Abstract
BACKGROUND
D-Cycloserine (DCS), a partial N-methyl-D-aspartate receptor agonist, has been shown to enhance the extinction of both cocaine and amphetamine-induced conditioned place preference (CPP). However, there have been no reports of the effects of DCS on the extinction of ethanol-conditioned behaviors in mice. Thus, the current experiments examined the effects of DCS on the extinction and subsequent reconditioning of ethanol-induced CPP in mice.
METHODS
Male DBA/2J mice received either 2 or 4 pairings of ethanol (2g/kg) with a CS+ floor cue (and an equal number of saline pairings with a CS− floor cue on alternate days) resulting in either a weak or strong ethanol CPP, respectively. Following conditioning of a strong ethanol CPP mice received saline or 30 mg/kg DCS prior to each of the twelve 30-min choice extinction trials administered at 48-hr intervals. Mice that had received conditioning of a weak ethanol CPP received saline, 30 or 60 mg/kg DCS immediately before each of the six 30-min choice extinction trials. Following successful ethanol CPP extinction, mice received reconditioning trials similar to the initial conditioning trials. A final experiment examined the effects 12 DCS pre-exposures (15, 30, and 60 mg/kg) on initial conditioning of ethanol CPP.
RESULTS
First, we showed that two doses of DCS (30 and 60 mg/kg) did not have aversive properties that could confound the effects on extinction of CPP (Exp. 1). Second, we showed that DCS (30 and 60 mg/kg) had no effect on the rate of extinction of either strong (Exp. 2) or weak (Exp. 3) ethanol-induced CPP. Interestingly, DCS administered during extinction interfered with reconditioning of ethanol-induced CPP—an effect specific to reconditioning, as DCS pre-exposure did not influence initial ethanol CPP conditioning (Exp. 4).
CONCLUSIONS
These experiments show that although DCS showed no effect on extinction behavior, when given during extinction it interfered with subsequent reconditioning of ethanol CPP. The mechanisms of this effect were not, however, due to nonspecific interference with learning because repeated DCS pre-exposures did not impair initial conditioning of ethanol CPP.
Keywords: ethanol, conditioned place preference, extinction, d-cycloserine, relapse
INTRODUCTION
Successful attenuation of alcohol-seeking behavior, as well as prevention of relapse to these behaviors, continues to be a considerable challenge in the rehabilitation process in alcoholic patients. Pharmacotherapies, as an adjunct to cognitive behavioral therapy, may serve as a means by which the extinction of alcohol-seeking behaviors can be enhanced and strengthened, thereby reducing the liklihood of future relapse. Although a number of pharmacotherapies have recently been explored, one such drug, D-Cycloserine (DCS), has received a great amount of attention for its ability to augment exposure-based behavioral therapy in a number of cognitive disorders including social anxiety (Hofmann et al., 2006), obsessive-compulsive disorder (Kushner et al., 2007), and acrophobia (Ressler et al., 2004). However, to our knowledge, no published clinical studies have examined the effects of DCS on the extinction of alcohol-seeking behaviors. As such, the current set of experiments was aimed at examining the effects of DCS on extinction of, and relapse to, ethanol-seeking behavior using ethanol-induced conditioned place preference (CPP) in mice.
Because it has been firmly established that glutamatergic transmission via the N-methyl-D-aspartate (NMDA) receptor is important for the encoding and recall of memories (for review see Riedel et al., 2003), NMDA-receptor transmission may be an ideal target for pharmacological enhancement of the extinction learning process. Enhancing extinction of conditioned behavior, both fear and drug-related, may improve the rehabilitation process and increase the long-term persistence of the extinguished behavior in humans, thereby reducing the potential of relapse.
One possible way to facilitate extinction via NMDA transmission may involve partial receptor agonists at the glycine site, as these compounds are capable of modulating channel activity (Johnson & Ascher, 1987) without resulting in the cellular toxicity seen with direct NMDA agonists (Deupree et al., 1996). One such partial agonist, DCS, has shown some promising results in its ability to enhance extinction in a variety of associative learning paradigms in rodents. For example, Walker et al. (2002) showed that DCS facilitated the extinction of freezing behavior in a conditioned fear procedure. This effect, however, was specific to the extinction phase, as DCS given during acquisition had no affect on the initial learning of the freezing behavior (Davis et al., 2006). These effects of DCS on the extinction of conditioned fear have been successfully reproduced using a variety of conditioning procedures (for review see Davis et al., 2006).
The majority of the reported experiments showing extinction-facilitating effects of DCS have involved conditioned fear and only a few studies have reported the effects of DCS on the extinction of learned behavior involving appetitive stimuli. Recently, however, DCS has been shown to accelerate extinction of a cocaine-induced place preference in rats when administered immediately after repeated nonreinforced preference tests (Paolone et al., 2008; Botreau et al., 2006). Additionally, Kelley et al. (2007) showed that a DCS injection administered immediately before a 20-min preference test reduced the expression of cocaine-induced CPP, an effect that persisted to a second CPP test 7 days later. Further, Sakurai et al. (2007) found that a single set of bilateral, intrahippocampal DCS injections prior to the first of four non-reinforced amphetamine CPP preference tests facilitated extinction of the place preference compared to saline-injected controls—an effect that was significant only on the fourth test. Although these studies appear to indicate that DCS may facilitate the extinction of cocaine- and amphetamine CPP, this effect has not been examined using other more commonly abused drugs such as ethanol. Recently, however, it was reported that DCS facilitated extinction of an ethanol-associated operant behavior in rats (Vengeliene et al., 2008). Thus, it appears that the effects of DCS exist across a variety of drugs and drug-seeking behaviors.
The current set of experiments was conducted to determine the effect of DCS on the extinction of both strong and weak ethanol-induced CPP (achieved by varying the number of conditioning trials) in DBA/2J mice. Given that DCS has been shown to facilitate extinction of both cocaine- and amphetamine CPP and conditioned fear, we hypothesized that DCS would also facilitate extinction of ethanol-induced CPP. Additionally, these experiments examined the long-term effects of injections of DCS during extinction by examining the subsequent reconditioning of the initial place preference. Rapid reconditioning has been used to show that extinguished behavior can be reinstated by a single presentation of the original Pavlovian association (Pavlov, 1927). This phenomenon, when examined in a CPP procedure, can be viewed as one of many models of relapse to drug-seeking behavior (Kehoe & Macrae, 1997). Because previous reports have shown that exposure to DCS during extinction hinders subsequent reinstatement (Ledgerwood et al. 2004), but not reconditioning (Ledgerwood et al. 2005) of conditioned fear, it was hypothesized that DCS given during extinction would have no effect on reconditioning of ethanol-induced CPP after extinction.
Also, to assess possible confounding effects of DCS on extinction of preference, we characterized the potential hedonic effects of two doses of DCS using our CPP procedure. This control study was included to confirm that any extinction-enhancing effects of DCS are due to its actions on extinction learning and not simply from counterconditioning the initial preference (as may be the case with the anxiogenic agent yohimbine, which has been shown to enhance extinction: File, 1986; Morris & Bouton, 2007). Finally, in order to examine the effects of chronic DCS exposure on ethanol-conditioned learning, animals were exposed to one of three doses of DCS prior to conditioning of ethanol CPP. The results of these experiments will serve to further elucidate the effects of DCS on extinction and reconditioning in hopes of characterizing potential clinical pharmacotherapies for aiding alcohol addiction rehabilitation and prevention of relapse.
MATERIALS AND METHODS
Subjects
Male DBA/2J mice were obtained from the Jackson Laboratory (Bar Harbor, ME) at 6–7 weeks of age. DBA/2J mice were used because of the extensive literature showing the robustness with which this strain acquires ethanol-induced CPP (e.g. Cunningham et al., 2006). Upon arrival in the animal colony, mice were given a minimum of 2 weeks to acclimate before any experimental procedures. Mice were housed in groups of three to four with ad libitum food and water in polycarbonate cages that were ventilated in a Thoren rack. The colony temperature was maintained at 21±1° C and lights were on a 12-hr cycle with experimental procedures performed during the light cycle (between 0700 and 1900 hr). The Oregon Health & Science University IACUC approved all experimental procedures.
Apparatus
The conditioning apparatus used in all experiments was identical to that described in detail by Cunningham et al. (2006). Briefly, acrylic and aluminum rectangular conditioning boxes (measuring 30 × 15 × 15 cm) were housed in sound and light attenuating chambers (Model E10–20, Colbourn Instruments, Allentown, PA). Each conditioning box was equipped with six, equally spaced, infrared emitter/detector pairs running the length of the box, 2.2 cm above the floor and 5 cm apart. These detectors provided activity counts (expressed as beam breaks per min) and side preference (expressed as time spent on the left and right sides) during all conditioning and test sessions. Activity and side preference data were collected using a computer. The conditioning boxes were equipped with removable grid and hole floors that served as the conditioned stimuli (CSs) for all experiments. The grid floors consisted of 2.3 mm stainless steel rods (spaced 6.4 mm apart) encased in an acrylic frame whereas the hole floors were made up of 16 gauge stainless steel sheets with 6.4 mm diameter holes on 9.5 mm staggered centers. Previous reports from our laboratory have shown that these cues are equally preferred when mice are given a pretest prior to any conditioning (i.e., the apparatus is unbiased: Cunningham et al., 2003).
Drugs
Ethanol (20 % v/v in isotonic saline) was administered intraperitoneally at a dose of 2 g/kg (12.5 ml/kg). Vehicle injections consisted of isotonic saline administered in a volume of 12.5 ml/kg. D-Cycloserine (DCS) solutions were prepared daily in 1.5, 3, and 6 mg/ml concentrations by dissolving the powder (Sigma Aldrich, St. Louis, MO) in isotonic saline. DCS was administered in a volume of 10 ml/kg, yielding doses of 15, 30, and 60 mg/kg.
Procedure
Each experiment consisted of some, or all, of the following experimental phases: habituation, conditioning, preference tests, extinction and reconditioning. For a detailed review of the standard conditioning procedure used in our laboratory see Cunningham et al. (2006).
Experiment 1: Unconditioned stimulus properties of DCS
The purpose of Experiment 1 was to determine if DCS injections induce any confounding aversive or appetitive effects. Mice (n = 47) were randomly divided into two experimental groups (30 and 60 mg/kg DCS groups) that received habituation, conditioning and preference test phases. Beginning 24 hr after a 5-min habituation session (animals injected with saline and placed in a conditioning chamber equipped with smooth paper flooring), mice received 8 days of place conditioning, with preference tests occurring 24 hr after the fourth and eighth days. Each conditioning day consisted of one 20-min exposure to the conditioning chamber with either all grid or all hole flooring (i.e., a one compartment training procedure). This session duration was chosen in order to allow ample time for the drug to be absorbed and distributed as it has been shown that a dose of 400 mg/kg DCS has a half-life of 23 min in mice (Conzelman & Jones, 1956). Mice were injected with either saline or DCS (30 or 60 mg/kg) immediately before placement on the CS− or CS+ floor, respectively. Exposure to the CS− and CS+ floors, as well as type of injection, alternated over the course of the conditioning sessions in a counterbalanced manner. Animals in the Grid+ (G+) group received drug paired with the grid floor and saline paired with the hole floor. Alternatively, animals in the Grid− (G−) group received saline paired with the grid floor and drug paired with the hole floor. Twenty-four hr after the fourth conditioning session, mice were injected with saline and placed in the center of the conditioning chamber prepared with both grid and hole flooring (preference test). Activity and side preference were monitored for 30 min. Beginning 72 hr after this test, mice received four additional conditioning sessions (two CS+ and two CS− trials) followed by another preference test.
Experiment 2: Effect of DCS on choice extinction of a strong preference
The purpose of Experiment 2 was to examine the effects of DCS pretreatments on the extinction of a strong ethanol-induced CPP. Mice (n = 48) were randomly divided into two experimental groups (Saline and 30 mg/kg DCS) that received habituation, conditioning, extinction and reconditioning. Animals received our standard ethanol-induced place conditioning procedure (Cunningham et al., 2006) that included a single 5-min habituation session, 8 days of ethanol place conditioning (5 min trials) with 2 g/kg ethanol and 12 preference tests (30-min each). These repeated preference tests, which served as the extinction trials, were given every 48 hr. Mice in the Saline group received a saline injection immediately before each extinction trial whereas mice in the DCS group received a DCS injection before each extinction trial. Seventy-two hr after the final preference test (Test 12), the two groups were further divided such that one half of each group received DCS before each reconditioning session and the remaining half received saline. Thus, four extinction-reconditioning groups (DCS-DCS, DCS-Sal, Sal-DCS, Sal-Sal) underwent the 2 days of reconditioning. Reconditioning consisted of one pairing of the CS+ floor with ethanol and one pairing of the CS− floor with saline (counterbalanced order) followed 24 hr later by a 30-min preference test. Analysis of the preference test after reconditioning revealed that DCS, when given immediately before reconditioning with ethanol, did not affect reconditioning (i.e., no significant interaction between Extinction Pretreatment and Reconditioning Pretreatment, p > 0.3). Specifically, the DCS-DCS group did not differ from the DCS-Sal group and the Sal-DCS group did not differ from the Sal-Sal group. Thus, data from these sets of groups were collapsed to the original two extinction treatment groups for analysis of Experiment 2 (Sal and DCS). Furthermore, because there were no effect of reconditioning treatment in Experiment 2, DCS injections were not administered before reconditioning trials in Experiment 3.
Experiment 3: Effect of DCS on choice extinction of a weak preference
The purpose of Experiment 3 was to extend the findings of Experiment 2 to the extinction of a weak ethanol-induced CPP using multiple doses of DCS. Mice (n=72) were randomly divided into three groups (Saline, 30 mg/kg DCS, and 60 mg/kg DCS) and, as in Experiment 2, underwent habituation, conditioning, extinction and reconditioning. However, animals received a shortened version of our standard ethanol-induced place conditioning procedure that involved only 4 days of conditioning (i.e., two CS+ and two CS− sessions) in contrast to the 8 days given in Experiment 2. After the fourth trial, animals received six extinction trials (30-min each) at 48-hr intervals. Each trial was preceded by either saline or DCS using the same procedures described for Experiment 2. Extinction was followed by a reconditioning cycle (i.e., one CS+ and one CS-trial) and another 30-min choice test. Because no significant reconditioning was seen in the control group, two additional reconditioning cycles (i.e., a total of two CS+ and two CS− sessions over 4 days) were conducted, each followed by a 30-min preference test.
Experiment 4: Effect of chronic DCS pre-exposure on development of ethanol CPP
The purpose of Experiment 4 was to investigate the effects of repeated DCS injections, administered in the home cage before conditioning, on initial conditioning of ethanol CPP. Mice (n=96) were randomly divided into four groups (Saline, 15 mg/kg DCS, 30 mg/kg DCS, and 60 mg/kg DCS) and received chronic exposure to saline or drug before ethanol CPP conditioning. Specifically, animals received either saline or DCS injections every 48 hrs for 24 days, resulting in a total of 12 injections. This procedure was designed so that animals in Experiment 4 were matched for total amount and pattern of DCS exposure with animals from Experiment 2—the experiment that showed the strongest effect of DCS on reconditioning. Forty-eight hrs after the final pre-exposure injection, all animals underwent standard CPP conditioning for 4 days (2 CS+ and 2 CS− trials) followed by a 30-min preference test. Forty-eight hrs after this first preference test, animals underwent another 4 days of conditioning followed by a final, 30-min preference test.
Data Analysis
The primary dependent variable in these studies was the amount of time spent on the grid floor (“grid times”) during the post-conditioning, extinction and post-reconditioning preference tests. In this unbiased counterbalanced CPP procedure, differences between the G+ and G− conditioning subgroups in time spent on the grid floor are used to index strength of place conditioning (Cunningham et al., 2003, 2006). However, to simplify examination of the time-course of extinction across repeated preference tests (Experiments 2 and 3), we also converted these data to percent time spent on the drug-paired floor (collapsed across conditioning subgroups). All data were evaluated using analysis of variance (ANOVA). DCS dose (30 vs. 60 mg/kg in Experiment 1), Drug (Saline vs. DCS in Experiments 2, 3, and 4) and Conditioning Subgroup (G+ vs. G− in all experiments) were treated as between-group factors, whereas Trial Type (CS+ vs. CS−), Trial (conditioning phase) and Test (extinction phase) were treated as within-group factors. Alpha-level was set at 0.05 for all analyses. Additional information about follow-up strategies is reported in the results section for each experiment.
Because of two recent reports suggesting that the effects of DCS on extinction are more apparent in individual animals that showed the greatest amount of extinction learning, we also analyzed the results of Experiments 2 and 3 utilizing the “median-split” method described in these reports (Bouton et al., 2008; Weber et al., 2007). Specifically, a median-split was performed on extinction data such that each drug-treatment group (Saline and DCS groups) was separated into “extinguishers” (those animals that showed above-median extinction, based on measures described below) and “non-extinguishers” (animals that showed below-median extinction). This median-split analysis was performed for three different measures of extinction including 1) Percent Time spent on the ethanol-paired floor on the final test of extinction (Extinction Tests 12 and 6 for Experiments 2 and 3, respectively), 2) Decrease in Percent Time spent on the ethanol-paired floor from the beginning (Test 1) to the end of extinction (Test 12 or 6 for Experiments 2 or 3, respectively), and finally, 3) Percent Time spent on the ethanol-paired floor during the second extinction trial (Test 2) for animals that showed above or below-median levels of within-session extinction during Test 1 (as indicated by the difference between the First 10-min and the Last 10-min blocks of the 30-min session). This latter analysis was performed because it has been shown that the ability of DCS to facilitate extinction is most robust during the first few drug exposures. However, because these multiple statistical analyses did not reveal any new, significant effects of DCS on extinction and therefore added no additional insight, these data were not reported.
RESULTS
Experiment 1: Unconditioned stimulus properties of DCS
Experiment 1 was performed to assess the stimulus qualities of DCS by using two doses of DCS (30 or 60 mg/kg) as the unconditioned stimulus in a standard CPP procedure.
Conditioning Activity
Examination of activity levels during conditioning revealed decreases in activity for both groups over trials, but no effects of DCS dose or trial type. This observation was supported by a three-way repeated measures ANOVA (Dose Group × Trial Type × Trial) that revealed a significant main effect of Trial [F(3,135) = 12.3, p < 0.0001], but no effects of dose, trial type or interactions (p’s > 0.4). Post-hoc analyses showed that activity for both dose groups was lower on the fourth trial (DCS mean = 34.0±1.3; Saline mean = 34.9±1.3) than on the first trial (DCS mean = 40.7±1.0; Saline mean = 40.2±0.9) on both CS+ [DCS: t(46) = 5.2, p < 0.0001] and CS− [saline: t(46) = 5.5, p < 0.0001] trials.
Preference Tests
There was no evidence of place conditioning on either the first or second test, with mice spending an average of 48.1±2.8% time on the DCS paired floor (averaged across both dose groups and tests). Two-way (Dose Group × Conditioning Subgroup) ANOVAs applied separately to the times spent on the grid floor in each test failed to yield any significant main effects or interactions, supporting the conclusion that DCS, at doses of either 30 or 60 mg/kg, did not condition either a place preference or aversion.
Test Activity
Prior exposure to DCS during conditioning trials did not affect activity during either drug-free preference test. Mean activity rates (counts/min) for the 30 and 60 mg/kg groups were 33.6±1.5 and 32.0±1.2, respectively (averaged across both conditioning subgroups and tests). One-way ANOVAs showed no group difference during either test.
Experiment 2: Effect of DCS on extinction of a strong preference
Experiment 2 was performed to assess the effects of DCS on the extinction of a strong, ethanol-induced CPP. DCS (30 mg/kg) or saline was administered before each preference test during the extinction phase. Three mice (from the Saline group) were removed from the study due to poor health.
Conditioning Activity
Consistent with previous studies in DBA/2J mice (e.g., Cunningham et al., 2003), activity was higher during CS+ (ethanol) trials than during CS−(saline) trials. Mean activity rates across all CS+ trials were 174.7±6.2 and 170.8±4.6 counts/min for the Saline and DCS groups, respectively. On CS− trials, activity rates for those groups were 45.9±2 and 46.6±1.7 counts/min, respectively. A two-way (Drug Group × Trial Type) repeated measures ANOVA confirmed the significant main effect of Trial Type [F(1,43) = 930.6, p < 0.0001] and the absence of a significant drug group effect or interaction. Thus, the groups did not differ in activity before the extinction phase.
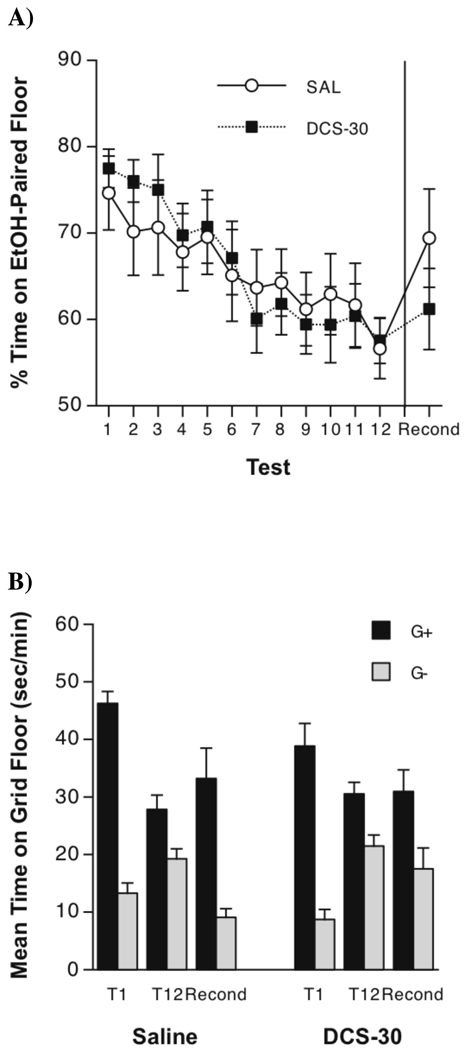
First Preference Test
Performance on the first post-conditioning test is depicted in Figure 1 both as percent time spent on the ethanol-paired floor (Panel A) and as time (sec/min) spent on the grid floor (Panel B). As can be seen, both drug groups expressed a robust conditioned preference, with the Saline and DCS groups spending 74.6±4.3% and 77.5±2.2% of the session on the ethanol-paired floor, respectively (averaged across conditioning subgroups; Test 1, Panel A). Conditioned preference was also apparent in the grid times, which showed that G+ subgroups spent more time on the grid floor than G− subgroups (T1, Panel B). Two-way (Drug Group × Conditioning Subgroup) ANOVA of grid time scores confirmed the development of a significant CPP [Conditioning Subgroup effect: F(1,41) = 154.5, p < 0.0001] that did not differ between drug groups [interaction: F < 1]. Unexpectedly, this ANOVA also yielded a significant main effect of Drug Group [F(1,41) = 5.5, p < 0.05], reflecting the fact that Saline-treated mice spent more time on the grid floor than DCS treated mice, regardless of conditioning subgroup. Overall, these data showed that DCS did not affect the initial magnitude of ethanol-induced CPP and that both groups showed a similar initial preference.
Figure 1.
(A) Mean ± SEM percent time spent by Saline and DCS (30 mg/kg) groups on the ethanol-paired floor over the course of 12 extinction tests (Tests 1–12) and after reconditioning (Recond). Significant extinction of the strong preference occurred in both groups and was not affected by DCS. The DCS group showed impaired reconditioning of ethanol CPP when compared to the Saline group (a statistical trend toward a significant Group × Test interaction, p = . 051). (B) Mean ± SEM time spent on the grid floor for Grid+ (G+) and Grid− (G−) mice on the first extinction test (T1) and before (T12) and after reconditioning (Recond). DCS given before extinction trials impaired reconditioning of a strong ethanol-induced CPP.
Extinction
Performance over the 12 extinction tests is shown as percent time on the ethanol-paired floor in Figure 1A. Preference declined steadily with repeated testing, but there was no effect of DCS pretreatment on extinction. A two-way (Drug Group × Test) ANOVA of percent time spent on the ethanol-paired floor during each test revealed a significant main effect of Test [F(11,473) = 18.2, p < 0.0001], indicating that repeated testing produced a decrease in place preference (i.e., extinction). However, there was no significant effect of drug group or interaction, suggesting that DCS did not affect CPP strength or the time course of extinction. A three-way (Drug Group × Conditioning Subgroup × Test) ANOVA comparing grid times on the first and 12th tests (i.e., T1 and T12 in Figure 1B) confirmed conclusions from the percent time analysis, yielding a significant Conditioning Subgroup × Test interaction [F(1,41) = 78.2, p < 0.0001] that reflected the decrease in CPP magnitude across tests. This analysis also produced significant main effects of Conditioning Subgroup [F(1,41) = 109.2, p < 0.0001] and Drug Group [F(1,41) = 4.8, p < 0.05], but no main effect of Test or other interactions. Thus, the analyses of both dependent variables indicated that the DCS and saline pretreatment groups performed similarly during extinction of ethanol-induced CPP.
Reconditioning
Results of the preference test conducted after the reconditioning cycle are shown on the right side of the panels in Figure 1 (Recond). As can be seen, DCS pretreatment on extinction trials impaired reconditioning when compared to the Saline group. A two-way (Drug Group × Test) repeated measures ANOVA of percent time spent on the ethanol-paired floor before and after reconditioning (i.e., Test 12 vs. Recond) revealed a significant Test effect [F(1,43) = 13.1, p < 0.001] and a trend toward a significant Drug Group × Test interaction [F(1,43) = 4.0, p = 0.051], consistent with the conclusion that the Saline group showed stronger reconditioning than the DCS group. A three-way (Drug Group × Conditioning Subgroup × Test) ANOVA applied to grid times (Figure 1B, T12 vs. Recond) offered additional support for this conclusion, yielding a significant three-way interaction [F(1,41) = 4.3, p < 0.05], as well as significant main effect of Conditioning Subgroup [F(1,41) = 24.4, p < 0.0001] and a Conditioning Subgroup × Test interaction [F(1,41) = 13.6, p < 0.001]. Two-way (Conditioning Subgroup × Test) follow-up ANOVAs conducted separately for each drug group showed a significant interaction in the Saline group [F(1,19) = 15.9, p < 0.001], but not in the DCS group [F(1,22) = 1.4, p > 0.25]. Thus, these analyses suggested that pretreatment with DCS during extinction interfered with subsequent reconditioning after extinction of a strong CPP.
Test Activity
Activity for both groups increased from the first to last of the 12 extinction tests, but there was no difference between groups (Table 1). These conclusions were supported by a two-way (Drug Group × Test) ANOVA of T1 and T12 test activity that yielded a significant main effect of Test [F(1,43) = 39.1, p < 0.0001], but no significant main effect of drug group or interaction. Also, there was no effect of prior drug treatment during the reconditioning test. Thus, interpretation of the drug effect on reconditioning of a strong CPP was not complicated by group differences in test activity (Gremel & Cunningham, 2007).
Table 1.
Experimental Designs, Drug Doses, and Test Trial Activity (counts/min ± SEM)
| Exp. | Cond. CS+ drug |
Conditioning Subgroup |
n | CS+ dose (g/kg) |
DCS Dose (g/kg) |
First Extinction Trial Activity (counts/min) |
Last Extinction Trial Activity (counts/min) |
Post- Reconditioning Test Activity (counts/min) |
|---|---|---|---|---|---|---|---|---|
| 1 | DCS | Grid+ | 12 | .03 | -- | -- | -- | -- |
| Grid− | 12 | |||||||
| DCS | Grid+ | 12 | .06 | -- | -- | -- | -- | |
| Grid− | 11 | |||||||
| 2 | EtOH | Grid+ | 11 | 2 | 0 | T1= 29.7±1.9 | T12= 41.4±2.2 | TRecond= 33.5±2.5 |
| Grid− | 10 | |||||||
| EtOH | Grid+ | 12 | 2 | .03 | T1= 32.7±11.3 | T12= 43.9±2.1 | TRecond= 36.8±2.3 | |
| Grid− | 12 | |||||||
| 3 | EtOH | Grid+ | 12 | 2 | 0 | T1= 32±1.4 | T6= 30.2±1.7 | TRecond= 29.3±2.4 |
| Grid− | 12 | |||||||
| EtOH | Grid+ | 12 | 2 | .03 | T1= 30.7±1.9 | T6= 28.7±2 | TRecond= 23±1.9 | |
| Grid− | 11 | |||||||
| EtOH | Grid+ | 12 | 2 | .06 | T1= 32.7±1.2 | T6= 32.6±1.6 | TRecond= 26.7±2.1 | |
| Grid− | 12 | |||||||
| 4 | EtOH | Grid+ | 12 | 2 | 0 | -- | -- | -- |
| Grid− | 11 | |||||||
| EtOH | Grid+ | 12 | 2 | .015 | -- | -- | -- | |
| Grid− | 12 | |||||||
| EtOH | Grid+ | 11 | 2 | .03 | -- | -- | -- | |
| Grid− | 12 | |||||||
| EtOH | Grid+ | 12 | 2 | .06 | -- | -- | -- | |
| Grid− | 12 | |||||||
NOTE: Values in BOLD indicate a significant (p < 0.05) main effect of Test from Drug Group × Test repeated measures ANOVA comparing activity from the first and last extinction tests.
Experiment 3: Effect of DCS on extinction of a weak preference
Experiment 3 was performed to assess the effects of saline or two DCS doses (30 and 60 mg/kg) on extinction of an ethanol-induced CPP weaker than that induced in Experiment 2. Saline or DCS was injected only before each extinction test. Two mice were removed from the study, one because of a procedural error (DCS-30 group) and the other because of a health problem (DCS-60 group).
Conditioning Activity
As in Experiment 2, animals showed higher activity levels on CS+ (ethanol) trials than on CS− (saline) trials. Mean activity rates across both CS+ trials were 167.3±4.9, 171.6±4.0 and 173.6±5.5 counts/min for the Saline, DCS-30 and DCS-60 groups, respectively. On CS− trials, mean rates for those groups were 54.9± 3.4, 57.9±3.0 and 56.7±2.2, respectively. Ethanol’s activating effect was confirmed by a Drug Group × Trial Type repeated-measures ANOVA that revealed a significant main effect of Trial Type [F(1,67)=1891.3, p < .0001], but no effect of drug group or interaction.
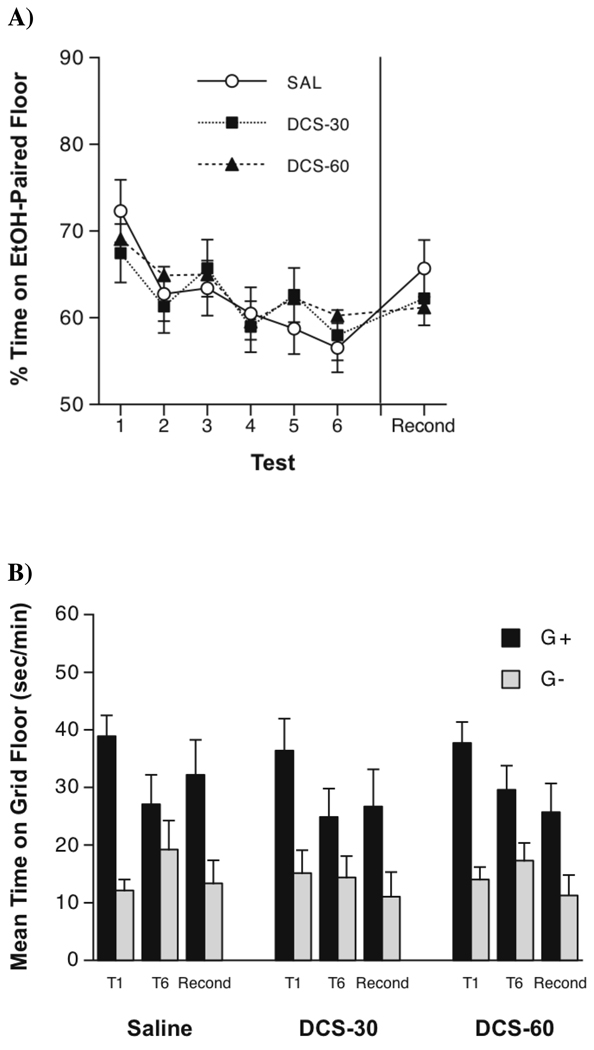
First Preference Test
Performance on the first post-conditioning test is shown in Figures 2A (percent time) and 2B (grid time). All three groups expressed a reliable preference, but (as expected) the preference produced by two CS+ and two CS− trials in Experiment 3 was generally weaker than that induced by four trials of each type in Experiment 2. Averaged across conditioning subgroups, the Saline, DCS-30 and DCS-60 groups spent 72.3±3.7%, 67.4±5.8% and 69.4±3.8% of the test session on the ethanol-paired floor, respectively (Test 1, Figure 2A). All three groups also showed significant place preference as indexed by higher grid time scores in the G+ subgroups than in the G− subgroups (T1, Figure 2B). Two-way (Drug Group × Conditioning Subgroup) ANOVA of grid times showed a significant Conditioning Subgroup effect [F(2,64) = 63.1, p < 0.0001], confirming development of CPP. However, there was no significant effect of drug group or interaction, indicating that DCS did not affect the initial expression of ethanol-induced CPP.
Figure 2.
(A) Mean ± SEM percent time spent by both groups on the ethanol-paired floor over the course of six extinction tests (Tests 1–6) and after reconditioning (Recond). Significant extinction of the weak preference occurred in both groups and was not affected by DCS. Further analysis indicated that significant reconditioning occurred in only the Saline group. (B) Mean ± SEM time spent on the grid floor for Grid+ (G+) and Grid− (G−) mice on the first extinction test (T1) and before (T6) and after (Recond) the final reconditioning session. DCS given before extinction trials impaired reconditioning of a weak ethanol-induced CPP.
Extinction
Figure 2A shows CPP expressed as percent time on the ethanol-paired floor over the six extinction tests (T1-T6). Although all groups showed a decrease in CPP across tests, neither DCS dose affected rate of extinction. A two-way (Drug Group × Test) repeated measures ANOVA of the percent time data supported this conclusion, yielding a significant main effect of Test [F(5,335) = 6.8, p < 0.0001], but no effect of drug group or interaction. This conclusion was also supported by a three-way (Drug Group × Conditioning Subgroup × Test) ANOVA of grid times on the first and sixth extinction tests (T1 and T6, Figure 2B), which produced a significant Conditioning Subgroup × Test interaction [F(1,64) = 19.7, p < 0.0001] and significant main effects of Conditioning Subgroup [F(1,64) = 32.7, p < 0.0001] and Test [F(1,64) = 5.5, p < 0.05], but no other effects. Thus, like Experiment 2, these data showed that DCS pretreatment had no effect on extinction of ethanol-induced place CPP.
Reconditioning
The right sides of the panels in Figure 2 show the outcome of the final post-reconditioning preference test. Visual inspection suggests that the Saline group showed a greater increase in CPP between the last extinction test (Test 6) and the reconditioning test (Recond). Although a two-way (Drug Group × Test) repeated measures ANOVA yielded a significant main effect of Test [F(1,67) = 5.4, p < .03], indicating that reconditioning was successful, the drug group × test interaction was not significant (p > 0.3). Nevertheless, in light of the reconditioning test results in Experiment 2, we examined the reconditioning effect separately for each drug group using a paired t-test (i.e., Test 6 vs. Recond) with the Bonferonni-corrected alpha level set at 0.017. These analyses indicated that only the Saline group showed significant reconditioning [t(23) = 2.7, p = 0.013].
Our analysis of grid times (Figure 2B, T6 vs. Recond) yielded similar results. A three-way (Drug Group × Conditioning Subgroup × Test) repeated measures ANOVA revealed a significant Conditioning Subgroup × Test interaction [F(1,64) = 5.9, p < 0.02] and a significant main effect of Conditioning Subgroup [F(1,64) = 12.7, p < 0.001], but no main effects of drug group or test and no other interactions. Conditioning Subgroup × Test ANOVAs applied separately to data from each drug group indicated that only the Saline group showed significant reconditioning, as confirmed by a significant interaction [F(1,22) = 7.0, p < .015]. Thus, as in Experiment 2, DCS administration during extinction trials prevented subsequent reconditioning of CPP.
Test Activity
Analysis of test activity from the first (T1) and last (T6) extinction tests showed no significant differences in any of the groups (Table 1). This was supported by a two-way (Drug Group × Test) ANOVA that revealed no significant interaction or main effects. Moreover, there was no significant group difference in activity during the preference test after the last reconditioning session, eliminating test session activity differences as an explanation for the group difference in reconditioning of a weak CPP.
Experiment 4: Effect of chronic DCS pre-exposure on development of ethanol CPP
Experiment 4 was performed to assess the effects of chronic pre-exposure (12 injections every 48 hrs) of three DCS doses (15, 30 and 60 mg/kg) on subsequent development and expression of ethanol-induced CPP. One mouse was removed from the study because of a procedural error (DCS-30 group).
Conditioning Activity
As in Experiments 2 and 3, animals in all groups showed higher activity levels on CS+ (ethanol) trials than on CS− (saline) trials. Mean activity rates across CS+ trials were 141.1±3.8, 142.9±4.8, 137.5±5.2 and 141.7±4.0 counts/min for the Saline, DCS-15, DCS-30 and DCS-60 groups, respectively. On CS− trials, mean rates for those groups were 49.6±2.1, 45.1±1.5, 48.0±1.7 and 49.6±1.9, respectively. Ethanol’s activating effect was confirmed by a Drug Group × Trial Type repeated-measures ANOVA that revealed a significant main effect of Trial Type [F(1,91)=1918.8, p < .0001], but no effect of drug group or interaction. Thus, DCS pre-exposure had no effect on either CS+ or CS− trial activity.
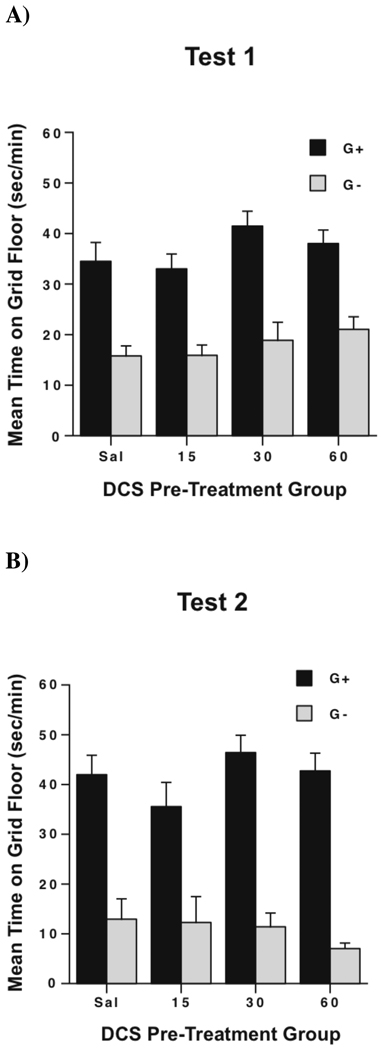
Preference Tests
Two CPP expression tests were conducted after the first two and then again after all four conditioning trials. Data from these two tests are shown in Figure 3 as time spent on the grid floor for animals in the G+ and G− conditioning subgroups. As can be seen, animals in the Saline group as well as all three DCS groups showed significant place preference on Test 1 (Figure 3A) that strengthened in Test 2 (Figure 3B). There was, however, no effect of DCS pre-exposure at any dose on the development of ethanol CPP. The Drug Group × Conditioning Subgroup × Test ANOVA revealed a significant main effect of Conditioning Subgroup [F(1,87)=141.3, p < .0001] and a significant Conditioning Subgroup × Test interaction [F(1,87)=26.4, p < .0001], confirming that all groups showed similarly significant place preference that strengthened over the course of conditioning. There was no significant three-way interaction, indicating that DCS pre-exposure had no effect on develoment of ethanol CPP.
Figure 3.
(A) Mean ± SEM time spent on the grid floor for Grid+ (G+) and Grid− (G−) mice on the first preference test (Test 1). Chronic DCS pre-exposure had no effect on development of ethanol CPP after 2 CS+ and 2 CS− trials. (B) Mean ± SEM time spent on the grid floor for Grid+ (G+) and Grid− (G−) mice on the second preference test (after a total of 4 CS+ and 4 CS− trials). All groups show signficantly stronger CPP on Test 2 when compared to Test 1 (a significant Conditioning Subgroup × Test interaction), but there was no effect of DCS pre-exposure at any of the tested doses.
DISCUSSION
Although DCS did not enhance the rate of extinction, there was evidence that DCS enhanced the persistence of extinction, i.e., reconditioning of the initial place preference was impaired in DCS-treated mice. The lack of a facilitating effect of DCS on the rate of extinction was consistent across two doses (30 and 60 mg/kg) and two experiments that manipulated the strength of initial place preference by varying the number of conditioning trials. In both studies, however, administration of DCS prior to each extinction trial impaired subsequent reconditioning of the extinguished place preference. This effect on reconditioning was not due to unconditioned effects of DCS because neither dose conditioned a place aversion (Experiment 1) or had any effect on activity levels. Furthermore, the effects of DCS on reconditioning were not due to a nonspecific effect of chronic DCS exposure on learning because DCS pre-exposure before initial conditioning did not impair development of ethanol CPP (Experiment 4).
In Experiment 2, the strongly conditioned place preference resulted in a relatively slow rate of extinction, which should have allowed any extinction-facilitating effects of DCS to be observed. However, because extinction took such a long time in this experiment, it was hypothesized that DCS might be more effective in facilitating the extinction of a weaker, more susceptible, place preference. Because previous work from our laboratory had shown that testing after only two CS+ and two CS− conditioning trials produced a weaker CPP than that seen after four CS+ and four CS− trials (Cunningham et al., 2002), Experiment 3 examined DCS effects on extinction of a weaker ethanol-induced CPP. The results of that experiment showed that the weaker CPP extinguished more rapidly than CPP in Experiment 2 as indexed by the number of test trials required to reduce overall mean preference below 60% (6 tests in Experiment 3 vs. 12 tests in Experiment 2). However, despite beginning extinction with a weaker CPP, mice given DCS before each extinction test did not show an enhanced rate of extinction. Nevertheless, as in Experiment 2, DCS injections administered during extinction impaired subsequent reconditioning.
The results of Experiments 2 and 3 are not consistent with previous reports of the facilitating effects of DCS on extinction of conditioned fear and cocaine-induced CPP. In contrast to our experiments, those studies reported effects of DCS on rate of extinction (e.g., Botreau et al., 2006; Walker et al., 2002). Additionally, the effects of DCS on the reconditioning of an extinguished place preference in the current experiments are not in agreement with previous reports showing that DCS given during extinction blocks reinstatement, but not reconditioning, of conditioned fear (Ledgerwood et al. 2004; Ledgerwood et al. 2005). Interestingly, in contrast to the results of Ledgerwood et al. (2004), Kelley et al. (2007) showed that extinction-specific DCS administration did not impair reinstatement of cocaine CPP. More recently, Paolone et al. (2008) showed that rats treated with DCS during extinction of cocaine CPP showed an inability to exhibit cocaine-induced reinstatement. However, because the control group (Saline-treated animals) showed no significant reinstatement of cocaine CPP (as indicated by a signficant increase in preference following the cocaine-priming injection), the interpretation of this effect is limited. Thus, considering the current reconditioning effects of DCS, as well as the data from Kelley et al. (2007), it appears that both reconditioning and reinstatement of drug-induced CPP may involve different mechanisms than those involved in the conditioned fear procedures used by Ledgerwood et al. (2004, 2005). However, a more systematic examination of the different methods of reinstatement and reconditioning within each of these behavioral procedures is required to clarify these discrepancies.
In contrast to most of the previously published reports of the extinction-facilitating effects of DCS, the current set of experiments involved examination of two doses of DCS (30 and 60 mg/kg) in the DBA/2J mouse strain. In previous reports, effective doses for the extinction-facilitating effects of DCS in rats have ranged from 5 to 30 mg/kg with the majority of experiments using 15 mg/kg, whereas in mice, effective doses have ranged from 15 to 30 mg/kg (e.g., Kelley et al., 2007; Tomilenko & Dubrovina, 2007). Interestingly, doses of 15 and 30 mg/kg DCS have been shown to equally enhance extinction of a food-associated operant behavior in C57Bl/6 mice (Shaw et al., 2008). However, because at high doses, DCS can exhibit antagonist-like characteristics (for review see Lanthorn, 1994) as well as the fact that the cognitive enhancing effects of DCS are eliminated at both very low (i.e., 2.5 mg/kg) and high DCS doses (i.e., 50 mg/kg) in mice (Flood et al., 1992), we hypothesized that the extinction-facilitating effects of DCS in DBA/2J mice would be greatest at a dose of 30 mg/kg. Therefore, although Experiment 1 revealed no detectable stimulus properties or locomotor effects of either 30 or 60 mg/kg DCS, we decided to examine only the lower of these two doses in Experiment 2. Although this dose resulted in no effect on extinction of ethanol CPP (Experiment 2), it did significantly impair subsequent reconditioning and, as such, we are confident that DCS did reach biologically relevant levels. This is further supported by previous reports showing that an even lower dose of 20 mg/kg DCS has anticonvulsant effects against audiogenic seizures in DBA/2 mice (De Sarro et al., 2000). Therefore, we feel strongly that the doses used in Experiments 2 and 3 should have been sufficient to produce biologically relevant, potentially extinction-facilitating, levels of DCS in the brains of the DBA/2J mice used in these studies. It has been suggested, however, that the cognitive-enhancing effects of DCS may be strain dependent (Sunyer et al., 2008), and therefore, it is possible that DBA/2J mice, although adept at expressing ethanol CPP, may not be as susceptible to the extinction-facilitating effects of DCS as other mouse strains and/or species.
Studies of DCS on extinction of ethanol-induced learning are particularly interesting because ethanol has direct interactions with the NMDA receptor. Ethanol inhibits glutamatergic transmission of the NMDA receptor by both glycine-reversible and glycine-independent mechanisms (Buller et al. 1995). Further, ethanol exposure can reduce the potency of glycine at its binding site, thereby inhibiting NMDA receptor transmission in rat cerebellar cells (Hoffman et al. 1994). In a study examining the effects of DCS on extinction during withdrawal from ethanol, Bertotto et al. (2006) showed that chronic ethanol exposure (14 days of a 6% v/v ethanol containing liquid diet) impaired the subsequent extinction of conditioned fear. Furthermore, the previous chronic exposure actually enhanced the extinction-facilitating effects of a sub-optimal dose of DCS. Similarily, in a recently published report, Vengeliene et al. (2008) showed that a low dose of DCS (5 mg/kg), administered 60 minutes prior to the extinction trial, facilitated the extinction of an ethanol-paired operant behavior in rats following extensive ethanol exposure during saccharine-fading, training, and discrimination training. However, because in the current study, mice were administered only four or two injections of 2 g/kg ethanol over eight and four days (Experiments 2 and 3, respectively), it is unlikely that these sub-chronic ethanol exposures significantly altered NMDA-receptor function in such a way that impaired the effects of DCS during the initial extinction trials.
Because extinction lasted for 12 trials in Experiment 2 and six trials in Experiment 3, we were able to examine the effects of DCS with different amounts of extinction. We found that DCS had no effect on rate of extinction during the initial few trials, when preference was highest, nor did it have effects during later trials, when preference was lower. These findings suggest that the failure to observe effects of DCS on extinction were not due to ceiling or floor effects on preference. However, it is possible that the absence of an effect on the later extinction trials was due to previous exposure to DCS during initial extinction trials. Pre-exposure to DCS has been shown to reduce the learning-enhancing effects of DCS in the Porsolt Swim Test (Lopes et al. 1997), a linear maze apparatus (Quartermain et al 1994), and extinction of conditioned fear (Parnas et al. in 2005). All of these reports hypothesized that pre-exposure to DCS caused a desensitization of the NMDA receptor at the glycine-binding site, thereby reducing the effects of subsequent DCS exposures. Therefore, in the current experiments, the effects of DCS, expected to be the greatest during the first few extinction trials when the greatest amount of learning should occur, were either non-existent or undetectable with our behavioral assay, whereas any effects of DCS injections later in the extinction phase were most likely hindered by NMDA-receptor desensitization caused by the initial DCS exposures.
The impairment of reconditioning by DCS, evident in both Experiments 2 and 3, may have resulted from an extinction-facilitating effect of DCS. Specifically, despite showing no effect on extinction behavior, DCS may have deepened the extinction learning, thereby impairing the subsequent reconditioning process. This hypothesis is supported, in part, by the finding that repeated exposure to DCS in Experiment 4 before conditioning had no affect on the new learning that occurs during initial ethanol CPP conditioning. Thus, it seems unlikely that the impaired reconditioning in the DCS groups was simply a result of NMDA-receptor desensitization caused by DCS exposure during extinction.
Given the complexity of the actions of DCS and the procedural sensitivity of the effects of DCS, it is not surprising that several reports have shown inconsistencies in the ability of DCS to enhance extinction. For example, extinction enhancing effects of DCS have not been observed in a variety of behavioral disorders including mild arachnophobia (Guastella et al., 2006) and obsessive-compulsive disorder (Storch et al., 2007). Further, in studies of rodents, DCS effects on extinction may sometimes be limited to low anxiety animals (Tomilenko & Dubrovina, 2007) or to animals that show large amounts of extinction within a session (e.g., Weber et al., 2007). In a recent report, Woods and Bouton (2006) demonstrated that DCS (30 mg/kg) enhanced the rate of fear extinction in rats, but did not weaken contextual renewal of conditioned fear. This effect, however, was not significant at a lower dose of 15 mg/kg. Moreover, when the authors attempted to replicate the significant findings using 30 and 60 mg/kg, they found no effect of either DCS dose.
In a more recent follow-up report, these authors showed that DCS did, in fact, have slight extinction-enhancing effects but only in animals that showed the greatest amount of overall extinction learning. Specifically, Bouton et al. reported that when analyzing only the rats that showed above-median extinction levels, DCS (30 mg/kg) showed signficant facilitation of extinction (Bouton et al., 2008). However, despite using multiple variants of this “median-split” technique to analyze our current set of experimental data, we were unable to detect any extinction-enhancing effects of DCS at any dose. In fact, we performed median-split analyses using three different measures of extinction including preference on the last test of extinction (Tests 12 and 6 for Experiments 2 and 3, respectively), decrease in preference over the course of extinction (calculated as a preference difference score from the first to the last test of extinction), and finally, preference on the second test of extinction (Test 2) for animals that showed above-and below-median within-session extinction on Test 1. This latter analysis was performed after considering previously published reports suggesting that the extinction-facilitating effects of DCS are greatest during the first few extinction trials. All in all, none of these median-split analyses revealed a signficant effect of DCS on extinction of ethanol CPP (data not shown).
In conclusion, these experiments demonstrated that DCS did not facilitate the rate of extinction of ethanol-induced CPP, regardless of the strength of the initial preference. Nevertheless, administration of DCS during extinction impaired subsequent reconditioning of ethanol-induced CPP, demonstrating that DCS did enhance some aspect of the extinction experience. This was further supported by the findings of Experiment 4 that revealed no effect of chronic exposure to multiple doses of DCS on the initial development and expression of ethanol CPP. These findings emphasize the general importance of using multiple measures of learning to assess pharmacological effects on extinction. Additionally, these findings suggest that DCS may not be capable of facilitating the behavioral therapy used in the rehabilitation of alcoholic patients, though it may provide a means by which to reduce the potential for relapse to alcohol-seeking behavior.
Acknowledgements
This research was supported by NIH grants AA007702, AA007468, MH077111, and DA025922.
REFERENCES
- Bertotto ME, Bustos SG, Molina VA, Martijena ID. Influence of ethanol withdrawal on fear memory: Effect of D-cycloserine. Neuroscience. 2006;142(4):979–990. doi: 10.1016/j.neuroscience.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172(1):173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90(3):504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller AL, Larson HC, Morrisett RA, Monaghan DT. Glycine modulates ethanol inhibition of heteromeric N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Mol Pharmacol. 1995;48(4):717–723. [PubMed] [Google Scholar]
- Conzelman GM, Jones RK. On physiological disposition of cycloserine in experimental animals. Am Rev Tuberc. 1956;74:802–806. doi: 10.1164/artpd.1956.74.5.802. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170(4):409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1(4):1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE, Meyer PJ. Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology (Berl) 2002;160(4):414–424. doi: 10.1007/s00213-001-0990-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60(4):369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- De Sarro G, Gratteri S, Naccari F, Pasculli MP, De Sarro A. Influence of D-cycloserine on the anticonvulsant activity of some antiepileptic drugs against audiogenic seizures in DBA/2 mice. Epilepsy Res. 2000;40(2–3):109–121. doi: 10.1016/s0920-1211(00)00113-3. [DOI] [PubMed] [Google Scholar]
- Deupree DL, Tang XW, Yarom M, Dickman E, Kirch RD, Schloss JV, Wu JY. Studies of NMDA- and non-NMDA-mediated neurotoxicity in cultured neurons. Neurochem Int. 1996;29(3):255–261. doi: 10.1016/0197-0186(96)00003-4. [DOI] [PubMed] [Google Scholar]
- File SE. Aversive and appetitive properties of anxiogenic and anxiolytic agents. Behav Brain Res. 1986;21(3):189–194. doi: 10.1016/0166-4328(86)90236-6. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Lanthorn TH. Effect on memory processing by D-cycloserine, an agonist of the NMDA/glycine receptor. Eur J Pharmacol. 1992;221(2–3):249–254. doi: 10.1016/0014-2999(92)90709-d. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology (Berl) 2007;191(2):195–202. doi: 10.1007/s00213-006-0651-5. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of d-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007;41(6):466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Snell LD, Bhave SV, Tabakoff B. Ethanol inhibition of NMDA receptor function in primary cultures of rat cerebellar granule cells and cerebral cortical cells. Alcohol Alcohol Suppl. 1994;2:199–204. [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63(3):298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Macrae M. Savings in animal learning: Implications for relapse maintenance after therapy. Behavior Therapy. 1997;28:141–155. [Google Scholar]
- Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: disruption and reinstatement. Neuroreport. 2007;18(8):777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62(8):835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Lanthorn TH. D-Cycloserine: Agonist turned antagonist. Amino Acids. 1994;6:247–260. doi: 10.1007/BF00813745. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118(3):505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57(8):841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Lopes T, Neubauer P, Boje KM. Chronic administration of NMDA glycine partial agonists induces tolerance in the Porsolt swim test. Pharmacol Biochem Behav. 1997;58(4):1059–1064. doi: 10.1016/s0091-3057(97)00302-x. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci. 2007;121(3):501–514. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Paolone G, Botreau F, Stewart J. The facilitative effects of D: -cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83(3):224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. New York: Dover Publications; 1927. [Google Scholar]
- Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur J Pharmacol. 1994;257(1–2):7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140(1–2):1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Yu L, Tan SE. Roles of hippocampal N-methyl-D-aspartate receptors and calcium/calmodulin-dependent protein kinase II in amphetamine-produced conditioned place preference in rats. Behav Pharmacol. 2007;18(5–6):497–506. doi: 10.1097/FBP.0b013e3282ee7b62. [DOI] [PubMed] [Google Scholar]
- Shaw D, Norwood K, Sharp K, Quigley L, McGovern SF, Leslie JC. Facilitation of extinction of operant behaviour in mice by D: -cycloserine. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1312-7. [DOI] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, Yang MC, Jacob ML, Larson M, Hirsh A, Fernandez M, Geffken GR, Goodman WK. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2007;22(4):230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- Sunyer B, Patil S, Frischer C, Hoeger H, Lubec G. Strain-dependent effects of cognitive enhancers in the mouse. Amino Acids. 2008;34(3):485–495. doi: 10.1007/s00726-007-0511-6. [DOI] [PubMed] [Google Scholar]
- Tomilenko RA, Dubrovina NI. Effects of activation and blockade of NMDA receptors on the extinction of a conditioned passive avoidance response in mice with different levels of anxiety. Neurosci Behav Physiol. 2007;37(5):509–515. doi: 10.1007/s11055-007-0044-1. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Kiefer F, Spanagel R. D-cycloserine facilitates extinction of conditioned alcohol-seeking behaviour in rats. Alcohol Alcohol. 2008;43(6):626–629. doi: 10.1093/alcalc/agn067. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22(6):2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem. 2007;87(4):476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120(5):1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]