Abstract
Some rats spinally transected as neonates (ST rats) achieve weight-supporting independent locomotion. The mechanisms of coordinated hindlimb weight support in such rats are not well understood. To examine these in such ST rats and normal rats, rats with better than 60% of weight supported steps on a treadmill as adults were trained to cross an instrumented runway. Ground reaction forces, coordination of hindlimb and forelimb forces and the motions of the center of pressure were assessed. Normal rats crossed the runway with a diagonal trot. On average hindlimbs bore about 80% of the vertical load carried by forelimbs, although this varied. Forelimbs and hindlimb acted synergistically to generate decelerative and propulsive rostrocaudal forces, which averaged 15% of body weight with maximums of 50% . Lateral forces were very small (<8% of body weight). Center of pressure progressed in jumps along a straight line with mean lateral deviations <1 cm. ST rats hindlimbs bore about 60% of the vertical load of forelimbs, significantly less compared to intact (p<0.05). ST rats showed similar mean rostrocaudal forces, but with significantly larger maximum fluctuations of up to 80% of body weight (p<0.05). Joint force-plate recordings showed forelimbs and hindlimb rostrocaudal forces in ST rats were opposing and significantly different from intact rats (p<0.05). Lateral forces were ~20% of body weight and significantly larger than in normal rats (p<0.05). Center of pressure zig-zagged, with mean lateral deviations of ~ 2cm and a significantly larger range (p<0.05). The haunches were also observed to roll more than normal rats. The locomotor strategy of injured rats using limbs in opposition was presumably less efficient but their complex gait was statically stable. Because forelimbs and hindlimbs acted in opposition, the trunk was held compressed. Force coordination was likely managed largely by the voluntary control in forelimbs and trunk.
Introduction
About 20% of neonatal rats subject to T8–T10 complete spinal transections develop good independent hindlimb weight support in locomotion as adults (Stelzner et al., 1975). Fetal spinal cord transplants placed into the transection cavity in neonatal rats increase the number of spinalized adult rats achieving weight support to 50– 60% (Miya et al. 1997). Howland and colleagues (1995a,b) obtained similar results in cats. A fraction of rat pups or kittens thus show a limited recovery of weight support following transection, improved by interventions. However, the mechanisms of such weight support are not well understood.
Cortical motor representations in neonatally injured rats with and without transplants show improved trunk representation but no hind-limb representation in the weight supporting rats compared to non-weight supporting (Giszter et al. 1998). This suggested that there is no direct control of hindlimb stepping motions even after transplants. Our data from stance (Giszter et al. 2007) leads us to speculate that stepping in such rats was largely autonomous, and was initiated and controlled voluntarily via trunk, acting through the available mechanical and reflex couplings. To further test this idea, we examined kinetic features of locomotion in detail in intact and SCI animals.
Individual limbs’ ground reaction force patterns and kinetics have been well described in cats in locomotion (Lavoie et al. 1995; Gregor et al 2006; Kaya et al. 2006), stance (Macpherson 1988, Fung and Macpherson, 1995), reaching (Schepens and Drew, 2003), and jumping (Zajac et al., 1981), and in stance after adult spinal transection (Fung and Macpherson, 1999, Deleon et al., 1998). Howland et al. did not examine ground reaction forces in the cats spinalized as kittens which achieved weight support. Vertical support forces of fore and hind-limbs in rats have been described by Clarke (1986, 1994) in normal rats. Analysis of forces of individual limbs in trot carefully controlled for speed and consistency in intact or partial injuries have been described by Muir and Whishaw (1999a, b; 2000), and by Webb et al. (2003), and Webb and Muir (2003,2004). Mulligan et al., 2002 and Zumwalt et al., 2006 provide novel recording designs for single plates. Howard et al. 2000 used a single force plate to assess different limbs after peripheral injuries. Joint recordings of multiple limbs on multiple plates have not generally formed the basis of analysis of injury in rats, but have been used in Giszter et al. 2007 for stance in neonatal spinalized rats.
In neonatal spinal transected (ST) rats that achieved weight-support as adults, we found the gait exhibited was too variable to allow standard gait analysis, with the usual averaging of many cycles of locomotion or runs in a meaningful way. Similarly, the gait was rarely if ever constant velocity. To compare statistically between normal and ST rats we therefore used a different strategy. In a runway task, we tested ground reaction forces and their cycle by cycle coordination and variance in normal rats without the usual velocity constraints imposed in gait analysis and we made similar measures in ST rats with weight support. In this paper we present an examination of these simultaneously recorded net ground reaction force vectors, those simultaneously generated by forelimbs and hindlimbs within a the locomotor cycle. We also present a motion of the center of pressure which was possible in our design. We performed these analyses both in the weight supporting locomotion of normal rats, and of transplant and transect rats. Our methods allowed us to examine the coordination of the hind-limb and fore-limb generated forces within each step-cycle. The simultaneous paired records allowed meaningful comparisons of the coordination statistics, although there were not infrequently accelerative and decelerative transients in the rats crossing the runway. In this way we examined how support and locomotive forces during the support phases of locomotion were organized when compared to the normal unconstrained range of task variations despite the high locomotion variability in the groups of fully spinalized rats.
Our data will show that the patterns of force generation in the gaits of normal and injured rats are qualitatively different. The data are consistent with the notion that control of weight-bearing hindlimb locomotion in spinalized rats is achieved through their skilled voluntary control of trunk and forelimb generated forces coupled to autonomous stepping of lumbar pattern generators. Forces delivered via the trunk may act to steer, coordinate and contain the effects of involuntary and autonomous hindlimb stepping, predominantly through mechanical and reflex interactions.
Methods
Neonatal transection/transplantation
36 Sprague Dawley rats received midthoracic (T8–T9) transections on postnatal day 1 or 2 (P1 or P2). One or two segments of spinal cord tissue were removed with sharp dissection and gentle aspiration in all 36 rats. In 24 of the 36 rats, a 1–2 mm section of E14 fetal thoracic spinal tissue was then immediately transplanted into the lesion cavity (transplant rats, see below). The remaining 12 rats underwent transection only with no transplantation, but gelfoam in the lesion cavity (spinal rats). (Methods described previously in Miya 1997). 12 additional rats served as normal controls (Normal rats). All procedures were approved by the Drexel University IACUC and were in accordance with USDA guidelines and regulations.
Fetal transplantation techniques
Some rats received E14 fetal spinal transplants as described in Miya et al. 1997 and Giszter et al. 1998. However, their gait and force here and responses in other studies (Giszter et al. 2007) did not differ from ST alone, and all rats were combined as an ST group.
Diet restriction
Food pellets were available ad libidum from P21–P120 or until the rats reached a body mass of 190g. Thereafter the rats in this study were food restricted and limited to 15g of food daily so that body mass did not limit their motor performance (by exceeding their muscle power, control and coordinative abilities). The rats' body mass stabilized at 200–250 g for TP and TX rats and <300g for normal rats as a result of this diet.
Locomotor training and animal maintainance
Normal and neonatally injured rats were trained on two locomotor tasks (runway and treadmill) beginning at weaning, 3 weeks post partum. Briefly, rats were trained to walk on a motorized treadmill at 4–8 cm/s speeds. The treadmill was slowed if ST rats walked poorly at 8cm/s but locomotion was improved at the lower speeds. Test animals were water deprived each evening preceding training or testing and rewarded on the treadmill with a dilute sucrose solution delivered through a sipping tube 5cm above the treadmill surface. Animals drank at the tube while maintaining the body at a constant velocity relative to the treadmill in order to remain at the tube. Treadmill training provide more intense exercise than runways. Animals were also trained to walk across the instrumented narrow runway for a water reward.
Test animals were videotaped in both tasks and trained before and after electrode implantation. At the end of training sessions the rats were given ad libidum access to water for either 1 hour (P21–P60) or 20 minutes (P60 on).
Animals were qualitatively evaluated during each session for (a) weight supported step cycles, (b) consistent drinking at the water tube, (c) posture, (d) tail position, (e) hip and hindpaw usage.
Runway experiment design
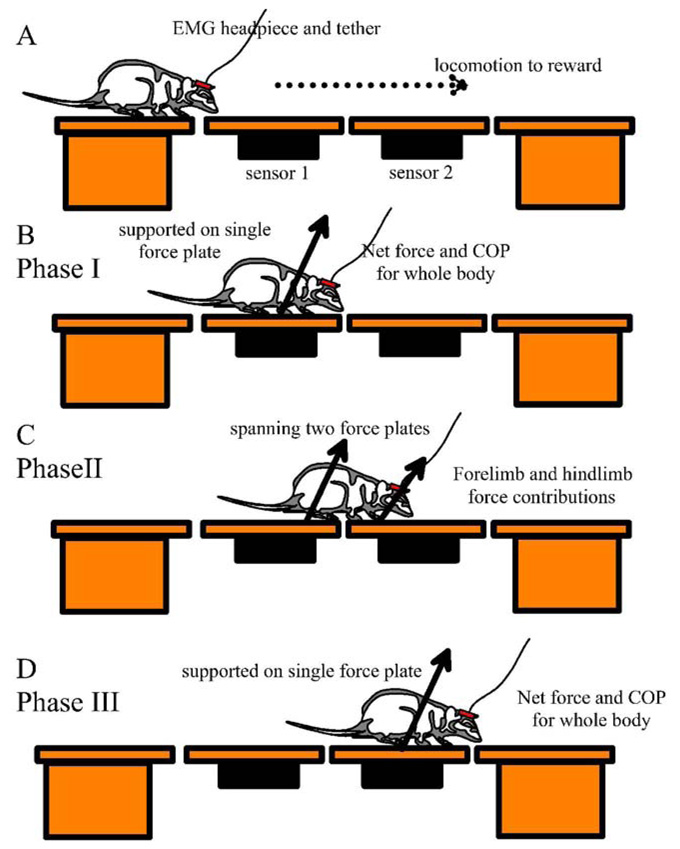
The runway was comprised of four thick stiff plexiglass oblong plates, separated from one another by under 2mm gaps. Each was covered with a thin (0.7 mm) silicone rubber sheet to aid traction. The end plates were mounted on supports. The middle two plates were each mounted on an individual ATI 3/10 force sensor (ATI Industrial Automation, Garner, NC; range of force = 13.35N, range of torque = 1.1Nm, resolution of force = 1.1E-2 N, resolution of torque = 5.65E-4 Nm) supported by rigid frames. Sensors were allowed to warm up for 1hr and calibrated with 100g weights. As the rats locomoted across the runway the force sensors recorded the three translational force components (vertical, rostrocaudal and mediolateral) and torques about these axes at 125Hz. Using a synchronizing light pulse to indicate trial onset, video data was collected from a camera with a shutter time of 2ms, mounted perpendicularly to the plane defined by the midline of the runway and the vertical. Using a 45 degree mirror, a ventral view of the rat on the runway was also collected on videotape. This allowed accurate evaluation of footfall and weight support during the passage across the central part of the instrumented middle of the runway (Phase II in Figure 1).
Figure 1.
Basic Experimental arrangement: A: Four plates form a runway. The gaps between plates were each under 2mm. The central two plates are instrumented with ATI 3/10 gamma sensors. A rat with or without implanted EMG electrodes traverses the runway and video data views of body motion and footfall are collected synchronized +/− 16.7ms with forces. The data collection can be qualitatively divided into three phases shown B–D. B: Phase I. When the rat is wholly supported by the first plate, its sensor can resolve net translational force and center of pressure. C:Phase II. While spanning the central two plates forelimb and hindlimb contributions to net force can be measured. D: after full transfer to the second instrumented plate Phase III begins.
Data Analysis
Data was selected in which rats moved continuously across the runway. Complete stops and turns on the runway were excluded from analysis. There was no application of a speed range constraint beyond continuous forward motion or a uniformity of speed constraint. In this way we obtained distributions of normal rats force and gait patterns on the runway. This approach gave significantly broader statistical distributions for the normal rats than would be observed in standard gait analysis, and showed variations from the mean pattern for fixed uniform speed progression using a single gait. We did this because it was clear that the gait of ST rats showed great variation. We wished to compare features that differed across the range of gait and kinetic behavior seen in both normal and ST rats. A highly constrained statistical comparison of any single normal gait would likely always show many statistically significant different features, partly because of the low variance arising from the rigorous data selection. By allowing variation in the normal rats we tested more rigorously which features of limb force coordination differed systematically in ST rats.
1: Kinematics
Times of individual foot contact and lift were measured from digitized video fields (field rate 60Hz). Due to the viewing field used in the video acquisition, individual limb motion had too small an aspect on the screen to allow accurate digitizing of limb kinematics. Video and force were aligned through the timing light triggered from the force collection computer. Gait was analyzed into toe touch, toe off cycles for each limb, and the gait pattern for each rat measured crossing the runway. Gait patterns varied in spinalized rats. High variance in phasing did not allow individual animal or group spinalized gaits to be cleanly classified. However, the dominant features of their stepping gaits and how they differed from normal gaits were captured by examining limb cycle durations, stance durations, and forelimb hindlimb delays and quantified with parametric statistics.
2: Force
The position coordinate frame for recording Z was oriented vertically, Y rostrocaudally along the runway and X laterally to the right. Forces were expressed as follows: Positive Z was supportive (accelerated up), positive Y was decelerating, positive X was accelerative to the left. For this study, only the data collected from normal rats and weight supporting spinal or transplant rats (operates) were used. A weight-supporting rat is defined as a rat that underwent transection or transplantation surgery as a neonate, but could stand and walk unassisted during more than 60% of its step cycles, i.e., without its belly, knees or hips touching the surface.
The three dimensional forces and torques collected from the force sensors were used to calculate the center of pressure (CoP) for the rat on each sensor. The center of pressure is the point of application of a single translational force that will act of the Center of Mass equivalently to the forces of all legs in contact with the ground. Calculations of CoP were made first in the sensor frame and then transformed into the world frame. X and Y coordinates of center of pressure due to foot placements on each sensor were obtained using the following formula. The x and y coordinates, for each foot placement, were based on the center of the respective sensor. Offsets were added to the individual coordinates, based on the location of the sensors to each other, to have them all related to the same world coordinate system. The z value is the offset needed due to the thickness of the plexiglass.
The individual foot placements on the two sensors and the ground reaction forces were combined to obtain the resultant CoP of the entire rat if the rat straddled the two instrumented sensors.
As a check on the completeness of our description and analysis, we recovered the locations of CoP and the mass of test objects placed on the system, either on a single plate or spanning both plates. Dwell time of center of pressure was estimated by centering gaussian radial basis functions of standard deviation 0.5cm at each measured center of pressure at each time point, and displaying the summation at each point in space of these distributions over time. This provided graphical display of the degree of control and precision of the motions of the center of pressure achieved.
3: Statistical testing
Regression coefficients of forces recorded on the two plates during transitions between plates were calculated using the S-plus or R software packages. Peak, and mean absolute force components on each plate were averaged within groups and compared between them with parametric t-tests. Mean regression coefficient of force correlations through the transitions were compared with parametric t-tests and distribution differences also tested with Kolmogorov Smirnov tests. Step durations, stance durations, interlimb swing initiation and termination delays and phases were calculated in Excel and compared with parametric statistics. Mean forces, and mean absolute forces while rats were fully on force plates were calculated and compared directly and after normalization to body weight (the mean vertical force). Absolute forces were used to examine horizontal components. Using absolute forces was especially useful comparing lateral components of force, where force oscillates and the mean force should be close to zero. We also applied the transformation for rostral components where both acceleration and deceleration occur each cycle. Center of pressure motions were compared statistically by obtaining the lateral variance, the range, the mean absolute deviations laterally, the lateral and rostrocaudal velocity component distributions, their variances and ranges, and the means and ranges of the absolute values of these velocities. Parametric statistics (t-tests for means, and F-tests for variances) were used to detect significant differences in these metrics.
Results
In this study 12 normal rats were tested, 12 spinal (TX) rats were prepared of which 2 had better than 60% weight support, and 24 transplant (TP) rats were prepared, of which 6 had better than 60% weight support. Analysis will focus on these 8 weight supporting rats from the TX and TP groups, and the intact rats as controls and for comparison. We were unable to distinguish any differences in biomechanics between the 2 weight-supporting (WS) TX rats and the weight-supporting 6 TP rats with the techniques here and we will combine their results in subsequent analysis referring to them as WS spinal transected (ST ) rats. Post-mortem histology of all WS ST rats used in this study confirmed them to be complete transections.
Both normal and the WS ST rats were trained to cross the narrow instrumented runway for a water reward. Normal rats crossed the runway efficiently, while WS ST rats were sometimes less motivated to cross. WS ST rats initially had to be actively dissuaded by us from using unusual strategies such as lateral gripping of the runway edges with the forepaws, or abandoning hindlimb weight support. After some training, all WS ST rats crossed repeatedly with a weight supporting gait indistinguishable from the open-field pattern they used except for a reduced yawing of the body (see Miya et al. 1998). This allowed force recordings to be collected routinely.
Gait and force patterns were collected from all rats and form the basis of comparison of normal and injured coordination strategies for ground reaction forces. We analyzed the total ground reaction forces, the forelimb and hindlimb contributions and the motion of the center of pressure of the rats during locomotion. We first describe the normal rats’ force behavior on our apparatus and then compare the spinal injured rats.
Ground reaction force patterns in normal rats : whole body pattern and gait
Normal rats crossed the runway with a trot, a consistent alternating diagonal gait as described by several authors (Muir and Webb 2000, Webb and Muir 2004, Clarke, 1995; and Gillis and Biewener, 2001) but also with walks, and mixtures of these. Antigravity forces showed patterns in keeping with two elastic exchanges of energy within a step cycle (Cavagna, Heglund and Taylor 1977). We divided the analysis of force patterns into vertical or antigravity force components, rostro-caudal directed force components involved in forward progression, and lateral directed force components potentially affecting yaw and roll.
Vertical antigravity forces
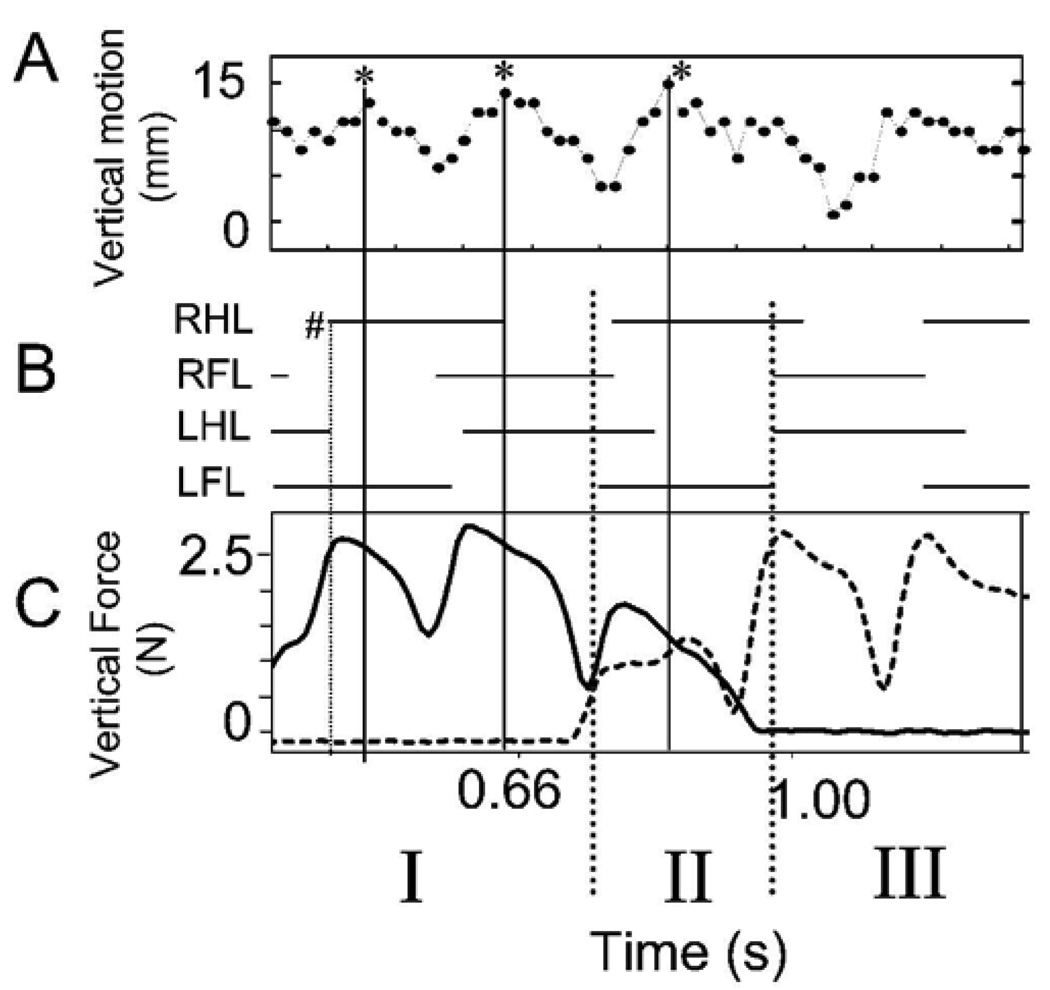
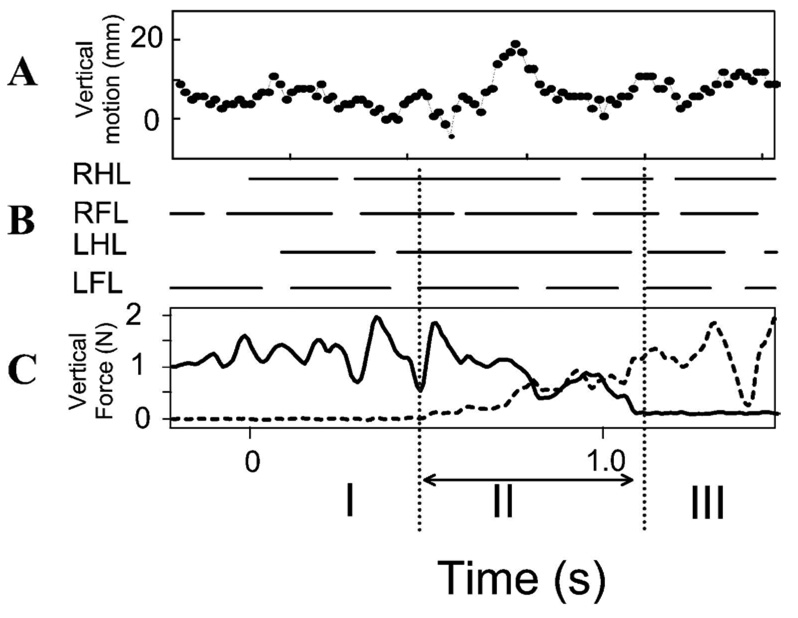
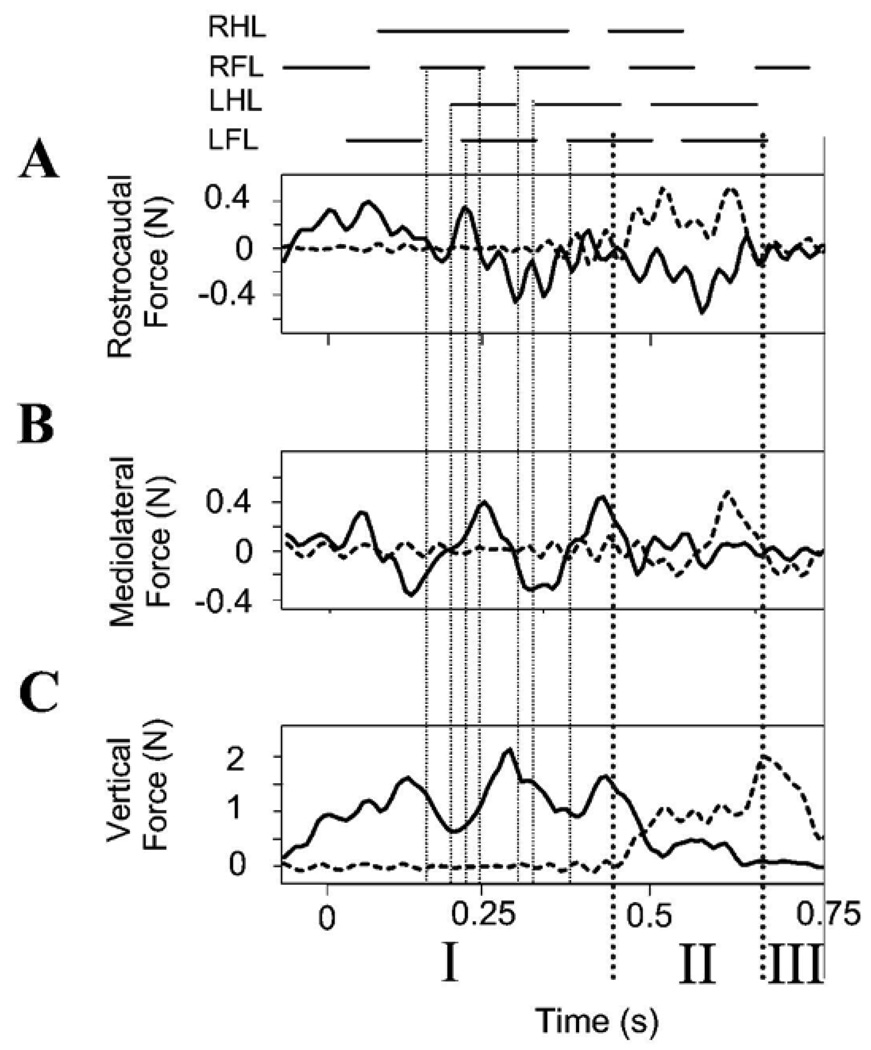
Figure 2A shows the vertical motion of a point on the vertebral column at mid thoracic level as a normal rat progressed across the runway. The gait cycle foot fall pattern is also plotted. The gait is a variable diagonal trot (Figure 2B). Figure 2C shows the simultaneous Z or vertical components of force in a normal rat through time. These forces represent antigravity components responsible for maintained weight support. It can be seen that the vertical force has two peaks per step cycle which are associated with the diagonal hind-limb / fore-limb steps during step cycles in which the rat was wholly on either one of the two force plates (i.e. in Figure 1 diagram, phase I and III). Peak vertical force occurred shortly after step transitions (Figure 1, first dotted vertical line indicated #) . Matching video measures of the height of a midpoint of the back in Figure 1A showed that the back of the animal was also undergoing a vertical motion, in a pattern consistent with elastic energy storage and exchanges (peaks indicated by asterisks). We did not attempt to further analyze the vertical energy exchanges here as they are well described elsewhere (see Webb et al. 2003) and these are probably not relevant to the WS ST rats (see below).
Figure 2.
Normal rat. Vertical (weight bearing) force and motion. A. video measure of the vertical excursion of a fixed point on the rat’s back (units in mm). B. stance phases (horizontal solid bars) of each leg obtained from video. Note that the gait is a variable diagonal trot merging with a lateral walk, as shown by LFL RHL near synchronous footfall, with LFL leading. C. vertical weight support forces. Solid bold trace: sensor 1 vertical force measurement. Dotted bold trace: sensor 2 vertical force measurement. The dotted vertical lines extending below indicate transition between sensors (Phase II). Phase I: rat wholly on sensor 1. Only solid line force is non-zero. Phase II spanning sensors. Net vertical force is sum of solid and dotted traces. Phase III: wholly on sensor 2. Only dotted trace is non-zero. Abscissa for all plots: time in seconds. RHL : right hindlimb. RFL: right forelimb. LHL: left hindlimb. LFL: left forelimb.
Propulsive and decelerative (rostrocaudal) forces
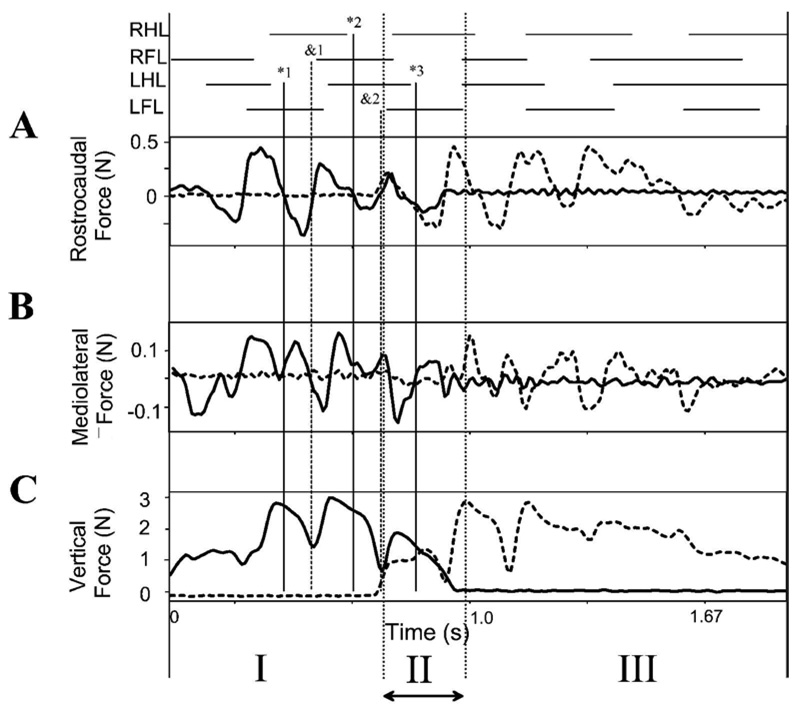
Figure 3 shows the rostrocaudal and mediolateral components of force production in relation to the vertical forces and step pattern in the rat shown in Figure 2. In the step cycles in which the rat is wholly on one force plate, in phases I and III, there were two accelerating and decelerating force cycles accompanying each step cycle (Figure 3A). The zero crossing of rostrocaudal forces occured around the times of the peak and the minimal vertical forces (vertical solid line *1, and dashed line &1). Peak rostrocaudal forces are about 0.4N or about 15% of the antigravity forces. Substantially less effort was thus expended on control of forward progression than on weight support in normal rats.
Figure 3.
Normal rat. Gait and translational force components. Note that in A, B and C each vertical scale differs. Top: stance phase of each leg. A: Propulsive (rostrocaudal) force: solid line is recording of sensor 1, dotted line is recording of sensor 2. Several zero crossings of the propulsive forces are indicated by fine vertical lines. These are extended to show alignment of rostrocaudal, mediolateral and vertical force patterns with gait pattern. *1, *2 and *3 (solid vertical lines with asterisks indicating negative going zero crossings in rostrocaudal forces) align approximately with hindlimb stance ends. Transitions &1, and &2 (dotted vertical lines aligned to positive zero crossing in rostrocaudal forces and minima in vertical force) align with forelimb stance onsets. B: Mediolateral force: solid line is sensor 1, dotted line is sensor 2. C: Antigravity (vertical) force: solid line is sensor 1, dotted line is sensor 2. Phase I : rat only on sensor 1. Phase II: rat spanning sensors. Phase III: rat only on sensor 2. See Figure 1. Abscissa: Time in seconds.
Stabilizing (lateral) forces
The mediolateral forces showed a more variable and complex pattern which is shown in Figure 3B (note the scale difference of A,B, and C). These force variations were phaselocked to the other force component cycles. The mediolateral forces averaged 4 maxima and minima per step cycle. Minima in mediolateral forces were synchronized with zero-crossings in rostrocaudal forces. This can be seen in the comparing panel A and B in Figure 3 at the vertical lines *1, &1, and *2. The cycle to cycle variability was presumably associated with corrections and some speed variation as rats crossed the runway. However, the normal rats were very well-balanced. There were only very small mediolateral forces compared to other components. The peak mediolateral forces were substantially smaller than the other force components, peaking at about 0.1N or 3–4% of antigravity forces.
Ground reaction force patterns in normal rats : forelimb hindlimb coordination
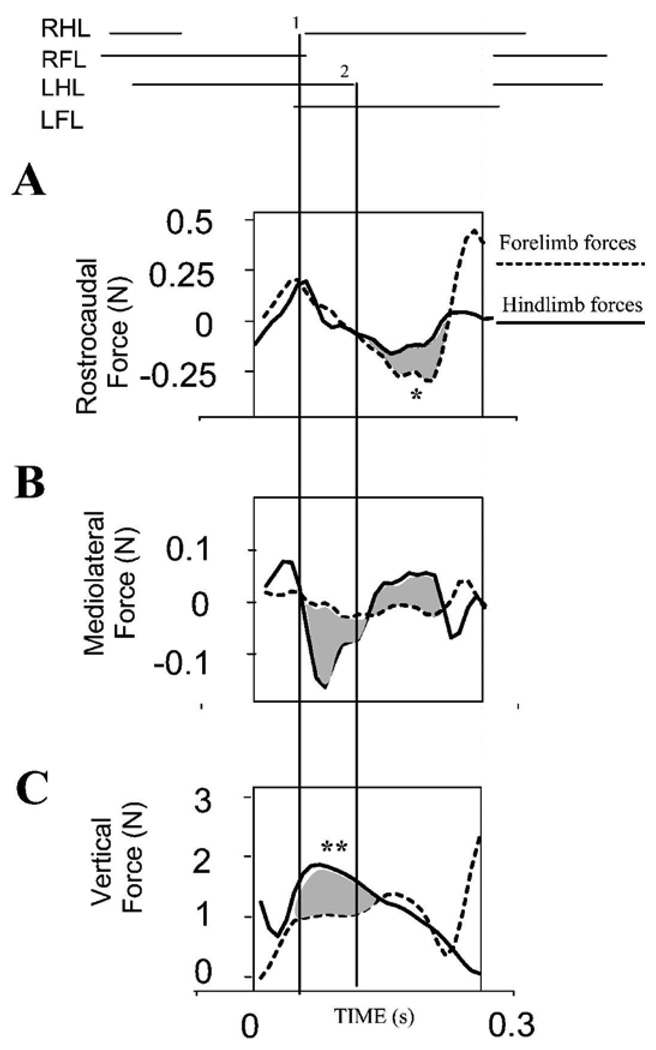
We examined forelimb and hindlimb force regulation within a single step cycle as the animal moved from one force plate to the second (Phase II in Figure 1C, and in Figure 3). This Phase II force pattern is expanded in Figure 4. Both antigravity, rostrocaudal and mediolateral force application on the two platforms and their covariations could be examined. One platform recorded hindlimb forces. The second platform recorded forelimb forces. The differences in forces between the caudal (solid line) and rostral (dotted line) plates are shaded.
Figure 4.
Normal rat. Forelimb and hindlimb force contributions to propulsion, stabilization and weight support. Phase II forces are shown expanded and shaded. Top : stance phase of limbs during transition across plates. A : synergistic decelerative and then propulsive actions of forelimbs (dotted line) and hindlimbs (solid line) is shown by overlapping and similarly directed forces. Rostrocaudal forces correlate well. Individual peak contributions (~0.25N) of forelimbs or hindlimbs are under 10% of body weight (2.7N). In the trial shown forelimbs play a larger part in acceleration (shaded and *). This is within the range of variability from the normal pattern observed in our runway task in which speed was not tightly controlled. B. Lateral forces: most mediolateral force (~0.15N peak, ~5% body weight) is exerted in hindlimbs (solid line). Difference of forelimb and hindlimb contributions are shaded. C: Antigravity forces: Hindlimbs (solid line) carry about 60% more body weight than forelimbs (dotted line). The difference is shaded, and indicated by **. Lines 1 and 2 indicate RHL foot strike and LHL lift.
We first examined antigravity forces. During transition from one plate to the second it was clear that sometimes the rearmost limb of the diagonal pair could bear more weight (up to about twice as much at peak forelimb occasionally). This difference between the hindlimb (solid line) and forelimb (dotted line) is shaded in Figure 4C, and indicated **. This greater hindlimb loading ratio was never seen in ST rats (see below). However, on average the hindlimbs in intact rats bore only about 80% of the forelimb vertical load as rats crossed between plates. This mean ratio in load bearing also differed significantly from ST rats (see below and final summary Figure 12). Examination of rostrocaudal force patterns (Figure 4A) revealed that the fore and hindlimbs could contribute equally to the deceleration phase (by convention here positive forces), as indicated by the overlap of the solid and dotted curves. Similarly, the fore limb sometimes contributed more to the acceleration phase (negative forces, shaded difference indicated *, between the dotted and solid lines, with dotted forelimb forces more negative). On average the pattern of force balance matched that reported in standard gait analyses, but cycle by cycle coordination variations clearly occurred in our task. Peak mediolateral forces generated were often substantially larger in the hindlimbs in either positive or negative directions (statistically significant t-test p<0.05, example Figure 4B, shaded). Forelimb mediolateral forces at peak were only 0.05N, which was less than half the peak hindlimb mediolateral force. Thus in our data here, hindlimbs bore less weight and but generated greater lateral forces, while forelimbs play a greater role in rostrocaudal deceleration in the normal rat as reported previously.
Figure 12.
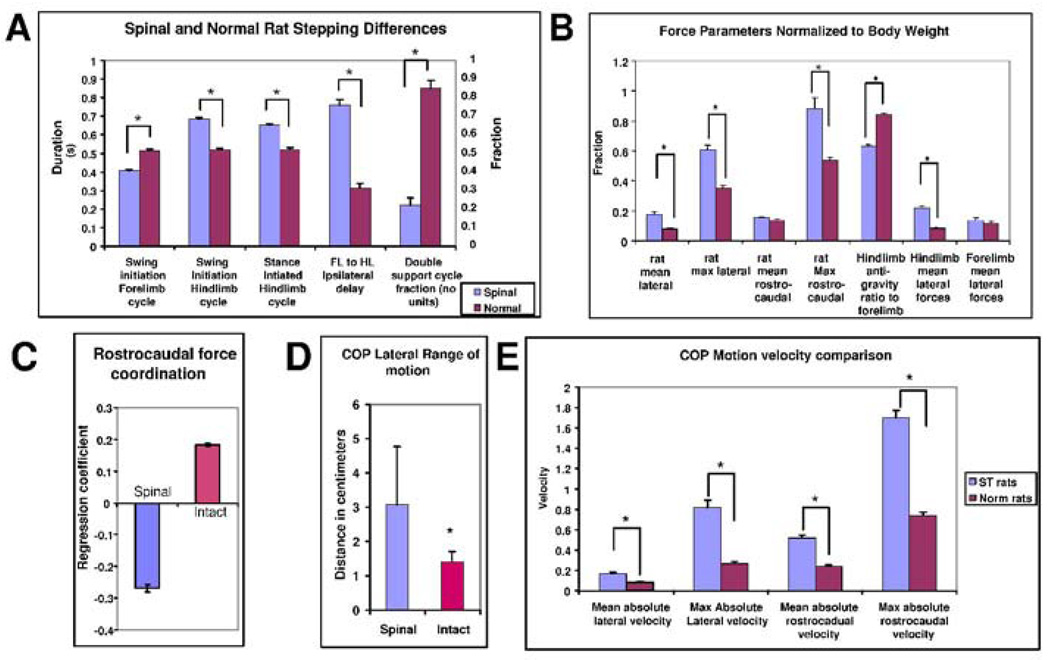
Summary statistical comparisons of Gait parameters, Ground reaction forces and Center of pressure motion between spinalized and intact rats. Asterisks indicate significant differences of p<0.05. A. Gait parameters: Cycle durations measured from swing initiation and stance initiation were examined for forelimbs (FL) and hindlimbs (HL). All parameters shown differed significantly (t-tests, p<0.05). ST rats had shorter forelimb cycles, longer hindlimb cycles, and very much longer ipsilateral forelimb to hindlimb delays. Whereas in trot intact rats spent about 80% of time in measured steps in double leg support, in spinal rats only 20% of the time or less was double support. Most of the time three or more legs were on the ground. B: Force comparisons: Absolute forces were compared across rats after conversion to fractions of body weight. The mean and maximum ground reaction forces in the lateral directions differed significantly between groups (t-tests p<0.05). The mean rostrocaudal force did not, but the maximum in ST rats was significantly larger. The last three comparisons in B examine two force plate measures. The ratio of HL vertical load to FL vertical load is shown. ST rats carried significantly less weight on the hindlimbs (60% of forelimb load) than intact (80% of forelimb load, t-test, p<0.05). Mean hindlimb lateral forces differed significantly between intact and ST rats, but forelimb forces did not. C: In transitions, rostrocaudal forces were significant positively correlated in intact rats, and negatively in ST rats and these differences were highly significant (t-test p<10E-6). D. The CoP in ST rats showed a significantly larger range of motion (t-test, p<0.05). E: The larger range of CoP motion was reflected in statistically significant differences in its motion pattern. ST rats had significantly larger mean absolute lateral velocity, and maximum velocity (t test, p<0.05). The rostrocaudal velocity and maximum also differed and were larger in ST rats, although their rate of actual body progression on the runway was slower. The CoP velocity also showed significantly greater variance in both lateral and rostrocaudal directions (not shown). The CoP velocity varied more and was probably more poorly controlled perhaps as a result of the triple and quadruple limb support patterns and uncontrolled lumbar generated forces.
Control of center of pressure (CoP) in normal rats
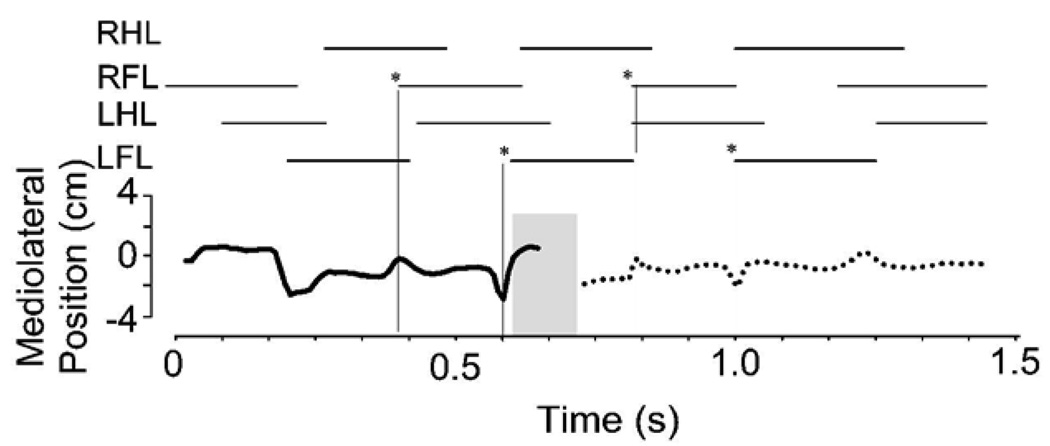
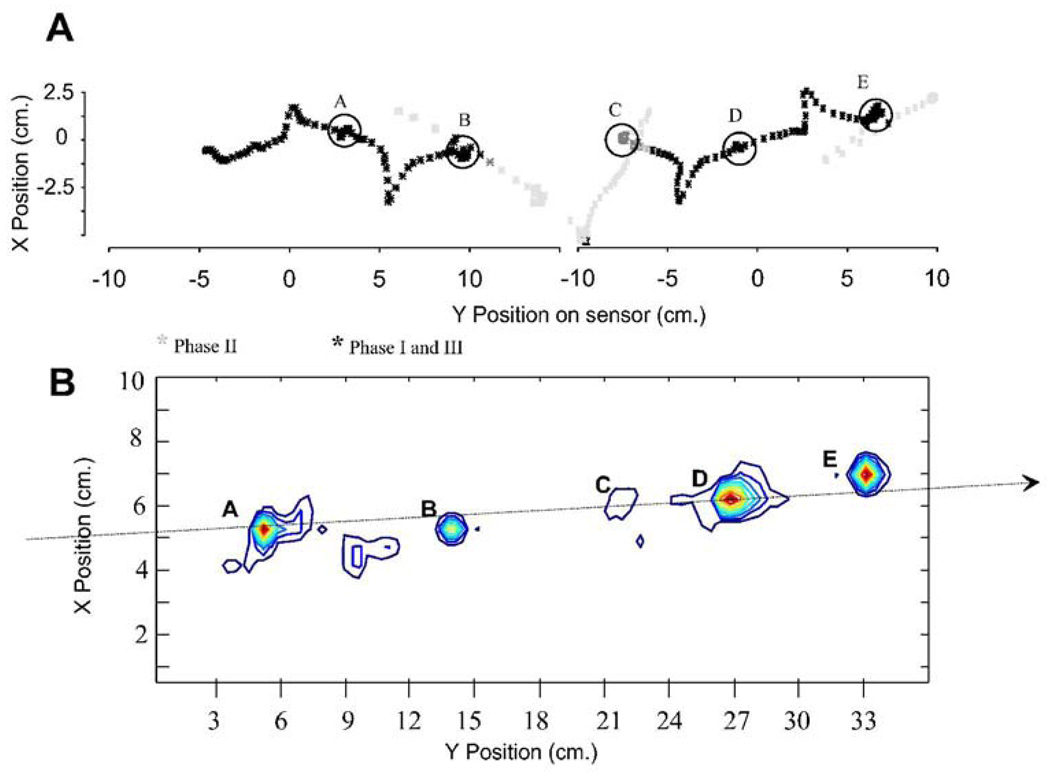
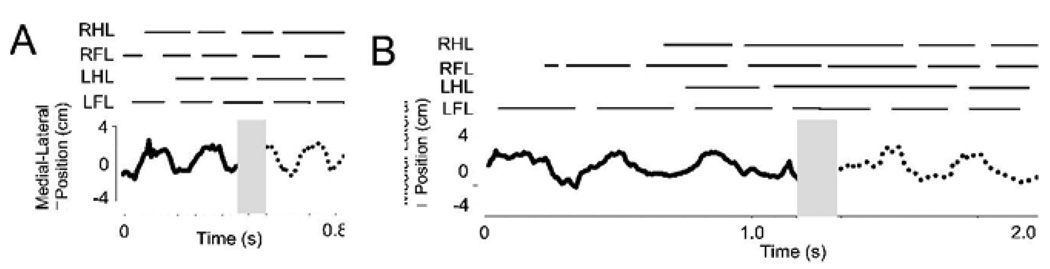
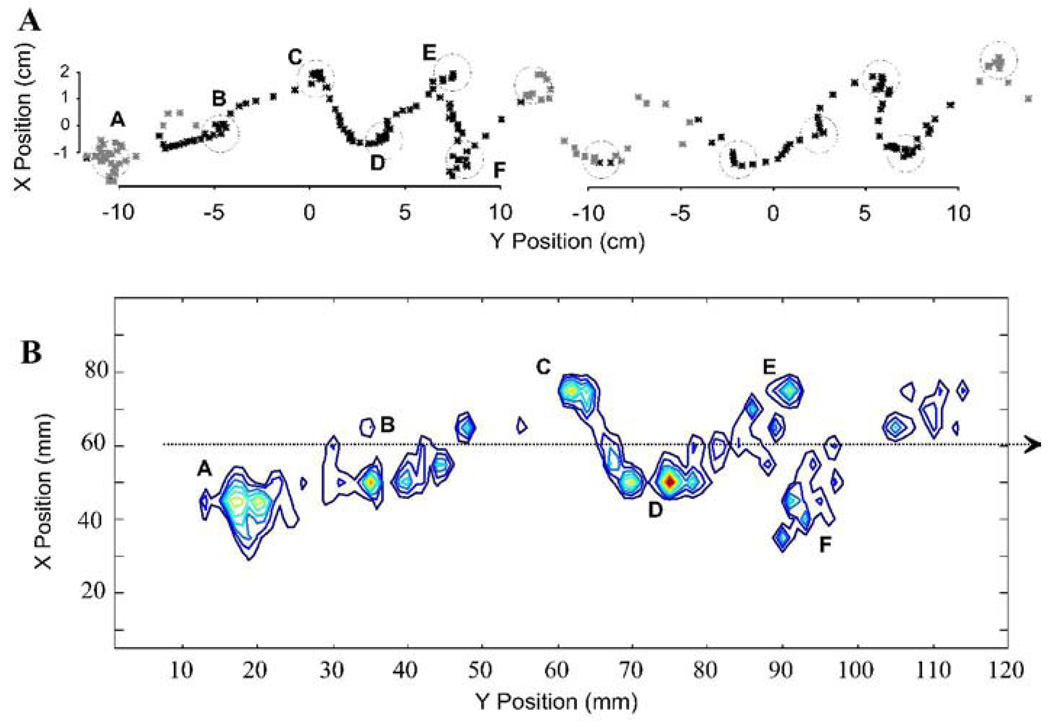
We combined the force and torque information from our force plates to estimate the motion of the CoP of the rat across the runway. The CoP is that point at which the application of an appropriate single force vector is equivalent to all the contact forces of the legs on the force plate surface. The CoP and net force can be considered the effect of a virtual leg. The net force represents the effect of all legs with ground contact. The placement of the CoP represents the balance of forces and how these interact with the inertia of the animal. This description can be useful in measuring postural stability, and stability and control in locomotion. It can also be computed as a basis for control and correction (e.g. see Raibert, 1986). The CoP should remain within the base of support to maintain static stability or must move in a specific relation to the momentum of the animal to ensure dynamic stability. Thus the CoP can be considered to provide a useful description of critical task variables in locomotion. Lateral deviation of the CoP from the direction of travel is a measure of stability and efficiency of progression. Smaller deviations indicate smaller lateral energy exchanges and less need to correct motion. We examined lateral excusions through time in normal and spinalized rats. Figure 5 shows how the CoP varies in its mediolateral deviation from the direction of progress through time. The plot is shown in relation to the footfall pattern as the rat crosses the runway. It can be seen that peak lateral deviations occurred during or shortly after transitions between support phases of diagonal pairs (indicated by vertical lines and asterisks). Maximum deviations off the line of progression were about 2cm. Figure 6A shows the pattern of spatial motion of the CoP in a normal rat. In the normal rats the center of pressure (CoP) showed a pattern of motion which was more precisely controlled than suggested by Figure 5 alone. The motion was in a straight line, but occured with prolonged pauses at points (A–E in Figure 6A) which were close to or along the line of progression. The CoP effectively jumped rapidly between these points. This is captured in 6B using a density plot. The peak deviations were only brief lateral excursions during the interlimb transfer of support and jump in CoP position. Each pause of the CoP along the line could be considered the contact point of a single virtual leg. Figure 6B shows the length of time spent, or density, of the CoP along its trajectory across the plate. This was calculated from the discrete time data by a smoothing technique using summing of gaussian basis functions with a standard deviation of 0.5 cm, located at each time-point’s observation. It can be seen that large lateral excursions of CoP are relatively insignificant in the normal rats. The densely contoured regions delineate the dwell points in Figure 6B. The correspondence to 6A is indicated by the letters. Phase II points were omitted in the smoothing, so point D is only weakly expressed. The degree of sharpness of these density plots shows the precision of the CoP dwell points. These points show deviations from a straight line by under 1cm, in keeping with the very small lateral forces in Figure 3 and 4.
Figure 5.
Normal rat. Center of Pressure motion: lateral deviations over time. The calculated mediolateral deviations of the center of pressure are plotted through time. The deviations are lateral, and the line of travel was down the long axis of the runway. If there were no deviations the plot would be a straight line at 0 through time. Stance phases are plotted above. Solid line : on sensor 1. Dotted line: on sensor 2. Shaded area is Phase II in which CoP calculation was not trustworthy. * and vertical hairlines indicate onset of forelimb stance: deviations in CoP peak around these transitions.
Figure 6.
Normal rat. Cartesian path of Center of Pressure. A: the X Y plane (horizontal plane viewed from above) showing the position of the trajectory of the center of pressure plotted each 8ms as a normal rat traverses the runway. Black asterisks show positions in which the rat was wholly on a sensor (Phase I and Phase III). Grey asterisks indicate transition onto, between or off force sensors (Phase II or other). Circles (A–E) show dwell points of center of pressure in which many tens of points are overlaid. B: contour plot of data in A subjected to smoothing with gaussian radial basis functions. A few dwell points (A–E) along a nearly straight line become evident in the segment calculated. These represent virtual points of contact of a single virtual leg. Their precision and placement reflects the precision and control of locomotion. Note their closeness to the dotted arrow indicating direction of locomotion in the normal rat. Point C is weakly represented because it overlapped Phase II.
Spinal transected rats with weight support
Whole body pattern and gait measured on a single force-plate
WS ST rats, with and without transplants, that achieved weight support did not differ from one another in their basic ground reaction force biomechanics or gait. Gait differed substantially from normal rats, as observed previously (e.g. see Murray et al. 2004). Limbs’ steps were sequenced so that there were normally at least three limbs in ground contact at any time, in contrast to the trot or lateral walk used on the runway in the normal rats. Steps were much more overlapping and variable (Figure 7B). The high step pattern variance meant gait orders as usually defined (pace, walk, trot, gallop) were to variable to classify normally. Because precise gait patterns were impossible to identify in the highly variable stepping from trial to trial in each individual rat, standard gait methods could not be applied. However, WS ST rats consistently had significantly shorter duration forelimb step cycles, significantly longer duration hindlimb step cycles, significantly longer delays between forelimb and hindlimb steps and very little time in double legged support (t-tests, p<0.05). These statistics of gait will be summarized with the other significant comparisons with normal rats and different biomechanical metrics in Figure 12 ( in 12A). Vertical force, height of back and gait were recorded as WS ST rats crossed the runway. An example is shown in Figure 7. Antigravity forces differed significantly from normal rats. Most WS ST rats showed no systematic and repeatable vertical force cycles or vertical body motion (e.g., Figure 7A). There were small higher frequency variations of the weight support forces (Figure 7C). When there were systematic variations in vertical forces these usually did not occur twice per step cycle as in normal rats. In general only one cycle of vertical force per step cycle occured or some value between 0.5 and 1.5 per step cycle as shown in Figure 8C. Taken together these data suggest the vertical elastic energy exchanges of the normal rat were completely absent in WS ST rats.
Figure 7.
WS ST rats. Gait and vertical force and motion. WS ST rats show little to no relation of midthoracic vertical motion and weight support forces compared to the normal rat. A: video measure of height of a fixed point on the rat’s back (mm). B: stance phases of each leg from video (solid bar: stance). Gait involves significant overlap of steps, very long hindlimb stance periods and rarely are only two legs on the ground. C: vertical support forces. Solid line: sensor 1 vertical force measurement. Dotted line: sensor 2 vertical force measurement. Phase I: rat wholly on sensor 1. Only solid line force is non-zero. Phase II spanning sensors. Net vertical force is sum of solid and dotted traces. Phase III: wholly on sensor 2. Only dotted trace is non-zero. Vertical forces do not relate clearly to vertical motion excursion in the back. Abscissa for all plots: time in seconds. RHL : right hindlimb. RFL: right forelimb. LHL: left hindlimb. LFL: left forelimb.
Figure 8.
WS ST rats. Gait and all translational force components, showing greater lateral and rostrocaudal forces in different patterns from normal rats. Top: stance phase of each leg. Vertical dotted lines from stance onset for RFL, LHL and LFL are drawn to see relation of force fluctuations to gait. A: Propulsive (rostrocaudal) force: solid trace is sensor 1, dotted trace is sensor 2. These amount to 25% of body weight compared to 15% in normal rats. Zero crossings and peaks align with stance phase transitions. B: Mediolateral force: solid trace is sensor 1, dotted trace is sensor 2. These forces amount to 25% of body weight compared to 4% in normal rats. Note that these are the most cyclic deviations in this rat, together with the weight support forces, and these two force component patterns can be out of phase some of the time. C: Antigravity (vertical) force: solid trace sensor 1, dotted trace sensor 2. Phase I : rat only on sensor 1. Phase II: rat spanning sensors. Phase III: rat only on sensor 2. Timescale in seconds is shown at bottom.
Propulsive (rostrocaudal) forces
In WS ST rats, the accelerative and decelerative rostrocaudal forces did not show variations at twice the step cycle frequency as was seen in the normal rat’s trot. In general the acceleration and deceleration variations that were seen occured at frequencies that were four or five times the whole step cycle frequency. This can be seen in Figure 8A. Our video data were not sufficiently precise to always allow us to clearly couple each oscillation of force to individual limb steps but it is likely that these rostrocaudal force oscillations represented these loadings and unloading (see vertical dotted lines in Figure 8, which align with oscillations in rostrocaudal force). Magnitudes of rostrocaudal forces in WS ST rats were similar to normal rats. However, because WS ST rats were always lower mass than normal rats (WS ST were 60–75% of normal littermate mass), this meant that these forces were larger as a proportion of body weight. They represented up to 25% of the magnitude of antigravity forces compared to 15% in normal rats. The injured rats showed a more stop-and-go progression with larger acceleration and decelerations than normal.
Stabilizing (Lateral) forces
Mediolateral forces in WS ST rats differed most dramatically in injured rats compared to normal rats. They were as large as rostrocaudal forces and represented up to 25% of peak antigravity forces as compared to 4–8% in the normal rat. There were less rather than more force peaks per step cycle compared with normal rats. This can be seen in Figure 8B when compared to Figure 3B. This larger peaked but simpler force pattern probably represented a rolling and yawing load transfer in the hindlimbs during locomotion (see below), which is also reflected in Center of Pressure measures below.
The differences in lateral and rostrocaudal forces between ST and intact rats seen in sample trials were statistically significant in pooled group data. The mean absolute lateral forces and maximum lateral forces during stepping on the force-plates were significantly larger in ST rats compared to normal (t-tests, p<0.05). The maximum rostrocaudal forces were also significantly larger in ST rats (t-test, p<0.05) as shown in Figure 12B, although the mean rostrocaudal forces did not differ.
Ground reaction force patterns in injured rats spanning two force-plates: forelimb hindlimb coordination
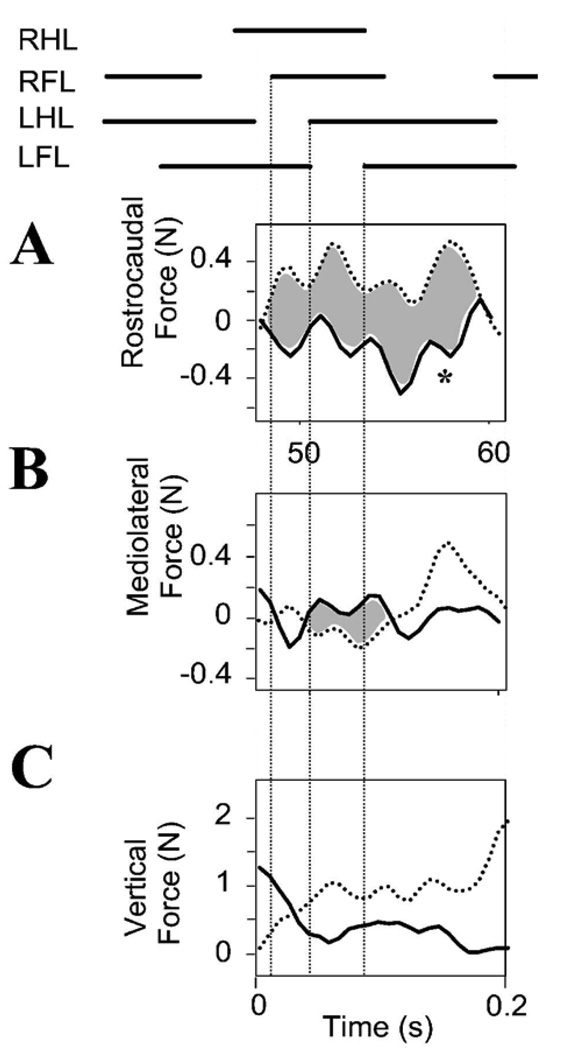
We examined the vertical, lateral and rostrocaudal forces as the WS ST rats crossed between the two force plates, i.e. phase II in Figure 8. The patterns of vertical load during these transitions between plates were consistent with much greater load bearing by the forelimbs compared to normals. (Compare Figure 9C with Figure 4C; in Figure 9C the dotted (forelimb) force is mostly above the solid hindlimb force trace or is similar to it, while in contrast, in the normal rat in Figure 4C it is mostly below ). Injured rats hindlimbs bore about 60% of the forelimb load on average, significantly less than intact rats (summary statistics in Figure 12B, t-test p<0.05).
Figure 9.
WS ST rats. Forelimb and hindlimb propulsive forces are coordinated in opposition in injured rats. Phase II forces are shown and differences shaded. Top : stance phase of limbs during transition across plates. A : antagonistic decelerative actions of forelimbs (dotted line) and propulsive forces from the hindlimbs (solid line). Rostrocaudal forces correlate negatively and in a manner significantly different from normal. Individual peak contributions (~0.5N) of forelimbs or hindlimbs are ~25% of body weight (<2N). The forces show several peaks per cycle. Not all can be related to stance transitions, e.g. peak at *. B. Lateral forces: mediolateral force (~0.4N peak) is exerted in both forelimbs (dotted line) and hindlimbs (solid line). C: Antigravity forces: Forelimbs (dotted line) carry about 60% of total body weight here, close to the typical mean of our ST rats, and significantly more than normal rats.
The pattern of forelimb and hindlimb rostrocaudal forces during transition between platforms also differed very strongly from the normal pattern. Normal rats had closely correlated force patterns on the two force plates with slightly differing peak values which varied around mean ratios matching those reported previously, albeit with high variability because a constant speed and gait were not demanded in data selection. In contrast, in all WS ST rats the rostrocaudal forces were strongly anticorrelated (shaded region in Figure 9A). Comparison of normal and WS ST rat rostrocaudal forelimb and hindlimb force correlations in Phase II data from all our rats (10 normal, 6 injured) showed ST rats had almost all negatively correlated forces and intact rats consistently positive correlated forces despite the variable speeds and gaits we allowed them to use. These were highly significant and repeatable differences in the correlation coefficients in all cases (Figure 12 C, t-test, p<10E-6, and cumulative distribution differences compared with Kolmogorov Smirnov test p<0.05). The WS ST rats torso was being (usually) pushed in opposing directions simultaneously by the forelimbs and hindlimbs as the rat walked. This internal trunk compression was over and above the net rostrocaudal accelerative forces, which arose from the resultant of limb forces. To reiterate, the force generation by forelimb and hindlimbs operated in an antagonistic relationship in WS ST rats, while in normal rats the two limb pairs were being used cooperatively as synergistic structures. Net propulsion forward in WS ST rats and CoP motion occured on this background of internal opposition of forces. This internal body loading was probably an active control strategy causing tight mechanical coupling of forelimbs and hindlimbs through the trunk.
Mediolateral forces also showed some local negative correlation of forelimbs and hindlimbs, albeit much less strongly than seen in the rostrocaudal forces. The rats also had periods of similarly directed forces and showed great variation. More important, the mean hindlimb lateral forces as the ST animals spanned the plates were larger than the normals (t-test p<0.05), while mean forelimb lateral forces were comparable to normal rats. The larger net forces exerted in the horizontal plane, thus appear to arise from these large force variations in the hindlimbs, which were significantly larger in ST rats than in intact rats, as discussed above.
Control of center of pressure in injured rats
The CoP showed large lateral excursions. Motion of the center of pressure in WS ST rats zig-zagged, consistent with the large hindlimb and net lateral force variations in the ST rats (Figure 10A). They were not able to control their CoP in the precise linear progression of the normal rat. The pattern was consistent with significant roll and yaw of the WS ST rats’ torsos and the forces noted above, which was observed in the video. It is likely that on wider runways these CoP excursions could be still wider (see Miya et al. 1996). WS ST rats spent most time at the lateral extremes, not in the center (Figure 10). Figure 10A and 11B show the time history of lateral position of CoP and footfall gait patterns. Compared to figure 5, the lateral deviations are somewhat larger and less time is spent on the line of travel. Lateral range of motion of CoP was significantly larger in ST rats (t test, P<0.05) double that of intact rats, Figure 12 D. The CoP velocity distributions of normal and injured rats differed significantly (Figure 12 E, t test, p<0.05). Both lateral and rostral velocity of CoP was large in injured rats although body progression was slower. Velocity variances also differed significantly (F-test, p<0.05). Figure 11 shows the length of time or density of CoP along its trajectory across the plate. This should be compared to Figure 6. These were calculated from the discrete time data by smoothing using summing of gaussian basis functions of 0.5cm standard deviation located at each observation. The spatial pattern of motion of the CoP consisted of a rapid sideways motion between the extreme lateral positions. The points at which the CoP paused thus formed a zig zag or sawtooth pattern along the force plates. In general there were about double the number sawtooth CoP pauses at vertices (11 circled in 12A) compared to the number of pauses (6–7 in Figure 6A) in the normal rats. These differences are likely to be associated with the substantial yaw and roll observed in the injured rats (e.g. Miya et al. 1997). The problem of deciding on forces responsible for CoP motion is ill-posed. However the CoP pattern of motion is consistent with the stepping patterns and the propulsive force differences in injured rats on the runway force plates. Usually, gait in ST rats appeared to be statically stable with three legs in ground contact. Force measurements indicated internally loaded support patterns. ST rat stepping involved more step transitions and weight transfers, and haunch kinematics had substantial roll and yaw motions. The ST rats employed a more statically stable but also much more strenuous, internally loaded, and presumably less efficient gait.
Figure 10.
WS ST rats. Center of Pressure motion: lateral deviations of CoP over time. This shows most time is spent at the lateral extremes of CoP motion compared to normal rat which only briefly exhibits lateral CoP motion. The calculated mediolateral deviations of the center of pressure from the line of travel are plotted through time (measured in seconds). Stance phases are plotted above. Solid line : on sensor 1. Dotted line: on sensor 2. Patterns are variable. Two examples are shown. Panel A: rat with considerable systematic deviations of about 2cm each side. Panel B: rat with asymmetric (note: scalloped pattern) slowly varying deviations over time. Grey shaded region: Phase II when CoP calculation was not trustworthy in this arrangement.
Figure 11.
WS ST rats. Cartesian path of Center of Pressure. A: the X Y plane (horizontal plane viewed from above) position of the center of pressure is plotted as the SCI rat traverses the runway. Black asterisks show positions in which the rat was wholly on a sensor. Grey asterisks indicate transition onto, between or off force sensors. Circles show dwell points of center of pressure in which many tens of points are overlaid. Note clearly zig-zag pattern of center of pressure indicated by circles. B: contour plot of a segment of data in A subjected to smoothing with gaussian radial basis functions. Dwell points more spatially spread than in the normal rats. The dwell points are arrayed on either side of a nearly straight line of progression (schematically indicated by the dotted arrow). These represent virtual points of contact of a single virtual leg. Their imprecision and lateral placement reflects the lower precision and control of locomotion in these rats and the energy which must be put into lateral as opposed to forward motions.
Discussion
In this study we examined how rats spinalized at birth that achieve independent weight support are able to do this. Our data are consistent with the view that they use their voluntary trunk control to couple and control largely autonomous hindlimb steps. We analyzed ground reaction forces and CoP motion and their coordination in normal and injured rats. We tested both transected and transplanted rats. Transect and transplant recipient locomotion were not significantly different in biomechanics, although cortical organization differed (Giszter et al. 1998) and they are grouped together in our analysis. All the WS ST rats used similar biomechanical strategies in their locomotion. However, very significant differences existed between the strategies of operates and the patterns of normal rat locomotion.
Previous studies of ground reaction forces have focused on single force plates. Most often, segments of constant speed locomotion are recorded and the results are averaged. Lavoie et al. 1995 examined ground reaction forces during normal stepping and then in stepping over an obstacle in the cat. They found differences in their average data between the relative rostrocaudal / parasagittal force contributions of the fore-limbs and hind-limbs in a diagonal gait. Our data for normal rats are in keeping with their results. In the present study rats also showed similar asymmetric contributions between forelimb and hindlimb as they crossed between the two sensor systems. Our mean data from intact rats match results in different rat strains (Webb et al. 2003; and Howard et al. 2000). Clarke 1995 examined vertical ground reaction forces in stance and during walking using an optical technique. Our results differ slightly from his in that the vertical load distributions measured as the normal rats crossed between plates here could sometimes show a bias of weight carriage to the hindlimbs. In Clarke's study this pattern was always reversed. It is possible that the differences in these two data sets arise from the runway design altering the rats strategy and the speed variations we permitted. Injured rats in our study always showed a strong biasing of weight to the fore-limbs, and never showed the pattern of greater hindlimb load sometimes seen in intact rats.
Muir and Whishaw have used force records in rats to assess Parkinsonism and effects of various partial lesions (Webb and Muir, 2003, 2004, Muir and Whishaw 1999a, b, 2000). The data here are the first description of spinal transected rats’ center of pressure and momentary coordination of ground reaction forces between the forelimbs and hindlimbs. Comparison of these data with normal rats suggests how differing strategies of control of locomotion allow function in these two groups which possess very different voluntary and propriospinal neural control and proprioceptive capabilities in lumbosacral spinal cord.
Normal rats walked across our runway using a diagonal trot or sometimes a walk gait. The motion of the center of pressure was largely a straight line. In trot the motion reflects the dynamic stability of this gait. The center of pressure made two rapid motions between two stable dwell points aligned along the path of motion in each complete step cycle. The precise control of center of pressure with two limbs in ground contact implies precise coordination of forelimb and hind-limb force application. Deviations of the balance of fore and hind limb forces would move the CoP off the controlled location and back and forth along the diagonal connecting the foot positions. This did not occur, except very briefly during weight transfer between diagonal pairs. The motion dwell points of the CoP formed a straight line down the center of the runway and deviations away from this line during movements between the dwell points were small, again during or close to weight transfer between pairs of limbs. Direct examination also showed that the forces in fore-limb and hind-limbs of a pair were closely correlated.
Injured rats contrasted strongly with normal rats in their gait, in their control of forces, and in the motion of the center of pressure. The gait adopted was highly variable rather than precise and repeatable. The gait range used led to more step overlap and significantly more time during the step cycle with three limbs in contact with the substrate. The forelimb step cycles were significantly shorter and hindlimb step cycles longer than each other, and than their matching equivalents in normal rats. There was stepping in an imprecise and highly variable 2 to 1 ratio (forelimbs to hindlimbs). The hindlimb initiation of steps were also significantly delayed relative to the ipsilateral foreleg. The rats appeared to use the multileg forces during the triple and quadruple leg support to control the net forces and center of pressure motion through the voluntary control in the forelimbs, somewhat similar to the compensation strategy observed during stance (Giszter et al. 2007). The center of pressure of ST rats progressed less rapidly overall and was less precisely centered on specific "dwell points". However, the lateral ranges and the velocities of CoP motion were significantly larger. Further, the motion of the center of pressure formed a diagonal along the platform, alternating back and forth across the center of the platform or direction of travel. The animals showed yaw and roll of the body mass along the platform as also reported in Miya et al. 1997. Ground reaction forces in injured rats reflected the altered pattern of motion and the hypothesized control strategy. The clean oscillations of the normal rats were absent in most injured rats. Lateral forces were larger in ST rats and at least some of this lateral force could be attributed to hindlimbs which had significantly higher mean lateral forces than normal rats. Interestingly, injured rats showed a variety of higher frequency variations in rostrocaudal forces not observed in normal rats. We attribute these to internal loading and stepping release of opposing forces because these could be associated to individual limb steps.
In contrast to normal rats, the pelvic and pectoral girdle seemed to act in opposition in our injured rats. During transition between force sensors the forces on the two sensors were uniformly negatively correlated. This observation fits with the notion of the forelimbs acting as brakes, or as initiators and stabilizers for the hind-limb movements and stepping pattern generation. Trunk control in such a situation would be critical to couple and direct the hindlimb generated forces appropriately for the task. The 2:1 stepping ratio of the forelimbs to hindlimbs we saw also favors the idea that the hindlimb force effects were controlled through the trunk and forelimbs stepping. In transitions between the plates the patterns of force opposition fit with the forelimbs braking and managing the hindlimb generated forces. The trunk was in compressive loading. The hindlimbs bore weight actively and stepped actively, presumably triggered and managed through the trunk. The trunk posture and loadings adopted likely delayed pattern generator initiated stepping until the voluntary controls were prepared. In keeping with this idea, the forelimbs stepped faster and the hindlimbs slower than the step cycles of intact rats trotting across the runways. The likely significance of trunk control in the autonomous weight support by injured rats spinal transected as neonates is a finding in both this and our preceding studies (Giszter et al, 1998, 2007, and submitted).
The rats with P1/P2 neonatal SCI develop or learn a strategy to cope with their neural deficit that adult or later injured rats never achieve. They have a significantly different developmental experience that is likely to affect function (Martin et al., 2004). The strategy of control used by the neonatal injured rats can allow both weight bearing stance and locomotion to be accomplished (Giszter et al., 2007). Although adult spinalized cats and rats can be trained to improve bipedal or quadrupedal locomotion, autonomous weight support is never achieved (DeLeon et al. 1999; Edgerton et al. 1992, 2004). A range of spinal reflex and pattern generation mechanisms may be engaged and must be managed in support of function (e.g., Forssberg et al. 1975; Hiebert et al., 1994, 1996; Poppele and Bosco, 2003). Some of these are likely to differ between stance and locomotion (e.g., Jankowska and Edgeley 1993). Much can also be achieved through mechanical coupling, and using fixed drives as well as feedback (Kuo, 2002; Kuo et al., 2005). Locomotor and stance motor behaviors may have predictable and possibly simplifying modular elements in both man and other mammals (e.g., Raasch and Zajac, 1999; Ting and Macpherson 2004,2005; Capellini et al. 2006; Ivanenko et al., 2003; Krouchev et al. 2006). Understanding whether it is possible for an adult injured rat to master the skills of the neonatal injured and how best to accomplish this training is an open question. Robotic systems may aid in this regard (De Leon et al., 2002a,b). We speculate that focusing on trunk- pelvis-leg mechanical interaction may be an important direction in rehabilitation (Udoekwere et al., 2006 ). The data here suggest the functional strategy for the ST rats is not an elegant and repeatable gait, but involves trunk constraints and integration and quite variable stepping of the haunches. Our results suggest that once plantar hindlimb stepping is achieved in ST rats, trunk use must be better understood for the next stages of functional rehabilitation, which will involve integrating hindlimb stepping into a functional strategy. Rats must be trained to best use the trunk so as to manage and coordinate the hindlimb and forelimb steps and forces.
In conclusion, our data show that injured rats adopt a novel strategy of locomotion control. This involves differences in gait, motion of center of pressure, opposing fore-limb hind-limb force coordination and presumably decreased efficiency. Trunk control is likely to play a crucial role in this gait strategy and skill.
Acknowledgments
Michel Lemay, Marion Murray provided critiques at various stages. Anonymous reviewers of early drafts are thanked for reviews leading to a clearer data presentation and analysis. Dr John Ditunno of Thomas Jefferson University Department of Rehabilitation Medicine provided encouragement, mentorship and protected time for V. Graziani. Supported by ASRI grants to SFG, NIH HD01127 to VG, and NINDS NS 24707, with equipment development and publication costs partly supported under NIH NS40412.
References
- Bouyer LJ, Whelan PJ, Pearson KG, Rossignol S. Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy. J Neurosci. 2001;21(10):3531–3541. doi: 10.1523/JNEUROSCI.21-10-03531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. Journal of Neurophysiology. 2006;95(6):3426–3437. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- Clarke KA. Differential fore- and hindpaw force transmission in the walking rat. Physiol Behav. 1995;58(3):415–419. doi: 10.1016/0031-9384(95)00072-q. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Kubasak MD, Phelps PE, Timoszyk WK, Reinkensmeyer DJ, Roy RR, Edgerton VR. Using robotics to teach the spinal cord to walk. Brain Res Brain Res Rev. 2002a;40(1–3):267–273. doi: 10.1016/s0165-0173(02)00209-6. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Reinkensmeyer DJ, Timoszyk WK, London NJ, Roy RR, Edgerton VR. Use of robotics in assessing the adaptive capacity of the rat lumbar spinal cord. Prog Brain Res. 2002b;137:141–149. doi: 10.1016/s0079-6123(02)37013-4. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J Neurophysiol. 1999 Jul;82(1):359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol. 1998;80(1):83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, Hodgson JA, Prober RJ, De Guzman CP, de Leon R. Potential of adult mammalian lumbosacral spinal cord to execute and acquire improved locomotion in the absence of supraspinal input. J. Neurotrauma. 1992;9:110–127. [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJK, Bigbee AJ, deLeon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Sjostrom A. Tactile placing reactions in chronic spinal kittens. Acta Physiol Scand. 1974 Sep;92(1):114–120. doi: 10.1111/j.1748-1716.1974.tb05727.x. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975 Feb 21;85(1):103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- Fung J, Macpherson JM. Attributes of quiet stance in the chronic spinal cat. J Neurophysiol. 1999;82(6):3056–3065. doi: 10.1152/jn.1999.82.6.3056. [DOI] [PubMed] [Google Scholar]
- Fung J, Macpherson JM. Determinants of postural orientation in quadrupedal stance. Journal of Neuroscience. 1995;15(2):1121–1131. doi: 10.1523/JNEUROSCI.15-02-01121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis GB, Biewener AA. Hindlimb muscle function in relation to speed and gait: in vivo patterns of strain and activation in a hip and knee extensor of the rat (Rattus norvegicus) J Exp Biol. 2001;204(Pt 15):2717–2731. doi: 10.1242/jeb.204.15.2717. [DOI] [PubMed] [Google Scholar]
- Gillis GB, Biewener AA. Effects of surface grade on proximal hindlimb muscle strain and activation during rat locomotion. J Appl Physiol. 2002;93(5):1731–1743. doi: 10.1152/japplphysiol.00489.2002. [DOI] [PubMed] [Google Scholar]
- Giszter SF, Kargo WJ, Davies MR, Shibayama M. Fetal transplants rescue axial muscle representations in M1 cortex of neonatally transected rats that develop weight support. J Neurophysiol. 1998;80:3021–3030. doi: 10.1152/jn.1998.80.6.3021. 1998. [DOI] [PubMed] [Google Scholar]
- Giszter S, Davies MR, Graziani V. Motor strategies used by rats spinalized at birth to maintain stance in response to imposed perturbations. J Neurophysiol. 2007 Feb 7; doi: 10.1152/jn.00308.2006. 2007, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change, and ankle extensor EMG patterns. J Neurophysiol. 2006;95(3):1397–1409. doi: 10.1152/jn.01300.2004. Epub 2005 Oct 5. [DOI] [PubMed] [Google Scholar]
- Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiological Reviews. 1975;55:247–304. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75(3):1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Gorassini MA, Jiang W, Prochazka A, Pearson KG. Corrective responses to loss of ground support during walking. II. Comparison of intact and chronic spinal cats. J Neurophysiol. 1994;71(2):611–622. doi: 10.1152/jn.1994.71.2.611. [DOI] [PubMed] [Google Scholar]
- Hodgson JA, Roland RR, deLeon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task? Medicine and Science in Sports and Exercise. 1994;26:1491–1497. [PubMed] [Google Scholar]
- Howard CS, Blakeney DC, Medige J, Moy OJ, Peimer CA. Functional assessment in the rat by ground reaction forces. J Biomech. 2000;33(6):751–757. doi: 10.1016/s0021-9290(00)00023-3. [DOI] [PubMed] [Google Scholar]
- Howland DR, Bregman BS, Tessler A, Goldberger ME. Development of locomotor behavior in the spinal kitten. Exp Neurol. 1995a;135:108–122. doi: 10.1006/exnr.1995.1071. [DOI] [PubMed] [Google Scholar]
- Howland DR, Bregman BS, Tessler A, Goldberger ME. Transplants enhance locomotion in neonatal kittens whose spinal cords are transected. Exp Neurol. 1995b;135:123–145. doi: 10.1006/exnr.1995.1072. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Zago M, Molinari M, Scivoletto G, Castellano V, Macellari V, Lacquaniti F. Temporal components of the motor patterns expressed by the human spinal cord reflect foot kinematics. J Neurophysiol. 2003;90:3555–3565. doi: 10.1152/jn.00223.2003. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Edgely S. Interaction between pathways controlling posture and gait at the level of spinal interneurones in the cat. Prog Brain Res. 1993;97:161–171. doi: 10.1016/s0079-6123(08)62274-8. [DOI] [PubMed] [Google Scholar]
- Kaya M, Leonard TR, Herzog W. Control of ground reaction forces by hindlimb muscles during cat locomotion. J Biomech. 2006;39(15):2752–2766. doi: 10.1016/j.jbiomech.2005.10.012. Epub 2005 Nov 28. [DOI] [PubMed] [Google Scholar]
- Krouchev N, Kalaska JF, Drew T. Sequential activation of muscle synergies during locomotion in the intact cat as revealed by cluster analysis and direct decomposition. J Neurophysiol. 2006;96(4):1991–2010. doi: 10.1152/jn.00241.2006. [DOI] [PubMed] [Google Scholar]
- Kuo AD. The relative roles of feedforward and feedback in the control of rhythmic movements. Motor Control. 2002;6(2):129–145. doi: 10.1123/mcj.6.2.129. [DOI] [PubMed] [Google Scholar]
- Kuo AD, Donelan JM, Ruina A. Energetic consequences of walking like an inverted pendulum: step-to-step transitions. Exerc Sport Sci Rev. 2005;33(2):88–97. doi: 10.1097/00003677-200504000-00006. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Maioli C, Fava E. Cat posture on a tilted platform. Experimental Brain Research. 1984;57(1):82–88. doi: 10.1007/BF00231134. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Maioli C. Independent control of limb position and contact forces in cat posture. Journal of Neurophysiology. 1994 Oct;72(4):1476–1495. doi: 10.1152/jn.1994.72.4.1476. [DOI] [PubMed] [Google Scholar]
- Lavoie S, McFadyen B, Drew T. A kinematic and kinetic analysis of locomotion during voluntary gait modification in the cat. Exp Brain Res. 1995;106(1):39–56. doi: 10.1007/BF00241355. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Gans C. Electromyography for Experimentalists. Chicago: The University of Chicago Press; 1986. [Google Scholar]
- Macpherson JM, Lywood DW, Van Eyken A. A system for the analysis of posture and stance in quadrupeds. J Neurosci Methods. 1987;20(1):73–82. doi: 10.1016/0165-0270(87)90040-9. [DOI] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. I. Forces at the ground. Journal of Neurophysiology. 1988a;60(1):204–217. doi: 10.1152/jn.1988.60.1.204. [DOI] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. II. Electromyographic activity. Journal of Neurophysiology. 1988b;60(1):218–231. doi: 10.1152/jn.1988.60.1.218. [DOI] [PubMed] [Google Scholar]
- Macpherson JM. Changes in a postural strategy with inter-paw distance. Journal of Neurophysiology. 1994 Mar;71(3):931–940. doi: 10.1152/jn.1994.71.3.931. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Fung J. Activity of thoracic and lumbar epaxial extensors during postural responses in the cat. Experimental Brain Research. 1998 Apr;119(3):315–323. doi: 10.1007/s002210050347. [DOI] [PubMed] [Google Scholar]
- Martin JH, Choy M, Pullman S, Meng Z. Corticospinal system development depends on motor experience. J Neurosci. 2004;24(9):2122–2132. doi: 10.1523/JNEUROSCI.4616-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley PA, Smith JL, Gregor RJ. Responses of elbow extensors to landing forces during jump downs in cats. Exp Brain Res. 1983;49(2):218–228. doi: 10.1007/BF00238582. [DOI] [PubMed] [Google Scholar]
- Miklyaeva EI, Martens DJ, Whishaw IQ. Impairments and compensatory adjustments in spontaneous movement after unilateral dopamine depletion in rats. Brain Research. 1995 May 29;681(1–2):23–40. doi: 10.1016/0006-8993(95)00277-w. [DOI] [PubMed] [Google Scholar]
- Miklyaeva EI, Woodward NC, Nikiforov EG, Tompkins GJ, Klassen F, Ioffe ME, Whishaw IQ. The ground reaction forces of postural adjustments during skilled reaching in unilateral dopamine-depleted hemiparkinson rats. Behavioural Brain Research. 1997 Nov;88(2):143–152. doi: 10.1016/s0166-4328(97)00043-0. [DOI] [PubMed] [Google Scholar]
- Miya D, Giszter S, Mori F, Adipudi V, Tessler A, Murray M. Fetal transplants alter the development of function after spinal cord transection in newborn rats. The Journal of Neuroscience. 1997;17(12):4856–4872. doi: 10.1523/JNEUROSCI.17-12-04856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir GD, Whishaw IQ. Complete locomotor recovery following corticospinal tract lesions: measurement of ground reaction forces during overground locomotion in rats. Behav Brain Res. 1999;103(1):45–53. doi: 10.1016/s0166-4328(99)00018-2. [DOI] [PubMed] [Google Scholar]
- Muir GD, Whishaw IQ. Ground reaction forces in locomoting hemi-parkinsonian rats: a definitive test for impairments and compensations. Exp Brain Res. 1999;126(3):307–314. doi: 10.1007/s002210050739. [DOI] [PubMed] [Google Scholar]
- Muir GD, Whishaw IQ. Red nucleus lesions impair overground locomotion in rats: a kinetic analysis. Eur J Neurosci. 2000;12(3):1113–1122. doi: 10.1046/j.1460-9568.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, Knapp E, Thompson B, Jung R. A method for assessing balance control in rodents. Biomed Sci Instrum. 2002;38:77–82. [PubMed] [Google Scholar]
- Mussa-Ivaldi FA, Hogan N, Bizzi E. Neural mechanical and geometric factors subserving arm posture. J Neurosci. 1985;5:2732–2743. doi: 10.1523/JNEUROSCI.05-10-02732.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang MY, Lam T, Yang JF. Infants adapt their stepping to repeated trip-inducing stimuli. J Neurophysiol. 2003;90(4):2731–2740. doi: 10.1152/jn.00407.2003. [DOI] [PubMed] [Google Scholar]
- Poppele R, Bosco G. Sophisticated spinal contributions to motor control. Trends in Neuroscience. 2003;26(5):269–276. doi: 10.1016/S0166-2236(03)00073-0. [DOI] [PubMed] [Google Scholar]
- Pratt CA, Fung J, Macpherson JM. Stance Control in the chronic spinal cat. Journal of Neurophysiology. 1994;71(5):1981–1985. doi: 10.1152/jn.1994.71.5.1981. [DOI] [PubMed] [Google Scholar]
- Raasch CC, Zajac FE. Locomotor strategy for pedaling: muscle groups and biomechanical functions. J Neurophysiol. 1999;82:515–525. doi: 10.1152/jn.1999.82.2.515. [DOI] [PubMed] [Google Scholar]
- Raibert MH. Legged Robots that Balance. MIT Press; 1986. [Google Scholar]
- Roerdink M, De Haart M, Daffertshofer A, Donker SF, Geurts AC, Beek PJ. Dynamical structure of center-of-pressure trajectories in patients recovering from stroke. Exp Brain Res. 2006;174(2):256–269. doi: 10.1007/s00221-006-0441-7. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Goldberger ME. The development and recovery of motor function in spinal cats. I. The infant lesion effect. Experimental Brain Research. 1986;62:373–386. doi: 10.1007/BF00238857. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Strategies for the integration of posture and movement during reaching in the cat. J Neurophysiol. 2003;90(5):3066–3086. doi: 10.1152/jn.00339.2003. Epub 2003 Aug 6. [DOI] [PubMed] [Google Scholar]
- Smith JL, Smith LA, Zernicke F, Hoy M. Locomotion in exercised and nonexercised cats cordotomized at two and twelve weeks of age. Experimental Neurology. 1982;76:393–413. doi: 10.1016/0014-4886(82)90217-5. [DOI] [PubMed] [Google Scholar]
- Stelzner DJ, Ershler WB, Weber ED. Effects of spinal transection in neonatal and weanling rats: survival of function. Exp Neurol. 1975;46:156–177. doi: 10.1016/0014-4886(75)90039-4. [DOI] [PubMed] [Google Scholar]
- Suter E, Herzog W, Leonard TR, Nguyen H. One-year changes in hind limb kinematics, ground reaction forces and knee stability in an experimental model of osteoarthritis. J Biomech. 1998;31(6):511–517. doi: 10.1016/s0021-9290(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, de Leon RD, Hoang TX, Roy RR, Edgerton VR, Tobin AJ. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J Neurosci. 2002 Apr 15;22(8):3130–3143. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoszyk WK, Nessler JA, Acosta C, Roy RR, Edgerton VR, Reinkensmeyer DJ, de Leon R. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res. 2005 Jul 19;1050(1–2):180–189. doi: 10.1016/j.brainres.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Timoszyk WK, De Leon RD, London N, Roy RR, Edgerton VR, Reinkensmeyer DJ. The rat lumbosacral spinal cord adapts to robotic loading applied during stance. J Neurophysiol. 2002 Dec;88(6):3108–3117. doi: 10.1152/jn.01050.2001. [DOI] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol. 2005 Jan;93(1):609–613. doi: 10.1152/jn.00681.2004. [DOI] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. Ratio of shear to load ground-reaction force may underlie the directional tuning of the automatic postural response to rotation and translation. J Neurophysiol. 2004 Aug;92(2):808–823. doi: 10.1152/jn.00773.2003. [DOI] [PubMed] [Google Scholar]
- Udoekwere UI, Mbi LT, Ramakrishnan A, Giszter SF. Robot applied elastic fields at the pelvis of the spinal transected rat: a tool for detailed assessment and rehabilitation; Proceedings of the IEEE/EMBC Conference; New York, NY: 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AA, Gowribai K, Muir GD. Fischer (F-344) rats have different morphology, sensorimotor and locomotor abilities compared to Lewis, Long-Evans, Sprague-Dawley and Wistar rats. Behav Brain Res. 2003;144(1–2):143–156. doi: 10.1016/s0166-4328(03)00076-7. [DOI] [PubMed] [Google Scholar]
- Webb AA, Muir GD. Unilateral dorsal column and rubrospinal tract injuries affect overground locomotion in the unrestrained rat. Eur J Neurosci. 2003;18(2):412–422. doi: 10.1046/j.1460-9568.2003.02768.x. [DOI] [PubMed] [Google Scholar]
- Webb AA, Muir GD. Course of motor recovery following ventrolateral spinal cord injury in the rat. Behav Brain Res. 2004;155(1):55–65. doi: 10.1016/j.bbr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Zajac FE, Zomlefer MR, Levine WS. Hindlimb muscular activity, kinetics and kinematics of cats jumping to their maximum achievable heights. J Exp Biol. 1981;91:73–86. doi: 10.1242/jeb.91.1.73. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Duarte M. Instant equilibrium point and its migration in standing tasks: rambling and trembling components of the stabilogram. Motor Control. 1999;3(1):28–38. doi: 10.1123/mcj.3.1.28. [DOI] [PubMed] [Google Scholar]
- Zumwalt AC, Hamrick M, Schmitt D. Force plate for measuring the ground reaction forces in small animal locomotion. J Biomech. 2006;39(15):2877–2881. doi: 10.1016/j.jbiomech.2005.10.006. Epub 2005 Dec 13. [DOI] [PubMed] [Google Scholar]