Abstract
The longitudinal assessment of episodic and semantic memory was obtained from 236 patients diagnosed with Alzheimer’s disease (n=128) and with FTLD (n= 108) including patients with a social comportment/ dysexecutive (SOC/ EXEC) disorder; progressive non-fluent aphasia (PNFA); semantic dementia (SemD); and corticobasal syndrome (CBS). At the initial assessment, AD patients obtained a lower score on the delayed free recall test than other patients. Longitudinal analyses for delayed free recall found converging performance, with all patients reaching the same level of impairment as AD patients. On the initial evaluation for delayed recognition, AD patients also obtained lower scores than other groups. Longitudinal analyses for delayed recognition test performance found that AD patients consistently produced lower scores than other groups and no convergence between AD and other dementia groups was seen. For semantic memory, there were no initial between-group differences. However, longitudinal analyses for semantic memory revealed group differences over illness duration, with worse performance for SemD versus AD, PNFA, SOC/ EXEC, and CBS patients. These data suggest the presence of specific longitudinal patterns of impairment for episodic and semantic memory in AD and FTLD patients suggesting that all forms of dementia do not necessarily converge into a single phenotype.
Keywords: Frontotemporal lobar dementia, longitudinal analysis, neuropsychological assessment, episodic memory, semantic memory, Pick’s disease
Introduction
Frontotemporal lobar degeneration (FTLD) is a progressive neurodegenerative condition associated with imaging and pathological evidence of frontal and temporal lobe disease (Forman et al., 2006; Snowden et al., 1996; Grossman et al., 2007). Clinical subgroups of patients within the FTLD spectrum have been described. This includes patients with a decline in social comportment, personality, and executive functioning (SOC/ EXEC); a fluent form of progressive aphasia associated with impaired word comprehension and poor object knowledge known as semantic dementia (SemD); a non-fluent aphasic syndrome associated with effortful speech and grammatical comprehension difficulty known as progressive non-fluent aphasia (PNFA); and a disorder of limb praxis, visuospatial processing, and executive functioning known as corticobasal syndrome (CBS).
Longitudinal observations of cognitive functioning in neurodegenerative diseases provide valuable information from several crucial perspectives. These include improved diagnostic accuracy, the development of endpoints for treatment trials based on natural history data, and the development of prognostic algorithms that can enhance patient and family knowledge. Longitudinal observations also can inform theories about the neuroanatomic and cognitive basis of memory functioning. However, quantitative studies of longitudinal decline in large group studies involving dementia patients are rare. In this report, we describe the longitudinal course of two central aspects of memory functioning – episodic memory and semantic memory.
Alzheimer’s disease (AD) is the most common age-associated dementing condition. The earliest clinical characteristic of AD is a disorder of episodic memory, i.e., problems in learning and recalling specific facts from a specific spatial or temporal context. Episodic memory declines longitudinally in patients with autopsy-confirmed AD (Rascovsky et al., 2002; Grossman et al., 2008). This form of memory is intimately dependent on functioning of medial temporal lobe structures such as the hippocampus. Prior research suggests that this brain area is compromised earliest in AD (Arnold et al., 1991). Episodic memory functioning can also be compromised in other neurodegenerative conditions such as FTLD, but only later in the course of the condition (Grossman et al., 2008). This may be related in part to the role that frontal and parietal regions appear to play in episodic memory (Cabeza et al., 2008; Lepage et al., 2002)
It has been suggested that neuropsychological test performance, including memory test performance, devolves into a common clinical end-point in neurodegenerative conditions, regardless of the initial diagnosis (Kertesz et al., 2005). However, recent work with a pathologically-confirmed series of patients suggests an alternate possibility. Grossman et al., (2008) showed that the relative severity of initial neuropsychological impairment profiles in AD and FTLD patients were maintained throughout the longitudinal course of these diseases. Performance on all measures thus worsened over time in all patients, but relative impairments were maintained between groups over time. Moreover, double dissociations observed in these patient groups emphasized that several potential confounding factors could not explain this pattern of non-converging longitudinal decline across neurodegenerative conditions. For example, executive control such as letter fluency was significantly more difficult in patients with autopsy-confirmed tau-positive pathology, while confrontation naming was significantly more difficult in patients with TDP-43 pathology. Patients with autopsy-proven AD were most impaired on a measure of verbal episodic memory. While all groups of patients worsened on all measures over time, these relative impairments were maintained throughout the entire course of the disease (Grossman et al., 2008). This conclusion was confirmed by a large longitudinal study of clinically-diagnosed AD and FTLD patients (Libon et al., 2009). These findings led to the conclusion that the relative anatomic distribution of histopathologic disease plays an important role in the initial clinical presentation of a neurodegenerative condition and that distinct patterns of relative cortical atrophy are maintained throughout the longitudinal course of these diseases.
Another way to test this observation is to examine specific forms of memory comparatively. Semantic memory is the long-term representation of knowledge about the meaning of words, objects, actions, ideas, and the like. Because semantic memory is complex and multi-faceted, semantic memory difficulties may be present in many neurodegenerative patients (Libon et al., 2007a & b), albeit for a variety of different reasons (Koenig & Grossman, 2007). Atrophic changes involving the left anterior and inferolateral temporal lobe extending into visual association cortex have also been reported in the SemD subgroup of FTLD patients with specific deficits in semantic memory (Söderlund et al., 2008). Semantic memory also may overlap conceptually and neuroanatomically with episodic memory. For example, some patients with AD have impairments for both episodic and semantic memory, suggesting that both forms of memory may decline longitudinally in a parallel manner. This would be consistent with claims that medial temporal structures such as the hippocampus contribute to episodic memory as well as semantic memory (Moscovitch et al., 2005). Alternatively, some AD patients present with striking episodic memory impairment but relatively less difficulty with semantic memory, while SemD patients have semantic memory deficits without significant episodic memory difficulty. This suggests that these two forms of memory can be dissociated.
In this study, we examined the longitudinal course of episodic and semantic memory in patients with a clinical diagnosis of AD or FTLD. These memory measures were not evaluated in the Libon et al., (2009) paper. Our primary hypothesis is that memory measures will present different longitudinal profiles and will diverge rather than converge into a common subtype. Convergence would be consistent with a shared neuroanatomic substrate involving medial temporal lobe structures as well as frontal and temporal neocortical structures in episodic and semantic memory. Alternatively, these forms of memory may diverge and follow different longitudinal paths in AD and FTLD. This would be more consistent with prior longitudinal work showing distinct neuropsychological patterns in different autopsy-confirmed conditions (Grossman et al., 2008). Moreover, this would partially dissociate the neural substrates for episodic and semantic memory.
Methods
Participants
Our study cohort included a group of 236 patients with clinical diagnoses of AD or FTLD. All patients were evaluated and recruited from the Department of Neurology, University of Pennsylvania. Subsequently, at least two trained reviewers of a consensus committee confirmed the presence of specific diagnostic criteria and also assigned patients to an FTLD subgroup based on an independent review of the semi-structured history, a detailed neurologic exam, and a recently developed, brief, but standardized mental status examination (The Philadelphia Brief Assessment of Cognition [PBAC], Libon et al., 2007a). The Mini-Mental State Examination (MMSE; Folstein, Folstein & McHugh, 1974) was also administered.
The subgroups were classified based on published criteria (Grossman & Ash, 2004; Neary et al., 1998) that have been modified to improve reliability. When there was disagreement between reviewers, the case was discussed by the entire committee to arrive at a consensus diagnosis. On a different occasion trained technicians administered a detailed neuropsychological protocol comprised of different tests. This formal neuropsychological evaluation, described in detail below, was not used to diagnose these patients and the diagnosing neurologist was blind to patients’ performance on the neuropsychological evaluation. These patients and their legal representatives participated in an informed consent procedure approved by the Institutional Review Board at the University of Pennsylvania.
Among the participants in this study, 108 patients were clinically diagnosed with FTLD, according to published criteria (Lund & Manchester, 1994; McKhann et al., 2001). Patients were subclassified based on a modification of published criteria (Grossman & Ash, 2004; Neary et al., 1998). Our sample included 33 SOC/ EXEC patients. These patients presented with significant social and behavioral difficulties and alterations of executive functioning. Our sample included 26 PNFA patients as well. These patients had effortful speech that may be associated with speech sound substitutions and impaired grammatical comprehension, but relatively good single word comprehension. Our sample also included 20 SemD patients. The language disorder of these patients was characterized by fluent and circumlocutory spontaneous speech that was often empty in content with a prominent naming deficit and was associated with difficulty understanding single words and objects. Finally, 29 patients were diagnosed with CBS based on criteria derived from clinical-pathological studies reported in the literature and our own autopsy series (Murray et al., 2007). These patients had apraxia, gait difficulty, and a lateralized extrapyramidal disorder (e.g. unilateral limb rigidity, myoclonus, dystonia, and/ or alien hand). CBS can present with PNFA. These patients were included in the PNFA group.
Another 128 patients were given a clinical diagnosis of AD based on National Institute of Neurologic and Communicative Disorders and Stroke - Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984). In brief, this included a progressive syndrome involving prominent episodic memory difficulty, associated with circumlocutory speech, a visual constructional impairment, or limited executive control. AD patients with either an unusual presentation or who were classified as presenting with an AD visual or frontal lobe variant (n= 26) were excluded.
The initial clinical diagnosis of a neurodegenerative disease was consistent with the results of serum studies, structural imaging studies such as MRI or CT, studies of cerebrospinal fluid (when available), and clinical functional neuroimaging studies such as SPECT or PET (these studies were not available to the consensus committee). Exclusion criteria included the presence of other neurologic conditions such as stroke or hydrocephalus (as determined by imaging studies reviewed by MG), primary psychiatric disorders such as depression or psychosis, or a systemic illness that can interfere with cognitive functioning. Some of these patients were taking a fixed dose of a cholinesterase inhibitor (e.g. donepezil, rivastigmine, or galantamine) or memantine. Some of these patients may also have been medicated with a low dose of a non-sedating anti-depressant (e.g., serotonin-specific re-uptake inhibitors such as sertraline) or an atypical neuroleptic agent (e.g., quetiapine), as indicated clinically, but none of the patients demonstrated any evidence of sedation suggesting over-medication. Table 1 summarizes the demographic features of these patients.
Table 1.
Demographic Characteristics (Mean ± SD)
| AD n = 128 |
SOC/ EXEC n = 33 |
PNFA n = 26 |
SemD n = 20 |
CBS n = 29 |
|
|---|---|---|---|---|---|
| Age | 71.76 (8.66) | 63.94 (10.14) | 67.52 (9.13) | 66.05 (9.43) | 66.17 (9.04) |
| Education | 13.81 (3.44) | 15.00 (3.13) | 14.15 (2.87) | 15.85 (3.22) | 14.93 (3.35) |
| MMSE | 20.80 (6.18) | 22.42 (5.17) | 24.67 (4.19) | 23.70 (5.33) | 23.07 (3.99) |
| Duration of Illness | 33.65 (21.16) | 33.91 (23.70) | 31.12 (18.41) | 42.10 (18.08) | 31.93 (14.44) |
| At Initial Evaluation |
MMSE = Mini-Mental State Examination; AD = Alzheimer's disease; SOC/ EXEC = social/ dysexecutive; PNFA = progressive non-fluent aphasia; SemD = semantic dementia; CBS = corticobasal syndrome.
The follow-up time for each patient was measured from the time of symptom onset. The longitudinal aspect of the design refers to the time since symptom onset, not since the initial clinic visit. Subjects were followed up to 100 months since symptom onset. All subjects had at least two visits during this study. The average follow-up time between two visits is 5.68 months (sd= 3.93). The average number of visits is 3.54 times (sd= 1.97). There were 95 subjects who had two visits (mean duration of illness= 46.55 months, sd= 22.63), 51 subjects who had three visits (mean duration of illness= 55.29 months, sd= 22.56), and 90 subjects who had four or more visits (mean duration of illness= 62.28 months, sd= 21.13). The average length of total time participating in this study (from the initial to the final observation) is 20.53 months (sd= 16.04). There is no statistical difference between drop-out rates at the end of the study among the 5 disease groups (Chi-square[4]= 7.82, p= 0.10).
Episodic Memory (Morris et al., 1987)
Verbal memory and learning was assessed with a 10-word list administered over three trials. Delayed free recall for this list was assessed after a 20-minute filled delay. The delayed free recall scores ranged from 0–10. This was followed by a delayed recognition test where the 10 original words were intermixed with 10 novel words. Patients were asked to simply identify which words were on the original word list. The dependent variable on the recognition test was the number of original test items correctly identified combined with the number of foils that were correctly rejected. On the recognition test, condition scores ranged from 0–20.
Semantic Memory (Grossman et al., 1996, 1997)
Semantic knowledge was assessed with a simple task that required little verbal expression and minimum executive resource demands. On this test patients were asked to judge the semantic category membership of 48 individually-presented stimuli in response to a simple probe (“Is it an X?”). One target category was tools and the other target category was vegetables. Half of the stimuli in each category were target category members and half foils, and half of each category of stimuli was printed words and half color photos (matched for frequency, familiarity, and visual complexity across categories). Stimuli were presented in a manner blocked by category and material. Patients were given as much time as they needed to complete the task. Performance on this task has been validated by behavioral and SPECT correlation studies in AD patients with impaired semantic memory (Grossman et al., 1996, 1997). The dependent variable (range 0–48) was the number of correct responses.
Statistics
Data were analyzed using methods reported by Grossman et al. (2008) and Libon et al. (2009). Raw scores from neuropsychological data were converted to z-scores based on the performance of 25-age and education matched healthy seniors for each individual patient group. Healthy seniors were screened for health-related issues (neurologic, psychiatric, or medical conditions that could contribute to cognitive difficulty). The rationale underlying the use of different normal control groups to calculate z-scores is that AD and FTLD patients can vary with respect to demographic parameters such as age and education. For example, it is well documented that FTLD subtypes tend to be younger than AD patients. To minimize the possibility of such confounding effects, individually matched normal control groups were recruited and used to calculate z-scores. Analyses of variance indicated that the age and education of these healthy controls do not differ between patient groups. A threshold of z= −2.32 (equivalent to a p-value of ≤0.01) was used to identify statistically impaired test performance relative to normal control participants at the initial evaluation.
An examination of histograms showed that all episodic and semantic memory measures were normally distributed. We chose to use graphical checks for normality rather than a statistical test such as the Shapiro-Wilk test (1965) because these tests can be overly sensitive to large sample size and may erroneously identify non-normality that is not practically important. One-way analysis of variance (ANOVA) was used to compare means of demographic variables, semantic memory and episodic memory at initial evaluation. Tukey’s post hoc test was used to perform pair-wise comparisons. Drop-out rates across groups were compared using a Pearson Chi-square test.
A mixed-effect model was used to examine the longitudinal patterns of the cognitive variables over time (Laird & Ware, 1982). This is essentially a linear regression model including three parts. The first part includes the random effect parameters. These are random variables and are assumed to follow a normal distribution with a correlation structure that can be estimated from the data. The second part includes fixed-effect parameters that are assumed to be non-random. The third part includes an error term that is assumed to follow a normal distribution. We can think of each subject in this mixed-effect model as having his/her own linear regression model, and the population parameters can be obtained by averaging across the individual regression coefficients. This statistical procedure accounts for the correlations that are due to the repeated measurements of cognitive variables over time in the same patients. This statistical procedure does not require that every subject has the same time points when repeated measures are obtained. It allows subjects to have missing data during the longitudinal follow-up. However, this procedure requires that missing data should be missing at random. In our analysis, we excluded subjects (n=151) who were only observed once because they do not contribute directly to the estimation of the rate of decline (slopes) and we do not want to impose a strong assumption that these 151 subjects behave similarly to other subjects who had at least two visits in the data set.
In our implementation of the mixed-effect model, the intercept and the regression coefficient for the follow-up time were treated as random effects such that each subject has a unique intercept and regression coefficient for the follow-up time. The population mean coefficient for the follow-up time was obtained by averaging across the subject specific regression coefficients for the follow-up time. This population mean coefficient estimated the average monthly change for each neuropsychological measure. We were specifically interested in examining whether disease diagnosis was related to the longitudinal measures of the neuropsychological variables after adjustment for covariates. The following covariates were adjusted in the mixed-effect models and their regression coefficients were treated as fixed effects: age, education, baseline MMSE, and disease diagnosis. The interaction between the disease group and the follow-up time was also examined. If the interaction term was significant, the rate of decline for each disease group was then estimated separately. Longitudinal decline may be linear or curvilinear, and we examined both longitudinal effects. The correlations among the random coefficients were estimated based on the actual data rather than using a pre-specified correlation structure. For between-group longitudinal analysis using the mixed-effect model, F-statistics were computed to assess for overall group effects and the effect of covariates. T-statistics were used to assess for differences between any two disease groups. For within-group longitudinal analyses using the mixed-effect model, t-statistics were reported when two tasks differed within a disease group. Analyses were performed using SAS software with the proc mixed program (v9.1, SAS Institute Inc, Cary, NC). All statistical tests were two-tailed. Statistical significance was set at the p< .05 level unless otherwise indicated.
Results
Demographic Data
One way analysis of variance (ANOVA) indicated that there were significant between-group differences for age (F[4, 229]= 6.96, p < .001). AD patients were older than SOC/ EXEC and CBS patients (p< .05). An ANOVA also indicated a difference in performance on MMSE (F[4, 228]= 3.65, p< .007) at the initial evaluation (Table 1). The AD group had a lower score on MMSE compared to PNFA patients (p< .05) when first seen. There was no significant difference for education among patient groups. There was no between-group difference for duration of illness at initial evaluation.
Between-Group Test Performance
Initial Delayed Free Recall Episodic Memory Performance
In the area of episodic memory a between-group difference was noted on the delayed free recall test (F [4, 224]= 10.74, p< .001, Table 2). Follow-up analyses found that AD patients obtained lower scores compared to SOC/ EXEC, CBS, and PNFA patients (p< .05, all analyses).
Table 2.
Semantic and Episodic Memory Scores at Initial Clinic Visit (Mean ± SD)
| Episodic Memory - Delay Free Recall | ||||
| AD | SOC/ EXEC | PNFA | SemD | CBS |
| raw score | raw score | raw score | raw score | raw score |
| z score | z-score | z-score | z-score | z-score |
| 1.25 (1.64) | 3.00 (2.75) | 3.46 (2.38) | 2.15 (2.35) | 2.86 (1.98) |
| −3.23 (0.92) | −2.24 (1.55) | −1.98 (1.34) | −2.72 (1.33) | −2.32 (1.12) |
| Episodic Memory - Delayed Recognition | ||||
| AD | SOC/ EXEC | PNFA | SemD | CBS |
| raw score | raw score | raw score | raw score | raw score |
| z score | z-score | z-score | z-score | z-score |
| 14.67 (4.01) | 16.00 (4.48) | 17.61 (3.01) | 15.65 (2.70) | 17.07 (2.93) |
| −2.13 (1.92) | −1.49 (2.14) | −0.72 (1.44) | −1.66 (1.29) | −0.98 (1.40) |
| Semantic Memory (word and picture total) | ||||
| AD | SOC/ EXEC | PNFA | SemD | CBS |
| raw score | raw score | raw score | raw score | raw score |
| z score | z-score | z-score | z-score | z-score |
| 41.53 (4.63) | 42.56 (5.38) | 42.86 (3.18) | 40.61 (7.45) | 43.15 (2.88) |
| −1.31 (1.87) | −0.89 (2.18) | −0.77 (1.29) | −1.68 (3.01) | −0.65 (1.17) |
AD= Alzheimer's disease; SOC/ EXEC= social/ dysexecutive; PNFA= progressive non-fluent aphasia; SemD= semantic dementia; CBS= corticobasal syndrome.
Longitudinal Delayed Free Recall Episodic Memory Performance
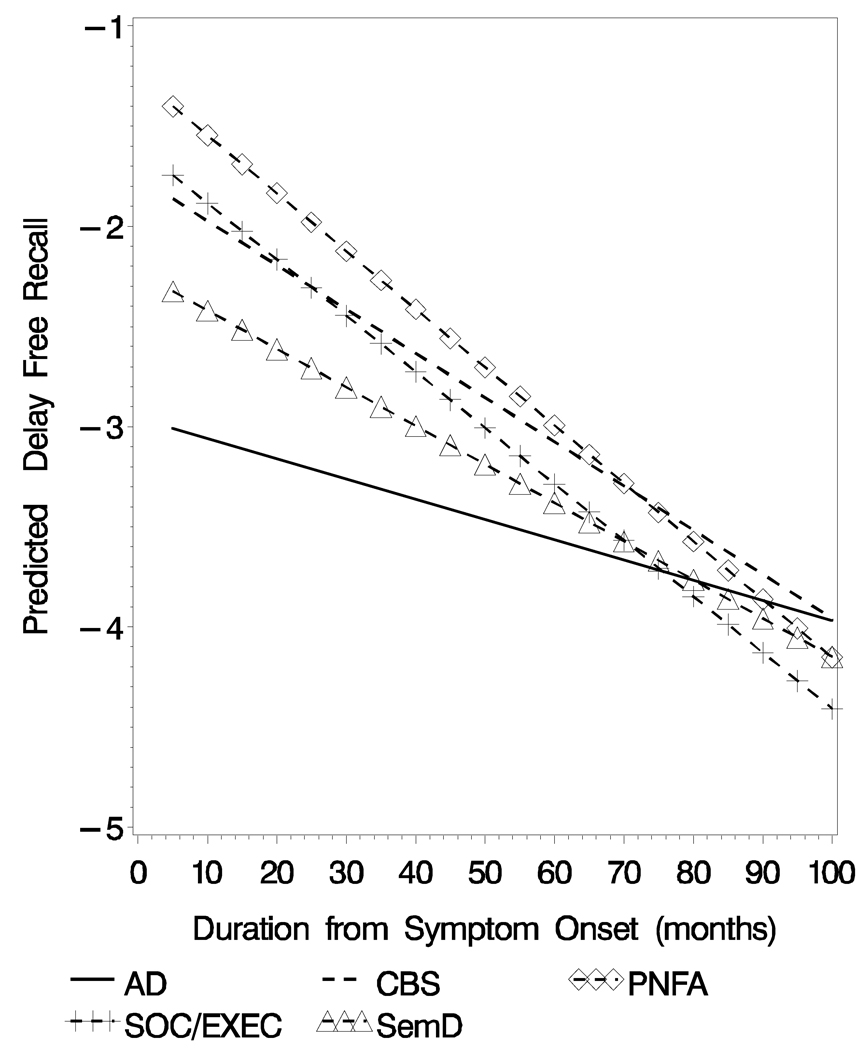
For delayed free recall there was a significant main effect for group (F[4, 327]= 8.53, p< .001; Table 3) and a significant linear main effect for illness duration (F[1, 214]= 71.36, p < .001). The linear group × illness duration interaction was also significant (F[4, 327]= 3.64, p< .007; Figure 1). The longitudinal rate of decline differed in that the SOC/ EXEC group declined faster than the AD group (t[327]= 3.05, p< .002) and the PNFA group declined faster than the AD group (t[327]= 2.65, p< .009).
Table 3.
Longitudinal Assessment: Effects of Group and Duration of Illness
| Test | Main Effect Group | Main Effect Illness Duration (linear or quadratic) |
Group × Illness Duration Interaction (linear or quadratic) |
|---|---|---|---|
| Delay Free Recall | F (4, 327) = 8.53, p< .001 | linear - F (1, 214) = 71.36, p<.001 | linear - F (4, 327) = 3.64, p< .007 |
| Delayed Recognition | F (4, 543) = 2.61, p< .04 | linear – F (1, 543) = 55.68, p<.001 | ns |
| Semantic Memory | F (4, 228) = 2.76, p< .03 | linear - F (1, 174) = 40.21, p<.001 | ns |
Figure 1.
Predicted Delay Free Recall (Z-Score) Obtained from the Mixed-effect model for the Five Diagnostic Groups
Initial Delayed Recognition Episodic Memory Performance
A between-group difference was noted on the delayed recognition test (F[4, 223]= 4.64, p< .002, Table 2). Follow-up analyses found that AD patients obtained lower scores compared to CBS and PNFA patients (p< .05, both analyses).
Longitudinal Delayed Recognition Episodic Memory Performance
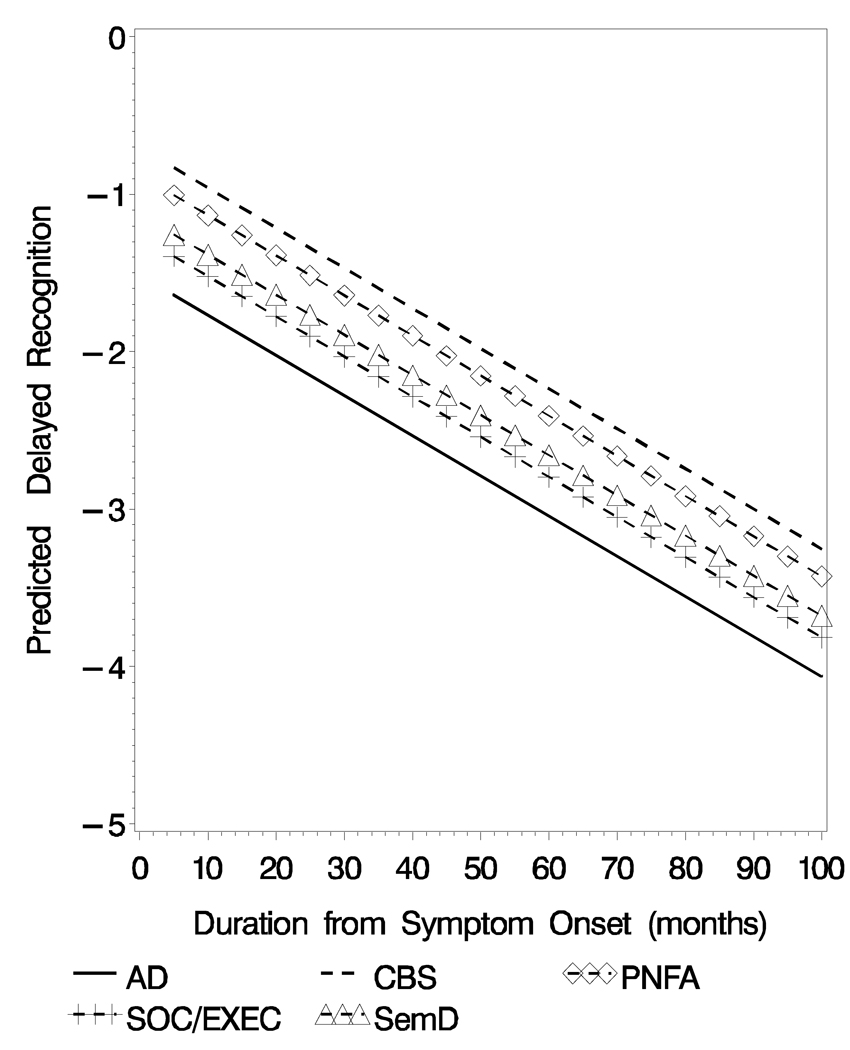
There were significant group differences over time (F[4, 543]= 2.61, p< .04; Table 3, Figure 2). Pair-wise comparisons revealed significantly worse delayed recognition test performance for AD versus PNFA patients (t[543]= 2.01, p< .05) and for AD versus CBS (t[543]= 2.83, p< .005) that was maintained throughout the course of the disease.
Figure 2.
Predicted Delayed Recognition (Z-Score) Obtained from the Mixed-effect Model for the Five Diagnostic Groups
Initial Semantic Memory Performance
There were no significant differences on the semantic memory test among the five groups at the initial evaluation (Table 2).
Longitudinal Semantic Memory Performance
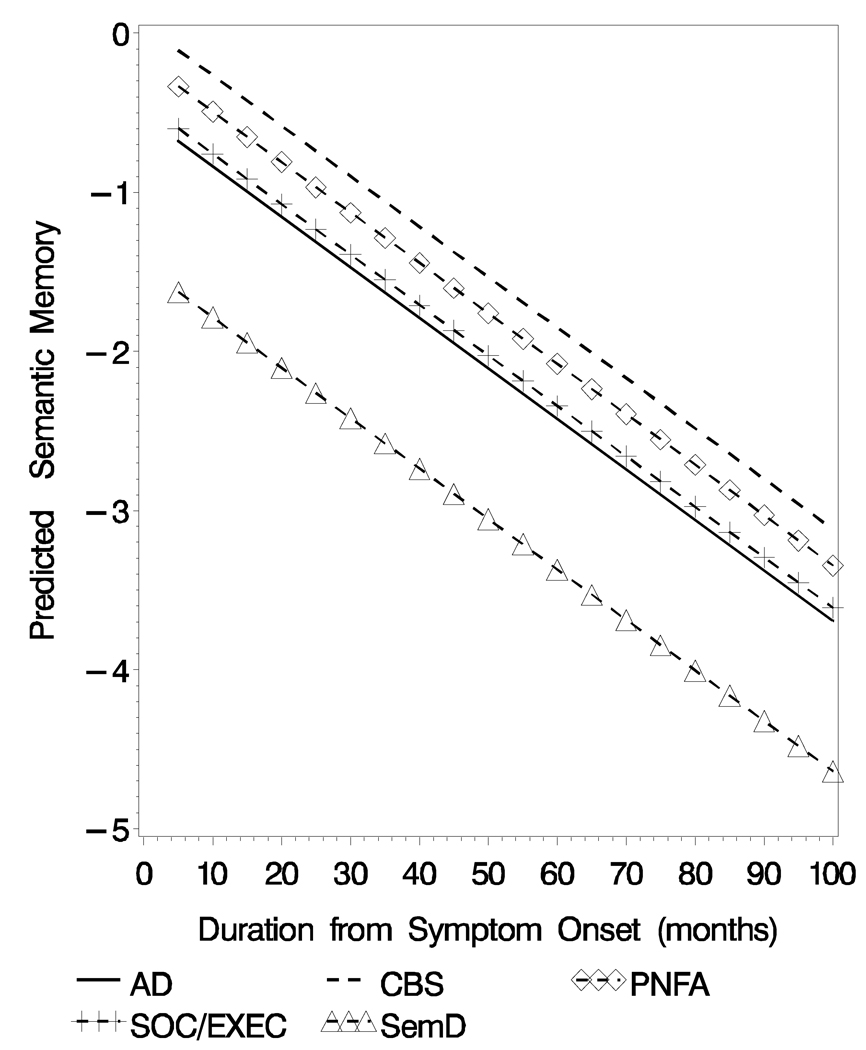
There were significant group differences that emerged over time (F[4, 228]= 2.76, p< .03; Table 3, Figure 3). Pair-wise comparisons revealed significantly worse semantic test performance for SemD versus AD (t[228]= 2.21, p< .03), for SemD versus CBS (t[228]= 3.16, p < .002), for SemD versus PNFA (t[228]= 2.57, p < .01), and for SemD versus SOC/ EXEC (t[228]= 2.11, p< .04) patients.
Figure 3.
Predicted Semantic Memory (Z-Score) Obtained from the Mixed-effect model for the Five Diagnostic groups
Within-Group Test Performance
Within-group analyses examined longitudinal pair-wise differences for all dementia groups on delayed free recall, delayed recognition, and semantic measures. These analyses demonstrated worse performance on episodic delayed free recall compared to scores from the semantic memory test for the following groups: AD - t[113] = 11.28, p< .001; SOC/ EXEC - t[28]= 2.39, p < .02; PNFA - t[21]= 2.84, p< .01; CBS - t[27]= 6.57, p < .001. These analyses also revealed worse performance on episodic delayed free recall compared to scores from the delayed recognition test for the following groups: AD - t[125]= 6.94, p< .001; SemD - t[19]= 6.56, p< .001; PNFA - t[23]= 4.78, p< .001; CBS - t[27]= 7.23, p< .001. Finally, we found that AD patients performed significantly worse on the delayed recognition test than on the semantic memory test (t[112]= 4.84, p< .001).
Discussion
FTLD is a major public heath problem often affecting people younger than age 65. Recent research into the neurobiology that underlies FTLD has resulted in newer schemes for the diagnosis of FTLD (McKhann et al., 2001). Moreover, there is now an accumulation of data suggesting that AD and the various FTLD phenotypes can be associated with specific neuropsychological and neuropathological syndromes (Forman et al., 2006; Grossman et al., 2007; Kramer et al., 2003; Libon et al., 2007a; Libon et al., 2007b; Murray et al., 2007; Rascovsky et al., 2002, 2007).
Two points of view have emerged regarding the longitudinal course in FTLD. Kertesz et al. (2005) have presented evidence suggesting that over time FTLD syndromes tend to converge or devolve into a single phenotype. Evidence for this assertion comes from a study conducted by Kertesz et al. (2000) who examined patients initially diagnosed with CBS. As the illness progressed, features of primary progressive aphasia (PPA) as well as a dysexecutive disorder developed. Upon autopsy these patients presented with a variety of pathological conditions. Similar findings were reported by Marczinski et al. (2004) who found that over time SOC/ EXEC and PPA patients both developed similar social comportment and behavioral disturbances. In a third study, Kertesz et al. (2005) found that autopsy-proven FTLD patients who initially presented with one neurobehavioral syndrome often developed features of other FTLD-neurobehavioral syndromes. Finally, Blair et al. (2007) observed that over time, a common pattern of impaired language tends to develop in FTLD patients.
An alternative point of view has been put forward by Grossman et al., (2008) who studied autopsy-proven FTLD patients longitudinally divided into tau-positive, tau-negative, and frontal variant-Alzheimer’s disease (fvAD) subgroups. Baseline neuropsychological/ behavioral measures differentiated between patient subgroups and these differences were maintained throughout illness duration. Also, a significant double dissociation involving relative difficulty on executive and visuoconstructional tests in the tau-positive group contrasted with relatively impaired performance on tests of visual confrontation naming in the tau-negative group. Libon et al. (2009) conducted a clinical study examining a large cohort of FTLD patients and found that the initial pattern of impairment on tests of executive control, lexical retrieval/ naming, and visuoconstruction was maintained throughout illness duration. Employing the same methods and data analysis techniques described above, Libon et al. (2009) found no statistical interactions between patient group and illness duration. Moreover, Libon et al. (2009) documented several striking dissociations on neuropsychological tests. For example, throughout the course of their illness AD patients showed persistently worse performance on the ‘animal’ fluency test, patients with PNFA were characterized by persistent difficulty on a test of letter fluency, the SemD group demonstrated profound impairment on a test of visual confrontation naming, and CBS patients consistently displayed poor performance on a visuoconstruction test. Thus, the results of the current study in conjunction with the data reported by Libon et al., (2009) indicate that these distinct patterns of neuropsychological impairment are maintained longitudinally, reflecting the unique anatomic distribution of relative disease burden in AD and FTLD, and that neuropsychological functioning does not converge into a common phenotype.
In the present research we used the same methodology to extend these findings by looking at the longitudinal course in AD and FTLD on tests of episodic and semantic memory. The present longitudinal analyses of memory test performance were designed to test competing hypotheses that the pattern of neuropsychological impairment seen on episodic and semantic memory tests at the initial assessment of patients with AD and FTLD would either diverge or converge, devolving into a single phenotype.
The longitudinal analyses of the delayed free recall test did show convergence over time. As seen in Figure 1, the initial delayed free recall test score produced by AD patients is very low (i.e., at floor) and changes very little over the course of their illness. An inspection of Figure 1 suggests that the significant group × illness duration interaction occurred as the illness progressed. This implies that the rate of decline for each group is different and patients with other clinical syndromes such as SemD, SOC/ EXEC and CBS eventually produced similarly impaired scores on this test. This could have occurred for a variety of reasons. For example, patients with a language disorder such as SemD may have had difficulty because of the verbal modality used to assess memory; patients with a SOC/ EXEC disorder may have had difficulty attending to the list of words; patients with CBS may have developed some frontal disease that interfered with their retrieval from memory. Regardless of the basis for these emergent episodic memory deficits, these issues should be examined in future studies. On the delayed recognition test longitudinal analyses demonstrated worse performance for AD patients compared to other patient groups, and this relative impairment was maintained over time. Juxtaposed with the findings on the delayed recall test, there was no convergence or interaction between patient group and illness duration for delayed recognition test performance. Possible language and attention-related problems that might have contributed to poor performance on the delayed free recall test are relatively minimal when episodic memory is assessed with a recognition format. Thus, delayed recognition test performance might provide a better overall measure of episodic memory for these dementia groups.
There were no baseline between-group differences on the semantic memory tests. However, statistical analyses did reveal a main effect for illness duration. As the illness progressed, distinct between-group differences emerged. For example, we saw lower semantic memory test scores for SemD compared to AD patients. In prior research we have consistently shown that SemD patients obtain lower scores on this test (Libon et al., 2007a&b). There may be several reasons that baseline analyses failed to show between-group differences in the current research. Among these are the multiple cognitive components that contribute to semantic memory (Koenig & Grossman, 2007), each of which can be compromised in different groups of patients. Another possibility is that patients with SemD develop a semantic memory deficit only after a period of time following disease onset, although we cannot rule out that our test of semantic memory has selective sensitivity to specific components of semantic memory. Regardless of the basis for the emergent semantic deficits in SemD patients, this pattern of performance is inconsistent with the common phenotype hypothesis (Kertesz et al., 2005), and emphasizes that distinct underlying pathologies may lead to different phenotypes (Grossman et al., 2008). As noted above the importance of these findings revolved around better differential diagnosis and prognostic information, endpoints for treatment trials as disease modifying medications become available, and a greater understanding of brain-behavior relationships that underlie dementia. Moreover, the discrepancy between SemD and AD for semantic memory calls to question recent claims that a common neural substrate supports both semantic memory and episodic memory (Kertesz et al., 2005). Episodic memory and semantic memory may share some neuroanatomic structures during processing. For example, this may support the process involved in exposure to multiple episodes about a particular object or word that may be needed to aggregate these episodes to form an entry in semantic memory (Moscovitch et al., 2005). However, these findings suggest that there are also important distinctions between these two forms of memory that must be honored in any comprehensive model of memory functioning.
The longitudinal within-group analyses demonstrated greater episodic memory versus semantic memory impairment. Thus, in addition to showing unique patterns of impairment in FTLD and AD over the duration of their illness, within-group analyses provide further evidence that the neurocognitive networks underlying episodic and semantic memory are at least partially distinct. Past research suggests several ways in which semantic memory diverges from episodic memory. This may be related, in part, to the way in which the features of a concept are represented cortically (Martin et al., 2007); to the way in which the network of knowledge about a concept represented in semantic memory is used to help categorize concepts (Koenig & Grossman, 2007); and/or to the way in which information represented in semantic memory is retrieved (Thompson-Schill et al., 1997). Additional work is needed to establish the common and divergent characteristics of episodic and semantic memory in these patient groups.
The current research is not without limitations, and several caveats should be kept in mind. First, no visual memory tests were used, and semantic memory was assessed with only one test. Different results could have occurred with a wider array of tests. This is particularly relevant since language problems typify several of our patient groups, and we assessed memory in the verbal modality. Second, although we examined longitudinal performance for a longer duration and later in the course of disease compared to most other research, it is possible that we did not examine patients sufficiently late in the disease process to demonstrate converging group profiles. Third, most AD patients were taking cholinesterase inhibitor, although this class of medications was rarely used in the FTLD patients, and this difference may have contributed to between-group findings. Fourth, we do not have histopathologic evidence for the diseases causing impairments in the patients participating in this study.
With these limitations in mind, the data reported above, in conjunction with the findings described by Grossman et al., (2008) and Libon et al., (2009), show that distinct profiles of converging and diverging longitudinal decline are present in AD and FTLD. Observations such as these suggest that AD and FTLD patients do not devolve into a single undifferentiated phenotype.
Acknowledgments
This work was supported in part by NIH (AG17586, AG15116, AG10124, NS44266, and NS53488).
Footnotes
There are no financial or other relationships that could be interpreted as a conflict of interest affecting this manuscript.
References
- Arnold SE, Hyman BT, Flory J, Damasio A, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Blair M, Marczinski CA, Davis-Faroque N, Kertesz A. A longitudinal study of language decline in Alzheimer's disease and frontotemporal dementia. Journal of the International Neuropsychological Society. 2007;13:237–245. doi: 10.1017/S1355617707070269. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli, Olson IR, Moscovitch M. The parietal cortex and episodic memory: An attentional account. Nature Review Neuroscience. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": A practical method for grading the mental state of patients for a clinician. Journal of Psychiatric Residency. 1974;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, Grossman M. Frontotemporal dementia: Clinicopathological correlations. Annals of Neurology. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Ash S. Primary progressive aphasia: A review. Neurocase. 2004;10:3–18. doi: 10.1080/13554790490960440. [DOI] [PubMed] [Google Scholar]
- Grossman M, Koenig P. Process and content in semantic memory. In: Hart J Jr., Kraut MA, editors. Neural Basis of Semantic Memory. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- Grossman M, Libon DJ, Forman MS, Wood EM, Moore P, Farmer J, Massimo L, Chatterjee A, Clark CM, Coslett HB, Hurtig HI, Lee V, Trojanowski JQ. Distinct neuropsychological profiles in pathologically-defined patients with Frontotemporal lobe dementia. Archives of Neurology. 2007;64:1601–1609. doi: 10.1001/archneur.64.11.1601. [DOI] [PubMed] [Google Scholar]
- Grossman M, D'Esposito M, Hughes E, Onishi K, Biassou N, White-Devine T, Robinson KM. Language comprehension profiles in Alzheimer's disease, multi-infarct dementia, and frontotemporal degeneration. Neurology. 1996;47:183–189. doi: 10.1212/wnl.47.1.183. [DOI] [PubMed] [Google Scholar]
- Grossman M, Payer F, Onishi K, White-Devine T, Morrison D, D'Esposito M, Robinson K, Alavi A. Constraints on the cerebral basis for semantic processing from neuroimaging studies of Alzheimer's disease. Journal of Neurology Neurosurgury and Psychiatry. 1997;63:152–158. doi: 10.1136/jnnp.63.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Xie SX, Libon DJ, Wang X, Massimo L, Moore P, Vesely L, Berkowitz R, Chatterjee A, Coslett HB, Hurtig HI, Forman MS, Lee VM-Y, Trojanowski JQ. Longitudinal decline in autopsy-defined frontotemporal lobar degeneration. Neurology. 2008;70:2036–2045. doi: 10.1212/01.wnl.0000303816.25065.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Canadian Journal of Neuroscience. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, Munoz DG. Clinical and pathological overlap between frontotemporal dementia, primary progressive aphasia, and corticobasal degeneration: The Pick Complex. Dementia and Geriatric Cognitive Disorders. 1999;10 suppl 1:46–49. doi: 10.1159/000051212. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Martinez-Lage P, Davidson W, Munoz DG. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55:1368–1375. doi: 10.1212/wnl.55.9.1368. [DOI] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lepage M, Ghaffer O, Nyberg L, Tulving E. Prefrontal cortex and episodic retrieval mode. Proceedings of the National Academy of Sciences of the USA. 2002;28:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Massimo L, Moore P, Coslett HB, Chatterjee A, Aguirre G, Grossman M. The Philadelphia Brief Assessment of Cognition (PBAC): A brief neuropsychological protocol that distinguishes Frontotemporal Dementia from Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2007a;24:441–447. doi: 10.1159/000110577. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, Cross K, Grossman M. Patterns of neuropsychological impairment associated with frontotemporal dementia: A factor analytic study. Neurology. 2007b;68:368–375. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Wang X, Massimo L, Moore P, Vesely L, Khan A, Chatterjee A, Coslett HB, Hurtig HI, Liang T-W, Grossman M. Neuropsychological decline in Frontotemporal Lobe Dementia: A longitudinal analysis. Neuropsychology. 2009;23:337–346. doi: 10.1037/a0014995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Davidson W, Kertesz A. A longitudinal study of behavior in frontotemporal dementia and primary progressive aphasia. Cognitive and Behavioral Neurology. 2004;17:185–190. [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadian EM. Clinical diagnosis of Alzheimer's disease: Report on the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann G, Trojanowski JQ, Grossman M, Miller BL, Dickson D, Albert M. Clinical and pathological diagnosis of frontotemporal dementia: Report of a work group on frontotemporal dementia and Pick's disease. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic, and spatial memory: A unified account based on multiple trace theory. Journal of Anatomy. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R, Neumann M, Forman MS, Farmer J, Miller BL, Johnson PhJK, Clark CM, Hurtig H, Gorno-Tempini ML, Lee M-Y, Trojanowski JQ, Grossman M. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology. 2007;68:1274–1283. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Ho GJ, Galasko D, Peavy GM, Hansen LA, Thal LJ. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology. 2002;58:1801–1808. doi: 10.1212/wnl.58.12.1801. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D. Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer's disease. Neuropsychology. 2007;21:20–30. doi: 10.1037/0894-4105.21.1.20. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- Snowden JS, Griffiths HL, Neary D. Semantic-episodic memory interactions in semantic dementia: Implications for retrograde memory function. Cognitive Neuropsychology. 1996;13:1101–1137. [Google Scholar]
- Söderlund H, Black SE, Miller BL, Freedman M, Levine B. Episodic memory and regional atrophy in frontotemporal lobar degeneration. Neuropsychologia. 2008;46:127–136. doi: 10.1016/j.neuropsychologia.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre G, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences of the USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]