria for MDD, agoraphobia, Generalized Anxiety Disorder (GAD), and posttraumatic stress disorder; another met criteria for GAD (see Supplementary Material for results excluding these participants). No other participants displayed current Axis-I disorder.
Table 2.
Current and Lifetime Axis-I Diagnoses for Maltreated Participants
| Subject | Period | Diagnoses |
|---|---|---|
| 1 | Current | Generalized Anxiety Disorder |
| Lifetime | Major Depressive Disorder; Alcohol Abuse; Cannabis Abuse | |
| 2 | Current | None |
| Lifetime | Major Depressive Disorder; Specific Phobia | |
| 3 | Current | None |
| Lifetime | Alcohol Abuse | |
| 4 | Current | None |
| Lifetime | Anxiety Disorder, Not Otherwise Specified | |
| 5 | Current | Major Depressive Disorder; Agoraphobia without Panic Disorder; Generalized Anxiety Disorder; Post-traumatic Stress Disorder |
| Lifetime | Major Depressive Disorder; Alcohol Dependence; Hallucinogen Dependence; Eating Disorders |
|
| 6 | Current | None |
| Lifetime | None | |
| 7 | Current | None |
| Lifetime | MDD | |
| 8 | Current | None |
| Lifetime | None | |
| 9 | Current | None |
| Lifetime | Alcohol Dependence; Bipolar I Disorder | |
| 10 | Current | None |
| Lifetime | Alcohol Dependence; Cannabis Dependence; Opioid Dependence | |
| 11 | Current | None |
| Lifetime | Panic Disorder without Agoraphobia; Cannabis Abuse | |
| 12 | Current | None |
| Lifetime | Generalized Anxiety Disorder; Social Phobia; Alcohol Abuse; Cannabis Abuse |
|
| 13 | Current | None |
| Lifetime | None |
The mean CES-D score for the maltreated group was low (Table 1). CES-D scores of 16–26 indicate mild depression, while scores above 26 indicate increasingly severe depression (42); by these criteria, the maltreated group was not depressed. Ten maltreated participants indicated no depression (CES-D<16), two indicated mild depression (CES-D=17, 23), and one indicated more severe depression (CES-D=49) (see Supplementary Material for results excluding these participants). However, despite absence of clinical depression, maltreated and control groups differed on MASQ GDD and AD scores (Table 1).
RT
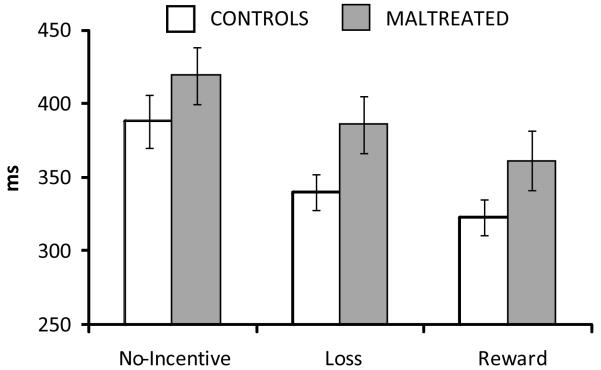
There was a significant Cue effect, F(2,80) = 23.40, p < .001. RT was fastest on reward trials (335.16±68.15 ms), intermediate on loss trials (354.54±69.50 ms), and slowest on no-incentive trials (397.90±88.82 ms) (all ps <.001) (Figure 1), indicating that participants were motivated to obtain gains and avoid penalties. There was a trend for Group, F(1,40) = 2.89, p = .097, as maltreated participants responded more slowly (389.07±68.69 ms) than controls (350.63±67.42 ms). However, contrary to predictions, group differences were not specific to reward trials, Group × Cue, F(2,80) < 1.
Figure 1.
Reaction time (RT) to the target by Group and Cue. Error bars reflect the standard error of the mean.
Affective Ratings
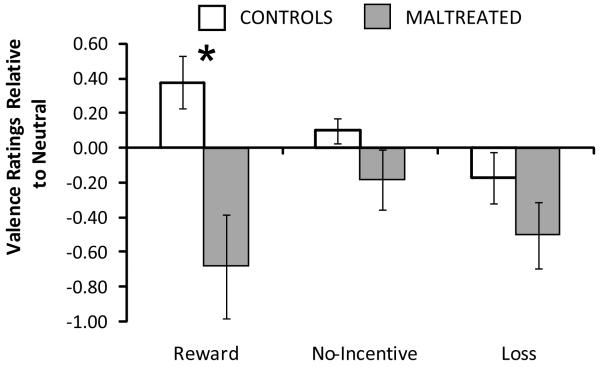
As predicted, analysis of cue-elicited valence revealed a Group effect, F(1,37) = 10.33, p = .003, and a Group × Cue interaction, F(2,74) = 4.14, p = .02. Reward cues were rated less positively by maltreated participants (2.32±.98) relative to controls (3.38±.78), t(37) = 3.55, p = .001, Hedges’ g = 1.24 (SE=.38; 95% CI=.49,−1.99) (Figure 2). Group differences for no-incentive and loss cues were non-significant (ps > .13).
Figure 2.
Valence ratings in response to cues. Data are plotted as change scores relative to neutral valence, which was 3 on the 5-point scale (1 = most negative, 3 = neutral, 5 = most positive). Maltreated participants rated reward cues significantly less positively than controls. Bars indicate the standard error of the mean. *p < .05.
No further evidence for group differences emerged, although there was a trend (p < .09) for maltreated participants to rate all outcomes as less positive than controls. Additional analyses revealed that cues and outcomes elicited intended affective responses (Supplementary Material).
Basal Ganglia Responses
Cues
There were two effects involving Group: a Group × Structure interaction, F(3,120) = 3.26, p < .05, and a Group × Cue × Hemisphere interaction, F(2,80) = 3.77, p = .03. To evaluate the triple interaction, Group × Cue ANOVAs were performed in each hemisphere. The interaction was significant in the left hemisphere, F(2,80) = 3.84, p < .04 (right hemisphere, F(2,80) < 1). Two steps were taken to decompose this interaction. First, within-group tests examined whether cues elicited differential activity in each group. In controls, a one-way ANOVA on data averaged across left hemisphere ROIs confirmed the predicted Cue effect, F(2,56) = 7.54, p = .005: responses to reward cues (.048±.06) were stronger (ps < .006) than responses to no-incentive (.006±.05) or loss cues (.021±.05), which did not differ (p = .17). By contrast, a similar ANOVA in maltreated participants was non-significant, F(2,24) = 1.18, p = .32: follow-up t-tests revealed no differences between responses to no-incentive (.021±.04), loss (.039±.04), or reward cues (.019±.05) (ps > .10). Second, a between-groups t-test investigated the predicted difference in reward cue responses averaged across left hemisphere ROIs; the test was non-significant, t(40) = 1.42, p = .16.
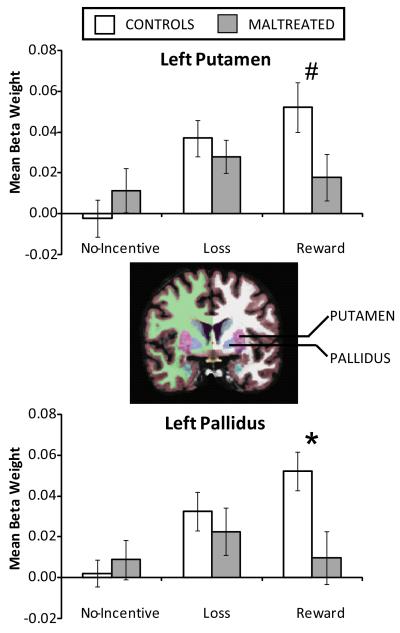
In light of the Group × Structure interaction, additional Group × Cue ANOVAs were conducted for each left hemisphere structure to investigate whether group differences were stronger in particular ROIs. The Group × Cue interaction was not significant in the left NAcc or caudate, Fs (2,80) < 2.74, ps > .08, but was significant in the left putamen, F(2,80) = 3.60, p < .05, and left pallidus, F(2,80) = 3.73, p = .03. Accordingly, between-groups t-tests were conducted in these regions (Figure 3).
Figure 3.
Left hemisphere reward anticipation deficit in the maltreated group. Mean beta weights in the left putamen (top) and left globus pallidus (bottom) by Group and Cue. The coronal image in the center depicts the FreeSurfer subcortical segmentation for a representative participant, with the putamen in pink and the globus pallidus in blue. The maltreated group showed a blunted response to reward cues in both structures. Furthermore, whereas controls showed significant modulation of activity as a function of Cue in both regions, the maltreated group did not. *p < .05; #p < .10.
For the left putamen, controls generated a marginally stronger response to reward cues (.052±.07) than maltreated participants (.018±.04), t(40) = 1.73, p = .09, Hedges’ g = .57 (SE=.34; 95% CI=−.10,1.23) , but responses to no-incentive and loss cues were similar (ps > .36). For the left pallidus, controls generated a stronger response to reward cues (.052±.05) than maltreated participants (.001±.05), t(40) = 2.55, p = .02, Hedges’ g =.83 (SE=.35; 95% CI=.16,1.51) , but responses to no-incentive and loss cues were again similar, ps > .53. Finally, within-group one-way ANOVAs confirmed that the Cue effect was significant in both regions for controls (Fs > 12.18, ps < .001), but in neither region for maltreated participants (Fs < 1.03, p > .36). Thus, the predicted group difference in reward cue response emerged for the left pallidus, with a similar trend in the left putamen.
Outcomes
The ANOVA revealed no evidence for between-group differences in outcome responses (all effects involving Group, ps > .18).
Basal Ganglia Volumes
There was a Group effect, F(1,40) = 18.60, p < .001, and several interactions involving Group: Group × Hemisphere, F(1,40) = 4.43, p = .04, Group × Structure, F(3,120) = 5.86, p = .003, and Group × Hemisphere × Structure, F(3,120) = 3.24, p = .04. To decompose the highest-order interaction, Group × Hemisphere ANOVAs were conducted at each structure. The interaction was only significant for the putamen, F(1,40) = 5.77, p = .02 [Fs(1,40) < 1.24, ps > .26 for other structures]. T-tests revealed larger putamen volumes for maltreated participants in the left hemisphere (maltreated: .406±.03; controls: .356±.04), t(40) = −3.80, p < .001, Hedges’ g = −1.24 (SE=.36; 95% CI=−1.95,−.54), and right hemisphere (maltreated: .385±.03; controls: .348±.04), t(40) = −2.98, p = .005, Hedges’ g = −0.95 (SE=.35; 95% CI= −1.66,−.29).
These results likely reflect the group difference in age. Indeed, among controls, age was significantly negatively correlated with putamen volume in the left (controls: r = −.70, p < .001; maltreated: r = −.47, p = .11) and right (controls: r = −.60, p = .001; maltreated: r = −.33, p = .27) hemispheres. Accordingly, hierarchical regressions predicting putamen volume by age (entered first) and group (entered second) revealed strong effects for Age (left putamen: beta = −.61, p < .001; right putamen: beta = −.56, p < .001), whereas Group was not a significant predictor of volume (left putamen: beta = .24, p = .06; right putamen: beta = .17, p = .22).
Regression Analyses
Additional regressions tested for effects of Group (entered second: 0=control, 1=maltreated) on reward cue valence ratings, MASQ AD, and MASQ GDD after accounting for age and education (entered first). For each variable, Group emerged as a significant predictor after accounting for age and education (MASQ AD: beta = .39, p < .05; MASQ GDD: beta = .37, p = .05; reward cue valence ratings: beta = −.47, p = .01). Furthermore, Group improved each model, ΔRs2 > .08, ΔFs > 4.02, ps < .053.
Next, two sets of regressions evaluated whether group differences in left putamen and left pallidus reward cue responses remained after accounting for other variables. In the first models, variables not hypothesized to relate specifically to reward processing (volumetric data, age, education, MASQ GDA, MASQ AA) were entered first, Group was entered second. Group predicted left putamen and left pallidus reward cue responses in these models (Table 3), although the putamen results narrowly missed significance (left putamen: Group beta = −.39, p = .06; left pallidus: Group beta = −.52, p = .04). Furthermore, Group improved the models (left putamen: ΔR2 = .08, ΔF = 3.80, p = .06; left pallidus: ΔR2 = .10, ΔF = 4.66, p = .04).
Table 3.
Hierarchical Regression Results for Left Putamen and Left Pallidus Reward Cue Response
| Predictor | Step | Beta | t-value | p-value |
|---|---|---|---|---|
| Left Putamen | ||||
| First Model | ||||
| Volume | 1 | .01 | .05 | .96 |
| Age | 1 | −.39 | −1.78 | .08 |
| Education | 1 | −.04 | −.20 | .85 |
| MASQ-GDA | 1 | .24 | .99 | .33 |
| MASQ-AA | 1 | −.43 | −1.74 | .09 |
| Group | 2 | −.39 | −1.95 | .06 |
| Second Model | ||||
| Volume | 1 | −.07 | −.28 | .78 |
| Age | 1 | −.38 | −1.65 | .11 |
| Education | 1 | −.10 | −.55 | .59 |
| MASQ-GDA | 1 | .31 | 1.14 | .26 |
| MASQ-AA | 1 | −.27 | −1.05 | .30 |
| MASQ-AD | 1 | −.27 | −1.08 | .29 |
| Reward Cue Valence Ratings | 1 | .26 | 1.37 | .18 |
| Group | 2 | −.18 | −.79 | .44 |
| Left Pallidus | ||||
| First Model | ||||
| Volume | 1 | .13 | .52 | .61 |
| Age | 1 | −.21 | −1.01 | .32 |
| Education | 1 | −.06 | −.31 | .76 |
| MASQ-GDA | 1 | .24 | 1.01 | .32 |
| MASQ-AA | 1 | −.42 | −1.75 | .09 |
| Group | 2 | −.52 | −2.16 | .04 |
| Second Model | ||||
| Volume | 1 | .12 | .43 | .67 |
| Age | 1 | −.19 | −.79 | .44 |
| Education | 1 | −.11 | −.55 | .58 |
| MASQ-GDA | 1 | .18 | .62 | .54 |
| MASQ-AA | 1 | −.31 | −1.20 | .24 |
| MASQ-AD | 1 | 0 | 0 | .99 |
| Reward Cue Valence Ratings | 1 | .28 | 1.43 | .16 |
| Group | 2 | −.34 | −1.21 | .24 |
Note. Group was coded 0 = controls, 1 = maltreated. See Table 1 for additional details.
In the second models, reward cue valence ratings and MASQ AD scores were added in step one. MASQ AD was used rather than MASQ GDD because the scales were correlated, r = .85, p < .001, and anhedonia is directly related to reward responsiveness (43). Not surprisingly, the Group effect was weakened (Table 3). Group no longer predicted reward cue response in left putamen (beta = −.18, p = .44) or left pallidus (beta = −.34, p = .24), and no longer improved the models (left putamen: ΔR2 = .02, ΔF < 1, p = .44; left pallidus: ΔR2 = .04, ΔF = 1.45, p = .24). These results indicate that group differences in reward cue valence ratings, MASQ AD, and left putamen/left pallidus reward cue responses share common variance. Indeed, left hemisphere basal ganglia reward responses were negatively correlated with MASQ AD across groups (putamen: r = −.31, p = .05; pallidus: r = −.26, p = .097), and positively correlated with reward cue valence ratings (putamen: r = .36, p = .02; pallidus: r = .29, p = .07).
Discussion
Consistent with findings in non-human animals (15-19), maltreated participants reported elevated depressive and anhedonic symptoms, rated reward-predicting cues less positively, and showed decreased anticipatory reward activity in the left pallidus relative to controls. Results indicate that childhood adversity that includes maltreatment is associated with impaired reward processing (13,14). Furthermore, the findings highlight a neural mechanism that could contribute to relationships between childhood adversity and psychopathology: decreased anticipatory reward activity in the left basal ganglia. The pallidus integrates reward information and conveys it to motor cortex via the thalamus (44). Thus, pallidus dysfunction might weaken the ability of reward-predicting cues to elicit goal-directed actions.
The relationship between childhood adversity and decreased subjective and neural responses to reward-predicting cues, rather than rewards themselves, was predicted based on findings in non-human animals. For example, early maternal deprivation in marmosets impaired motivation to work for liquid reinforcement but did not affect consummatory behavior (17). We expected similar results because DA neurons that project to the basal ganglia are susceptible to stress-related dysfunction (18,19) and critical for incentive motivation (21,27). However, it should be noted that early adversity can also weaken the hedonic impact of obtained rewards (16,19), possibly via effects on opioid systems (45). Accordingly, group differences in consummatory responses might emerge in larger samples or different paradigms.
The findings are consistent with the hypothesis that childhood adversity may have affected the development of DA systems. However, any strong causal interpretation of the data would be premature. In this small sample, we cannot disentangle effects of maltreatment per se from many potential correlates of maltreatment, such as inherited dysfunction in neural activity, parental depression or substance abuse, or the contribution of previous psychiatric issues (Table 2). Prospective studies using larger samples are needed to distinguish among such correlated factors.
Although the SCID and CES-D revealed little evidence of current clinical depression in maltreated participants, the groups differed on self-reported symptoms of depression and anhedonia. Moreover, when MASQ-AD scores and reward cue valence ratings were controlled, the Group effect on left pallidus reward cue responses became non-significant. One possibility is that the anhedonic symptoms and basal ganglia dysfunction are two manifestations of the same dysfunction. Indeed, MASQ-AD scores and reward cue responses in the left pallidus and left putamen were negatively correlated. In addition, the attenuated response to reward-predicting cues in the left pallidus is consistent with evidence of basal ganglia dysfunction in clinical depression. For example, relative to controls, depressed individuals showed weaker basal ganglia responses to reward-predicting cues and gains in the MID task (33), reduced ventral striatal responses to positive words (46), decreased caudate glucose metabolism (47) and blood flow (48), and reduced extracellular caudate and putamen DA (49).
The restriction of deficits to the left hemisphere was not predicted but echoes reports that post-stroke depression more often follows damage to the left versus right hemisphere (50), with globus pallidus lesions highly predictive of depression (51). Moreover, a study in healthy participants reported a positive correlation between D2-receptor availability in the left putamen and incentive motivation (52), consistent with the fact that left hemisphere group differences were specific to reward anticipation. Findings are also consistent with reported relationships between childhood maltreatment and electrophysiological abnormalities over the left hemisphere (53). The reason for this hemispheric asymmetry is unclear, but asymmetrical projections of DA neurons may play a role (54).
Critically, results do not reflect a global deficit in maltreated participants. There were no significant group differences in affective ratings to any stimulus except reward cues, and no differences in basal ganglia response to (a) loss or no-incentive cues in the left hemisphere, (b) any cue in the right hemisphere, or (c) any outcome. Furthermore, group differences in left pallidus reward cue responses remained after controlling for anxiety and general distress (Table 3).
The study possesses several limitations. First, several maltreated individuals were excluded due to active substance abuse, and the striatum is tonically hypoactive in substance abusers (55). Thus, we may have excluded individuals with severe reward processing dysfunction, yielding a conservative estimate of effects of maltreatment on reward processing. Second, the lack of group differences in response to loss cues and penalties may reflect a weakness of the MID task: because participants knew they would be paid for participation, the loss cues and penalties may not have been sufficiently aversive to elicit group differences. Notably, other studies report relationships between childhood adversity and sensitivity to emotionally negative stimuli (e.g., 56). Third, the current sample was too small to determine whether a dose-response relationship exists between extent or age of onset of maltreatment and responses to reward cues, or to examine whether specific types of maltreatment have different effects on reward processing. Larger studies are needed to address these issues, and to investigate whether particular genetic backgrounds or social supports can protect reward systems from adversity-induced dysfunction (57,58).
Fourth, because controls and maltreated participants were from different cohorts, variables besides maltreatment may have affected the results. Three considerations mitigate this concern. First, the loss and no-incentive conditions and cue/outcome design served as internal controls that allowed us to pinpoint the predicted differences in reward anticipation; the lack of differences in other conditions argues against a general deficit in maltreated participants. Second, the strong basal ganglia response to reward cues demonstrated by the controls is the norm in the MID task and has been demonstrated in samples differing in age, education, and sociodemographics (22,23,25,26). Thus, the findings reflect a deficit in maltreated participants rather than atypical results in the controls. Third, Group predicted left pallidus reward cue responses after adjusting for age, education, anxiety, and basal ganglia volumes. Nonetheless, groups may have differed on other variables not measured, especially because maltreated individuals tend to be exposed to multiple forms of childhood adversity (59). Consequently, results should be interpreted in terms of childhood adversity, rather than maltreatment per se.
Supplementary Material
Acknowledgements
This work was supported by grants from NIMH to Dr. Pizzagalli (R01MH68376, R21MH078979), and Dr. Lyons-Ruth (R01MH062030), as well as Harvard University’s Robert Wood Johnson Health and Society Scholars program and Talley Fund award to Dr. Pizzagalli. The authors are grateful to Kyle Ratner, Elena Goetz, James O’Shea, and Decklin Foster for their skilled assistance, to Drs. Lawrence L. Wald and Christopher Wiggins for their help implementing the optimized fMRI pulse sequence utilized in the present study, and to Dr. Douglas N. Greve for his expert assistance with the FS-FAST and FreeSurfer packages.
Footnotes
Financial Disclosures Dr. Pizzagalli has received research support from GlaxoSmithKline and Merck & Co. for studies unrelated to this project. Dr. Dillon, Mr. Holmes, Mr. Birk, Ms. Brooks, and Dr. Lyons-Ruth report no competing interests.
Supplementary material cited in this article is available online.
References
- 1.Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences study. JAMA. 2001;286:3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- 2.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the Adverse Childhood Experiences study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- 3.Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282:1652–1658. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- 4.Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999;156:1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- 5.Nelson EC, Heath AC, Madden PA, Cooper ML, Dinwiddie SH, Bucholz KK, Glowinski A, McLaughlin T, Dunne MP, Statham DJ, Martin NG. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Arch Gen Psychiatry. 2002;59:139–145. doi: 10.1001/archpsyc.59.2.139. [DOI] [PubMed] [Google Scholar]
- 6.Gladstone GL, Parker GB, Mitchell PB, Malhi GS, Wilhelm K, Austin MP. Implications of childhood trauma for depressed women: an analysis of pathways from childhood sexual abuse to deliberate self-harm and revictimization. Am J Psychiatry. 2004;161:1417–1425. doi: 10.1176/appi.ajp.161.8.1417. [DOI] [PubMed] [Google Scholar]
- 7.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 8.Navalta CP, Polcari A, Webster DM, Boghossian A, Teicher MH. Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. J Neuropsychiatry Clin Neurosci. 2006;18:45–53. doi: 10.1176/jnp.18.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 10.Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, Ernst M. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. J Am Acad Child Adolesc Psychiatry. 2006;45:1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- 11.Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry. 1983;40:79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 13.Lumley M, Harkness K. Specificity in the relations among childhood adversity, early maladaptive schemas, and symptom profiles in adolescent depression. Cogn Ther Res. 2007;31:639–657. [Google Scholar]
- 14.Harkness K, Monroe S. Childhood adversity and the endogenous versus nonendogenous distinction in women with major depression. Am J Psychiatry. 2002;159:387–393. doi: 10.1176/appi.ajp.159.3.387. [DOI] [PubMed] [Google Scholar]
- 15.Cabib S, Puglisi-Allegra S. Stress, depression and the mesolimbic dopamine system. Psychopharmacology (Berl) 1996;128:331–342. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- 16.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural response to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 17.Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol Psychiatry. 2004;56:72–79. doi: 10.1016/j.biopsych.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 20.Martin LJ, Spicer DM, Lewis MH, Gluck JP, Cork LC. Social deprivation of infant rhesus monkeys alters the chemoarchitecture of the brain: I. Subcortical regions. J Neurosci. 1991;11:3344–3358. doi: 10.1523/JNEUROSCI.11-11-03344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 23.Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2008;45:36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyons-Ruth K, Connell DB, Grunebaum HU, Botein S. Infants at social risk: maternal depression and family support services as mediators of infant development and security of attachment. Child Dev. 1990;61:85–98. doi: 10.1111/j.1467-8624.1990.tb02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samanez-Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Henize H-J, Zilles K, Duzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.George C, Kaplan N, Main M. Adult attachment interview. University of California, Berkeley; Berkeley, CA: 1985. [Google Scholar]
- 29.Lyons-Ruth K, Block D. The disturbed caregiving system: relations among childhood trauma, maternal caregiving, and infant affect and attachment. Infant Ment Health J. 1996;17:257–275. [Google Scholar]
- 30.Strauss MA, Hamby SL, Boney-McCoy S, Sugarman DB. The revised conflict tactics scale (CTS2) J Fam Issues. 1996;17:283–316. [Google Scholar]
- 31.Norris FH. Screening for traumatic stress: a scale for use in the general population. J Appl Soc Psychol. 1990;20:1704–1718. [Google Scholar]
- 32.Chapman LJ, Chapman JP. The measurement of handedness. Brain Cogn. 1987;6:175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- 33.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty D, Iosifescu D, Rauch S, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated subjects with Major Depressive Disorder. Am J Psychiatry. doi: 10.1176/appi.ajp.2008.08081201. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interviews for DSM-IV Axis I disorders (SCID) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- 35.Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- 36.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 37.Beck AT, Steer RA, Brown GK. Beck Depression Inventory manual. 2nd ed Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- 38.Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- 39.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 40.Tae WS, Kim SS, Lee KU, Nam E-C, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50:569–581. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- 41.Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- 42.Blumenthal JA, Lett HS, Babyak MA, White W, Smith PK, Mark DB, Jones R, Mathew JP, Newman MF, NORG Investigators Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362:604–609. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 43.Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biol Psychiatry. 2005;15:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- 45.Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of Long-Evans rats results in long-lasting changes in morphine tolerance and dependence. Psychopharmacology. 2001;157:305–312. doi: 10.1007/s002130100806. [DOI] [PubMed] [Google Scholar]
- 46.Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 47.Gabbay V, Hess DA, Liu S, Babb JS, Klein RG, Gonen O. Lateralized caudate metabolic abnormalities in adolescent major depressive disorder: a proton MR spectroscopy study. Am J Psychiatry. 2007;164:1881–1889. doi: 10.1176/appi.ajp.2007.06122032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff NP, Wilson AA, Houle S. Elevated putamen D(2) receptor binding potential in major depression with motor retardation: an [11C]raclopride positron emission tomography study. Am J Psychiatry. 2006;163:1594–1602. doi: 10.1176/ajp.2006.163.9.1594. [DOI] [PubMed] [Google Scholar]
- 50.Starkstein SE, Robinson RG, Price TR. Comparison of cortical and subcortical lesions in the production of poststroke mood disorders. Brain. 1987;110:1045–1059. doi: 10.1093/brain/110.4.1045. [DOI] [PubMed] [Google Scholar]
- 51.Vataja R, Leppavuori A, Pohjasvaara T, Mantyla R, Aronen HJ, Salonen O, Kaste M, Erkinjuntti T. Poststroke depression and lesion location revisited. J Neuropsychiatry Clin Neurosci. 2004;16:156–162. doi: 10.1176/jnp.16.2.156. [DOI] [PubMed] [Google Scholar]
- 52.Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol. 2008;77:98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, Ackerman E. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Ann NY Acad Sci. 1997;821:160–175. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- 54.Carlson JN, Fitzgerald LW, Keller RW, Glick SD. Lateralized changes in prefrontal cortical dopamine activity induced by controllable and uncontrollable stress in the rat. Brain Res. 1993;630:178–187. doi: 10.1016/0006-8993(93)90655-7. [DOI] [PubMed] [Google Scholar]
- 55.Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 56.Pollak SD. Mechanisms linking early experience and the emergence of emotions. Curr Dir Psychol Sci. 2008;17:370–375. doi: 10.1111/j.1467-8721.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charney D. Psychobiological mechanisms for resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 59.Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Loo CM, Giles WH. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl. 2004;28:771–784. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.