Abstract
Lymphatic filariasis characterized by the dysfunction of the lymphatics can lead to severe (and often) irreversible lymphedema and elephantiasis. Decades of research in the field shows that the establishment of the adult parasites in the lymphatics triggers a cascade of events that ultimately results in tissue scarring and fibrosis. In this minireview, we focus on the studies addressing the mechanisms underlying the parasite-induced lymphatic dilatation that suggests parasite-induced lymphatic remodeling and lymphangiogenesis may be the prelude towards developing chronic and irreversible filarial pathology.
The Lymphatics and Lymphatic Filariasis
The lymphatic system is a characteristic feature of higher vertebrates required both for the maintenance of tissue fluid balance and as a conduit for lymphocyte transport to the lymph nodes. Lymphatic vessels are also involved in tumor cell metastasis, wound healing, and chronic inflammation. Impairment of the lymphatic function because of physical damage or abnormal development can cause lymphedema, a progressive and often irreversible condition. Although primary lymphedema has a genetic basis, secondary lymphedema results most commonly from lymphatic filariasis ([LF] a filarial infection occurring in the tropical and subtropical regions of the world), or as a result of trauma, surgery, radiation, or any other process that can disrupt/block lymphatic flow.
Among the many parasitic nematodes that infect humans, those that cause LF (Wuchereria bancrofti, Brugia malayi, Brugia timori) cause considerable morbidity, in large part because of their effects on the lymphatics. These lymph-dwelling filariae have a complex life cycle that includes an infective larval stage carried by mosquitoes and an adult worm stage that resides either in the lymph nodes or adjacent lymphatics (typically the afferent lymphatics). The offspring of the adults, the microfilariae (200–250 μm long and 5–7 μm wide) circulate in the blood and can then be taken up by another mosquito during a blood meal. These microfilariae develop over 1–2 weeks into infective larvae that are capable of initiating the life cycle over again.
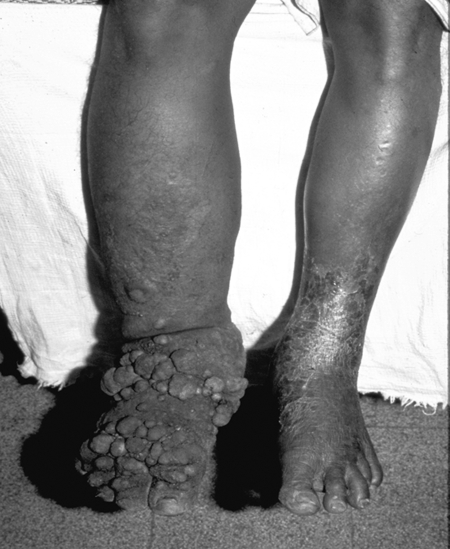
In its most severe clinical manifestation, LF is associated with hydrocele (in men), massive lymphedema, and elephantiasis (Fig. 1). Although much research has helped elucidate many aspects of filarial biology and immunology, its natural history, and the approach to diagnosis and treatment, there is a relative paucity of information on the mechanisms underlying the pathology seen in these infections. While it is known that the establishment of the adult filarial parasite within human lymphatic vessels may trigger a cascade of events that leads to abnormalities in lymphatic integrity and function, there remains a significant gap in our understanding of the pathogenesis of disease in lymphatic filariasis. In this mini-review, we focus on the basis of the lymphatic dysfunction and lymphangiectasia that is likely to be the prelude to irreversible lymphatic obstruction and/or elephantiasis.
FIG. 1.
Patient with severe lymphedema and secondary skin changes (elephantiasis).
Subclinical Lymphangiectasia and Inflammation
Lymphangiectasia and inflammatory reactions around adult worms are two independent components of LF. No direct evidence exists to demonstrate that adult filarial parasites are constrained by immune responses directed toward them in vivo. While treatment with antifilarial drugs with activity against the adult parasites (diethylcarbamazine (DEC) and albendazole) can trigger inflammatory responses against this stage of the parasite, clinically apparent lymphedema is rarely seen despite lymphangiectasia being a common feature of patent infection.1 This argues for the parasites having evolved mechanisms to both evade the host response and perhaps create an anatomical niche that is hospitable enough to ensure their own survival.
It is likely that host inflammatory responses contribute to the development of pathology. Acute inflammatory reactions to the living adult worms and their secretions have been observed in limited studies of filarial infections in both humans and animals.2,3 Although triggering the immune reactions by the death of adult worms and subsequent development of tissue pathology has been documented in several animal studies,4,5 it is also clear that even within an anatomical location in which multiple worms may be present (so-called “nest”) only some of the worms in any single nest may be susceptible to immune attack.6
Lymphatic dysfunction caused by filarial infection has been shown to predispose infected individuals to secondary bacterial infections 7,8 and to trigger inflammatory reactions in the skin and subcutaneous tissue that may accelerate the development of lymphedema and elephantiasis in patients from filarial-endemic areas.9 The development of fibrosis and cellular hyperplasia in and around the lymphatic walls in chronically infected animals parallels that which has been documented in humans.2
The marked differences observed in parasite-specific immune responses seen in clinically asymptomatic individuals and those with pathology (lymphedema, elephantiasis)10,11 however, fails to completely explain the presence of living adult worms in dilated lymphatics in the absence of inflammation. Recent studies have implicated lymphatic endothelial-specific vascular endothelial growth factor (VEGF)-induced responses felt to be mediated by the release of the rickettsial-like endosymbiont (Wolbachia) found intracellularly within the parasites that cause LF. The utility of doxycycline to target the Wolbachia has been shown to both alter plasma VEGF-C, VEGFR-3 levels, and to provide clinical improvement in those with lymphedema secondary to LF.12,13 The role of Wolbachia in the induction of the VEGF-mediated lymphatic damage in LF remains to be fully clarified, as other studies suggest that the levels of soluble lymphangiogenic factors are elevated in filaria-infected individuals, irrespective of their clinical status and in some filarial infections (e.g. Loa loa) in which there is no Wolbachia (Bennuru et al., unpublished).
It is clear that with patent infection and under circumstances of sufficient chronicity, individuals who harbor adult W. bancrofti or the two Brugia spp. will develop lymphangiectasia in the vicinity of the worm nests.1 Individuals carrying adult worms may be microfilaremic or amicrofilaremic, but typically have demonstrable lymphangiectasia.14,15 Subclinical lymphangiectasia of the vessels containing structurally intact worms have been shown to be distended with no apparent inflammatory responses in the wall,16 little response to living adult worms, with only a fleeting acute inflammatory reaction associated with the living parasites.17,18 Mice without an adaptive immune system such as nude or SCID mice infected with Brugia spp. have been observed to exhibit marked lymphatic dilatation,19,20 a dilatation that can be reversed by the removal or killing of the adult worms.5,21 Further, the general lack of adult translocation (i.e., the worms appear to remain in a particular anatomical location for their lifespan)6,14,22–24 and with the lymphangiectasia not being restricted entirely to the exact segment of lymphatics where the worms reside suggests that this process is mediated by soluble products excreted or secreted by the parasite that act on the lymphatic endothelial cells. All the available evidence suggest that the lymphatic dilatation is due to the parasite and its related products, whereas the more severe lymphatic obstruction may well be a consequence of immunologically mediated inflammation.1,25
Living or Dead Parasites
The role of the living adult worms in the pathological changes in the lymphatics of humans and animals has been much debated.2,3,21,25 Several reviews have suggested that the differences in clinical manifestations related to the lymphatics (clinically asymptomatic with lymphangiectasia vs. obstructive lymphedema and/or elephantiasis) are associated with the presence of live or dead parasites, respectively.25–27 A characteristic feature of chronic infections in both humans and animals with lymphatic disease is fibrosis and cellular hyperplasia in and around the lymphatic walls. Studies by Sakamoto et al. have shown that the endothelial cells from filarial-infected animals had a decreased number of vesicles (that presumably transport fluid) and an increase in the number of vacuoles (that presumably results from cell damage), suggesting alterations by the filarial parasites of the endothelial cells lining the lymphatics.28,29 Similarly, endothelial cells with bulging nuclei and many pinocytic vesicles and abundant collagen bundles aligned in multiple directions have also been observed30 in human lymph node specimens. These changes have been postulated to render these endothelial cells less effective in transporting interstitial fluid, thereby contributing to the edema and collagen accumulation seen in the disease processes associated with LF.
Several studies suggest that the responses leading to lymphatic blockage and gross pathological lesions are elicited by the dead and decalcifying adult worms (reviewed in Ref. 25). When coupled with pre-existing lymphatic dilatations, these lesions may lead to damage of the lymphatic valves that may in turn induce lymphatic backflow and lymphedema.31 However, animal studies in cats permissive to Brugia malayi indicate that the dilatations, valvular thickening, and inflammatory responses can be induced by both living and dead worms.2 Irrespective of the cause, collaterization of the lymphatics affected in filarial infections in humans and the presence of accessory lymphatics in cats suggests that lymphangiogenesis is a cardinal feature of lymphatic filariasis.32–34
Because the endothelium (be it vascular or lymphatic) is closely associated with cells mediating immune responses and inflammation, efforts have been targeted at understanding the interaction between lymphatic endothelial cells and the filarial parasites. The sequential alterations in the architecture of the lymphatics during the course of lymphatic-dwelling filarial infections occur along a continuum—from lymphangiectasia to granulomatous responses to development of collaterals—indicate that active lymphatic remodeling occurs that involve endothelial cell growth, migration, proliferation, and alterations of the extracellular matrix.35 Though initial in vitro studies using blood vascular human endothelial cells failed to demonstrate an effect of the filarial antigens36 on human umbilical vein endothelial cells, the ability to purify and culture primary human lymphatic endothelial cells has allowed the discovery that live filarial parasites (and their excretory/secretory products) induce the activation, proliferation and tube formation in lymphatic endothelial cells specifically.37 This lymphatic remodeling (that can occur in the absence of other cells) provides insight into the processes that occur early in infection and reconciles the disparate observations seen in immunodeficient mice5,19–21 and those seen in patients with subclinical patent infection.14,15
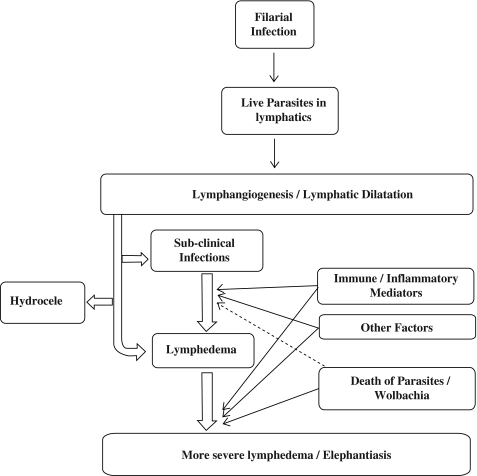
In summary, although several lines of evidence implicate Wolbachia and the host inflammatory responses in the development and aggravation of pathology in LF, it is also very likely that the filarial parasites (and their excreted and secreted products) cause lymphangiectasia and lymphatic remodeling that may be the prelude to lymphedema formation (Fig. 2). Recent advances in the biology of lymphatic endothelial cells associated with the availability of the filarial (and Wolbachia) genome and proteome should help us to delineate more clearly the molecules involved in those host-parasite interactions that lead first lymphatic dysfunction in LF and then to the more serious consequences of lymphatic filarial infections.
FIG. 2.
Establishment of filarial infection results in live adult parasites lodged in the lymphatics that result in lymphatic abnormalities. The transition from the asymptomatic microfilaremic state to chronic lymphatic obstructions varies widely in infected individuals. Lymphatic dilatation is, however, a prominent feature irrespective of the clinical manifestations. The interplay between the immune/inflammatory mediators, slow attrition of the parasites coupled with other factors affect the development of lymphedema. The death of the parasites stimulates strong inflammatory responses to the parasite and to the products of their Wolbachia endosymbionts, leading to tissue fibrosis. Secondary microbial infections further aggravate the pathology.
Disclosure Statement
No competing financial interest exist for either S. Bennuru or T.B. Nutman.
References
- 1.Dreyer G. Noroes J. Figueredo–Silva J. Piessens WF. Pathogenesis of lymphatic disease in bancroftian filariasis: A clinical perspective. Parasitol Today. 2000;16:544–548. doi: 10.1016/s0169-4758(00)01778-6. [DOI] [PubMed] [Google Scholar]
- 2.Rogers R. Denham DA. Studies with Brugia pahangi. 7. Changes in lymphatics of injected cats. J Helminthol. 1974;48:213–219. doi: 10.1017/s0022149x00022860. [DOI] [PubMed] [Google Scholar]
- 3.Schacher JF. Sahyoun PF. A chronological study of the histopathology of filarial disease in cats and dogs caused by Brugia pahangi. Trans R Soc Trop Med Hyg. 1967;61:234–243. doi: 10.1016/0035-9203(67)90162-9. [DOI] [PubMed] [Google Scholar]
- 4.Klei TR. Enright FM. Blanchard DP. Uhl SA. Specific hypo-responsive granulomatous tissue reactions in Brugia pahangi-infected jirds. Acta Trop. 1981;38:267–276. [PubMed] [Google Scholar]
- 5.Vickery AC. Vincent AL. Sodeman WA., Jr Effect of immune reconstitution on resistance to Brugia pahangi in congenitally athymic nude mice. J Parasitol. 1983;69:478–485. [PubMed] [Google Scholar]
- 6.Noroes J. Dreyer G. Santos A. Mendes VG. Medeiros Z. Addiss D. Assessment of the efficacy of diethylcarbamazine on adult Wuchereria bancrofti in vivo. Trans R Soc Trop Med Hyg. 1997;91:78–81. doi: 10.1016/s0035-9203(97)90405-3. [DOI] [PubMed] [Google Scholar]
- 7.Olszewski WL. Jamal S. Manokaran G. Pani S. Kumaraswami V. Kubicka U. Lukomska B. Dworczynski A. Swoboda E. Meisel–Mikolajczyk F. Bacteriologic studies of skin, tissue fluid, lymph, and lymph nodes in patients with filarial lymphedema. Am J Trop Med Hyg. 1997;57:7–15. doi: 10.4269/ajtmh.1997.57.7. [DOI] [PubMed] [Google Scholar]
- 8.Shenoy RK. Kumaraswami V. Suma TK. Rajan K. Radhakuttyamma G. A double-blind, placebo-controlled study of the efficacy of oral penicillin, diethylcarbamazine or local treatment of the affected limb in preventing acute adenolymphangitis in lymphoedema caused by brugian filariasis. Ann Trop Med Parasitol. 1999;93:367–377. doi: 10.1080/00034989958366. [DOI] [PubMed] [Google Scholar]
- 9.Foldi E. Foldi M. Weissleder H. Conservative treatment of lymphoedema of the limbs. Angiology. 1985;36:171–180. doi: 10.1177/000331978503600306. [DOI] [PubMed] [Google Scholar]
- 10.Babu S. Bhat SQ. Pavan Kumar N. Lipira AB. Kumar S. Karthik C. Kumaraswami V. Nutman TB. Filarial lymphedema is characterized by antigen-specific Th1 and th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl Trop Dis. 2009;3:e420. doi: 10.1371/journal.pntd.0000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babu S. Kumaraswami V. Nutman TB. Transcriptional control of impaired Th1 responses in patent lymphatic filariasis by T-box expressed in T cells and suppressor of cytokine signaling genes. Infect Immun. 2005;73:3394–3401. doi: 10.1128/IAI.73.6.3394-3401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debrah AY. Mand S. Specht S. Marfo–Debrekyei Y. Batsa L. Pfarr K. Larbi J. Lawson B. Taylor M. Adjei O. Hoerauf A. Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PLoS Pathog. 2006;2:e92. doi: 10.1371/journal.ppat.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debrah AY. Mand S. Toliat MR. Marfo–Debrekyei Y. Batsa L. Nürnberg P. Lawson B. Adjei O. Hoerauf A. Pfarr K. Plasma vascular endothelial growth Factor-A (VEGF-A) and VEGF-A gene polymorphism are associated with hydrocele development in lymphatic filariasis. Am J Trop Med Hyg. 2007;77:601–608. [PubMed] [Google Scholar]
- 14.Dreyer G. Addiss D. Santos A. Figueredo–Silva J. Noroes J. Direct assessment in vivo of the efficacy of combined single-dose ivermectin and diethylcarbamazine against adult Wuchereria bancrofti. Trans R Soc Trop Med Hyg. 1998;92:219–222. doi: 10.1016/s0035-9203(98)90754-4. [DOI] [PubMed] [Google Scholar]
- 15.Dreyer G. Figueredo–Silva J. Neafie RC. Addiss D. Lymphatic Filariasis. In: Nelson AM, editor; Horsburgh CR Jr, editor. Pathology of Emerging Infections. Washington: ASM Press; 1998. pp. 317–342. [Google Scholar]
- 16.Dreyer G. Noroes J. Addiss D. Santos A. Medeiros Z. Figueredo–Silva J. Bancroftian filariasis in a paediatric population: An ultrasonographic study. Trans R Soc Trop Med Hyg. 1999;93:633–636. doi: 10.1016/s0035-9203(99)90078-0. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenberg F. The early phase of endemic bancroftian filariasis in the male; pathological study. J Mt Sinai Hosp NY. 1957;24:983–1000. [PubMed] [Google Scholar]
- 18.Manson–Bahr P. The story of Filaria bancrofti. V. Description of W. bancrofti and pathology of filariasis. J Trop Med Hyg. 1959;62:160–173. [PubMed] [Google Scholar]
- 19.Nelson FK. Greiner DL. Shultz LD. Rajan TV. The immunodeficient scid mouse as a model for human lymphatic filariasis. J Exp Med. 1991;173:659–663. doi: 10.1084/jem.173.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent AL. Vickery AC. Lotz MJ. Desai U. The lymphatic pathology of Brugia pahangi in nude (athymic) and thymic mice C3H/HeN. J Parasitol. 1984;70:48–56. [PubMed] [Google Scholar]
- 21.Vickery AC. Albertine KH. Nayar JK. Kwa BH. Histopathology of Brugia malayi-infected nude mice after immune-reconstitution. Acta Trop. 1991;49:45–55. doi: 10.1016/0001-706x(91)90029-j. [DOI] [PubMed] [Google Scholar]
- 22.Amaral F. Dreyer G. Figueredo–Silva J. Noroes J. Cavalcanti A. Samico SC. Santos A. Coutinho A. Live adult worms detected by ultrasonography in human Bancroftian filariasis. Am J Trop Med Hyg. 1994;50:753–757. doi: 10.4269/ajtmh.1994.50.753. [DOI] [PubMed] [Google Scholar]
- 23.Dreyer G. Addiss D. Noroes J. Amaral F. Rocha A. Coutinho A. Ultrasonographic assessment of the adulticidal efficacy of repeat high-dose ivermectin in bancroftian filariasis. Trop Med Int Health. 1996;1:427–432. doi: 10.1046/j.1365-3156.1996.d01-79.x. [DOI] [PubMed] [Google Scholar]
- 24.Dreyer G. Amaral F. Noroes J. Medeiros Z. Ultrasonographic evidence for stability of adult worm location in bancroftian filariasis. Trans R Soc Trop Med Hyg. 1994;88:558. doi: 10.1016/0035-9203(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 25.Figueredo–Silva J. Noroes J. Cedenho A. Dreyer G. The histopathology of bancroftian filariasis revisited: The role of the adult worm in the lymphatic-vessel disease. Ann Trop Med Parasitol. 2002;96:531–541. doi: 10.1179/000349802125001348. [DOI] [PubMed] [Google Scholar]
- 26.Connor DH. Palmieri JR. Gibson DW. Pathogenesis of lymphatic filariasis in man. Z Parasitenkd. 1986;72:13–28. doi: 10.1007/BF00927731. [DOI] [PubMed] [Google Scholar]
- 27.von Lichtenberg F. The Wellcome Trust lecture. Inflammatory responses to filarial connective tissue parasites. Parasitology. 1987;94 Suppl:S101–122. doi: 10.1017/s003118200008584x. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto M. Meier JL. Folse DS. Ewert A. Perturbation of lymphatic endothelial cells in experimental Brugia malayi infections. Microcirc Endothelium Lymphatics. 1985;2:487–498. [PubMed] [Google Scholar]
- 29.Sakamoto M. Shimada M. Fujimaki Y. Ewert A. Degenerative changes in lymphatic endothelium of jirds infected with Brugia pahangi. J Parasitol. 1988;74:731–734. [PubMed] [Google Scholar]
- 30.Jungmann P. Figueredo–Silva J. Dreyer G. Bancroftian lymphadenopathy: A histopathologic study of fifty-eight cases from northeastern Brazil. Am J Trop Med Hyg. 1991;45:325–331. doi: 10.4269/ajtmh.1991.45.325. [DOI] [PubMed] [Google Scholar]
- 31.Cohen LB. Nelson G. Wood AM. Manson–Bahr PE. Bowen R. Lymphangiography in filarial lymphoedema, elephantiasis. Am J Trop Med Hyg. 1961;1961;10:843–848. doi: 10.4269/ajtmh.1961.10.843. [DOI] [PubMed] [Google Scholar]
- 32.Galliard H. Clinical, pathological and therapeutic aspects of bancroftian and malayan filariasis. International Congress of Tropical Medicine and Malariology Tehran. 2968:95–96. [Google Scholar]
- 33.Burn JI. Obstructive lymphopathy. Br J Hosp Med. 2969;2:755–763. [Google Scholar]
- 34.Hartz PH. Contribution to the histopathology of filariasis. Am J Clin Pathol. 1944;14:34–43. [Google Scholar]
- 35.Witte MH. Way DL. Witte CL. Bernas M. Lymphangiogenesis: Mechanisms, significance and clinical implications. EXS. 1997;79:65–112. doi: 10.1007/978-3-0348-9006-9_5. [DOI] [PubMed] [Google Scholar]
- 36.Rao UR. Zometa CS. Vickery AC. Kwa BH. Nayar JK. Sutton ET. Effect of Brugia malayi on the growth and proliferation of endothelial cells in vitro. J Parasitol. 1996;82:550–556. [PubMed] [Google Scholar]
- 37.Bennuru S. Nutman TB. Lymphangiogenesis and lymphatic remodeling induced by filarial parasites: implications for pathogenesis. PLoS Pathog. 2009;5:e1000688. doi: 10.1371/journal.ppat.1000688. [DOI] [PMC free article] [PubMed] [Google Scholar]