Abstract
A key feature in the recent widespread epidemic of the mosquito-borne alphavirus chikungunya virus (CHIKV) was the important role of Aedes albopictus, formerly regarded as a secondary vector, compared to the presumed primary vector Aedes aegypti. Ae. albopictus, a container-inhabiting mosquito, is an invasive species that occurs over a wide geographic range spanning tropical and temperate latitudes. In this study we examine the effects of a broad range of larval rearing temperatures on CHIKV infection, dissemination, and viral titer in Florida F1 Ae. albopictus. Adults from larvae reared at 18°C, 24°C, and 32°C differed significantly in size, development time, and CHIKV infection rate. Adult females with the largest body size were produced from the coolest temperature, took the longest to mature, and six times more likely to be infected with CHIKV than females reared at 32°C. There was also a significant effect of rearing temperature on viral dissemination, resulting in an increase in population dissemination at the coolest temperature. This study indicates that climate factors, such as temperature, experienced at the larval stage, can influence the competence of adult females to vector arboviruses.
Key Words: Arbovirus(es), Climate, Dengue, Entomology, Vector-borne
Introduction
Climate is one of the principal determinants of the distribution of vector-borne diseases (van Lieshout et al. 2004). In particular, vector development and survival, and arbovirus replication are greatly influenced by temperature. In mosquito vectors, temperature can influence larval development time, larval and adult survival, biting rates, gonotrophic cycle lengths, and vector size (Madder et al. 1983, Rueda et al. 1990, Scott et al. 1993). Ambient temperature can affect arboviral dynamics within the mosquito vector by altering the length of the extrinsic incubation period (EIP), which is the time between ingestion of an infectious blood meal and when transmission to a subsequent host is possible (Chamberlain and Sudia 1955, Reeves et al. 1994, Patz et al. 1996). Further, temperature often defines the latitudinal and altitudinal ranges of a vector. Species range may limit the distribution of disease when pathogens are species specific. Temperature may also limit viral transmission in areas where the vector is present, but the temperature precludes efficient transmission (Purse et al. 2005).
Vector competence, which is the capacity of an arthropod to acquire an infection and transmit it to a subsequent host, can greatly vary among individuals and between populations (Lorenz et al. 1984, Turell et al. 1992) and is influenced by genetics (Mercado-Curiel et al. 2008) as well as by climate variables, such as temperature (Davis 1932, Turell 1993, Dohm et al. 2002). An increase in environmental temperature for adult mosquitoes reduces the EIP (Davis 1932, Chamberlain and Sudia 1955), most likely due to an increase in the metabolism of the adult mosquito and the replication speed of the virus.
Although the majority of research on mosquito–virus interactions has focused on adult mosquitoes, temperature changes experienced in the immature stages of holometabolous vectors before infection may affect vector–virus interactions by changing physical and physiological characteristics of midgut and salivary gland barriers, which could have direct consequences on viral infection, replication, and transmission. Previous studies have shown that larval rearing temperature can effect mosquito competence for several arboviruses, including Murray Valley encephalitis virus (Kay et al. 1989b), Japanese encephalitis (Takahashi 1976), and western equine encephalitis (Hardy et al. 1990) viruses. In a specific study with Aedes taeniorhynchus, mosquitoes reared at 19°C had higher infection rates for Rift Valley fever and Venezuelan equine encephalitis viruses than counterparts reared at 26°C (Turell 1993). In view of global climate change models, which predict changes in temperature that will directly impact larval mosquito habitats, this study, which investigates a previously unexplored relationship between Aedes albopictus larval environmental temperature and chikungunya virus (CHIKV) susceptibility, could help increase the predictability of disease transmission patterns and future outbreaks.
The intercontinental dispersal of invasive arbovirus vectors, such as the Asian tiger mosquito Ae. albopictus, is accompanied by an increase in vulnerability to the exotic diseases vectored by these invaders (Juliano and Lounibos 2005). In 2005–2006 CHIKV, a single-stranded positive sense RNA–enveloped Alphavirus in the family Togaviridae emerged as an important pathogen in the Indian Ocean Basin. On the island of La Réunion alone, 241,000 clinical cases of chikungunya fever, representing 31% of the population, were reported (Paquet et al. 2006). A sylvan transmission cycle of CHIKV involving mosquitoes, such as Aedes furcifer and Aedes luteocephalus, and wild primates is limited to tropical Africa, and epidemic transmission of the virus is sustained though infection of the mosquitoes Aedes aegypti and Ae. albopictus in urban and peridomestic environments (Jupp and McIntosh 1990, Diallo et al. 1999).
A unique feature of the South West Indian Ocean CHIKV outbreak was the increased importance of Ae. albopictus as a vector. The enhanced role of Ae. albopictus as a CHIKV vector on La Réunion was in part due to the absence of the primary vector Ae. aegypti (Delatte et al. 2008); however, an amino acid substitution in La Réunion CHIKV isolates from alanine to valine at the 226 position of the E1 envelope structural protein present has been shown to increase Ae. albopictus, but not Ae. aegypti susceptibility to the virus in the laboratory (Tsetsarkin et al. 2007). After the Indian Ocean Basin epidemic, a strain of CHIKV nearly identical (99.9% nucleotide identity) to isolates from La Réunion emerged in India, where 1.3 million human cases were reported in 13 states in 2005–2006 (Arankalle et al. 2007). This widespread epidemic was not restricted to the tropics, with autochthonous transmission reported in Italy in 2007 (Rezza et al. 2007). The epidemic continued to spread in Indonesia, Sri Lanka, and Singapore, where cases were reported through 2008 and into 2009 (Seneviratne et al. 2007, International Society for Infectious Diseases 2009).
In this study we explore how variation in temperature during larval development affects the susceptibility of Ae. albopictus for CHIKV by measuring infection and dissemination rates, and viral titer. We also assess how larval temperature affects growth and survivorship by measuring larval mortality, development time to adulthood, and adult body size.
Materials and Methods
Mosquitoes and viruses
Ae. albopictus used in this experiment were generated from field collections of approximately 4000 larvae and/or eggs made from June to August 2007 in Palm Beach County, Florida. This population of Ae. albopictus was previously shown to be highly susceptible to the La Réunion (LR2006-OPY1) CHIKV strain (Reiskind et al. 2008). Females reared from field-collected immatures were given 20% sucrose ad libitum, blood fed weekly on live chickens, and kept in cages at 14 L:10 D, 26°C (±1°C SD) and >80% relative humidity (rh). Chicken care followed federally mandated animal use and care policies (University of Florida, IACUC Protocol VB-17). Eggs (F1) were hatched in tap water, and, within 24 h after hatching, individual larvae were placed in 50 mL Falcon™ conical tubes with 35 mL of tap water and 0.0105 g 1:1 yeast:albumin food. Based on preliminary studies, 0.0105 g of food at the beginning of the experiment was adequate for the completion of individual development but did not increase mortality at the higher temperature. Larvae were reared at 18°C, 24°C, and 32°C with a 14 L:10 D cycle in a Percival (Percival Corporation, Perry, IA) incubator. The experimental treatment units in this study were different incubators that were identical in all respects except for rearing temperature; thus, it was assumed that differences in treatments were caused by rearing temperature. These temperatures are within the temperature range of the treehole environment occupied by Ae. albopictus in Florida (Lounibos 1983). Larvae in each temperature treatment were from the same cohort of eggs whose hatch was staggered to synchronize adult emergence among all three temperature treatments. After the final larval instar, pupae were removed from rearing tubes, sexed, and stored in groups of 10 in water-filled vials to record adult emergences. After emergence, all adults were held at 24°C, 95–99% rh with a 14 L:10 D cycle in a biosafety level-3 facility and given 20% sucrose ad libitum.
The LR2006-OPY1 CHIKV strain (GenBank accession number DQ443544) was isolated in France from a febrile patient who had been infected on the island of La Réunion (Parola et al. 2006). This recently emergent strain of CHIKV contains the alanine to valine substitution at the 226 position of the E1 envelope structural protein that has been identified as a feature in many current CHIKV epidemics (Rezza et al. 2007) and has been shown to increase Ae. albopictus susceptibility (Tsetsarkin et al. 2007). To produce virus for infectious blood meals, a T-75 cm2 flask with a confluent monolayer of Vero cells in 10 mL of cell culture medium (M199 medium supplemented with fetal bovine serum, antibiotics, and antimycotics; Invitrogen™, Carlsbad, CA) was inoculated with 150 μL of previously frozen stock virus, and allowed to incubate in a 5% CO2 and 35°C atmosphere for 24 h.
Vector competence
Groups of fifty 5- to 7-day-old mosquitoes were placed in 1-L cylindrical, waxed cardboard containers (Dade Paper, Miami, FL) with mesh screening. Mosquitoes were starved for 24 h before being offered an infectious blood meal of 1:1000 dilution of freshly propagated CHIKV in citrated bovine blood (Hemostat Laboratories, Dixon, CA) supplemented with ATP (5 mM) as a phagostimulant. Water-jacketed glass membrane feeders (Rutledge et al. 1964) covered with Edicoll™ collagen film (Devro, Sandy Run, SC) and connected to a Haake Series F water circulator (Thermo Haake, Paramus, NJ) were used to maintain the blood meal at 37°C. Mosquitoes were given 30 min to feed. Low feeding success of Ae. albopictus made it necessary to conduct three consecutive days of feeding for individuals from all three temperature treatments. The blood meals from the three consecutive feeding days were assayed using quantitative real-time polymerase chain reaction (qRT-PCR), with copy number standardized to plaque forming units by plaque assay performed on 10-fold serial dilutions of virus stock (Bustin 2000). Virus titers in blood meals were log10 4.7, 4.5, and 3.4 plaque forming units/mL for the three feeding days, respectively. After feeding, mosquitoes were cold anesthetized, and fully engorged mosquitoes were removed, placed in new cages, and given 20% sucrose ad libitum.
After a 10-day EIP at 24°C, surviving Ae. albopictus females were killed by freezing. Females were stored at −80°C; after thawing, wings were removed for measurements, bodies were assayed to determine infection status and titer, and legs were tested to check for a disseminated infection (Turell et al. 1984). Wing length was measured in millimeters as an indicator of body size (Blackmore and Lord 2000) from alula to wing tip of digital images using a computer imaging and measurement program (i-Solution lite©; AIC, Princeton, NJ).
Bodies and legs were triturated separately in 2 mL microcentrifuge tubes containing 900 μL of BA-1 medium (Lanciotti et al. 2000) and two zinc-plated beads. Samples were homogenized at 25 Hz for 3 min using a Tissuelyzer® tissue homogenizer (Qiagen, Valencia, CA) and then clarified by centrifugation (3000 g for 4 min). Viral RNA was extracted from 250 μL of the sample with Trizol-LS (Invitrogen) following the manufacturer's instructions and using a final elution volume of 50 μL in diethylpyrocarbonate (DEPC)-treated water.
One-step qRT-PCR was used to determine infection and dissemination status and body titer by previously established protocols (Reiskind et al. 2008). Primers from the CHIKV E1 gene were designed with the following sequences: forward, 5′-ACC CGG TAA GAG CGA TGA ACT-3′; reverse, 5′-AGG CCG CAT CCG GTA TGT-3′. The probe sequence was: 5′-/5Cy5/CCG TAG GGA ACA TGC CCA TCT CCA/3BHQ_2/-3′ (IDT DNA, Coralville, IA).
Statistical analysis
Kruskal-Wallis tests followed by Dunn's pairwise multiple comparisons (family α = 0.05) were used to determine differences among treatments in distributions of wing lengths and development times to adulthood (SAS 9.1 for Windows, 2003; SAS Institute, Cary, NC). Males and females were analyzed separately because of sex-specific sizes and developmental times in this species. Chi-squared tests of independence were used to determine the effect of temperature on survivorship to adulthood. If significant effects were detected, post hoc pairwise comparisons were made with an alpha-adjusted (Bonferroni) correction to account for multiple comparisons (Minitab 15.1 for Windows, 2006; Minitab, State College, PA).
A generalized linear mixed model (PROC GLMMIX, SAS 9.1, Cary, NC) was used to describe relationships among temperature (fixed effect), feeding day (random effect), infection (# with virus/# fed), dissemination (# with virus in their legs/# with virus), and population dissemination (# with virus in their legs/# fed) specifying a logistic link function and a binomial error distribution. Odds ratios (OR) and 95% confidence intervals (CI) for infection at each temperature treatment were also calculated (SAS 9.1 for Windows, 2003). A linear mixed model (PROC MIXED, SAS 9.1, Cary, NC) was used to test for effects of temperature treatments (fixed) and feeding day (random), which were both class variables on body titer, a continuous variable. Titer data did not fit the model assumptions of normality, but approximate normality was achieved through a natural log transformation of titer values (SAS 9.1 for Windows, 2003). Wing length comparisons, pooled across all temperature treatments, between binomial variables, infected versus uninfected and disseminated versus nondisseminated, were analyzed with t-tests (Minitab 15.1 for Windows, 2006).
Results
Growth and mortality
Wing lengths and development times to adulthood were significantly affected by temperature for both females (wing length: H = 417.5; df = 2; p < 0.0001; development time: H =1279.6; df = 2; p < 0.0001) and males (wing length: H = 2127.4; df = 2; p < 0.0001; development time: H = 1697.3; df = 2; p < 0.0001) (Table 1). There was an inverse relationship between wing length and temperature with the largest adult mosquitoes produced at 18°C and the smallest mosquitoes produced at 32°C. Mosquitoes reared at the lowest temperature (18°C) took over two times longer to develop than those at 24°C and 32°C. Juvenile mortality rates at 18°C, 24°C, and 32°C were 9.16% (n = 1454), 7.34% (n = 1520), and 16.90% (n = 1473), respectively. There was a significant relationship between survivorship to adulthood and temperature (χ2 =54.123, df = 2, p < 0.0001). Ae. albopictus reared at 32°C were significantly more likely to die as larvae when compared with individuals reared at the 18°C and 24°C, with no differences between the two lower temperatures.
Table 1.
Treatment Medians and Interquartile Ranges (25th Percentile–75th Percentile) for Development Time to Adulthood and Wing Length
| 18°C | 24°C | 32°C | |
|---|---|---|---|
| Female time to adulthood (days) | 24.5a | 11.0b | 9.0c |
| Interquartile range (n) | 23.0–26.0 (515) | 10.0–11.5 (556) | 8.5–9.5 (445) |
| Male time to adulthood (days) | 22.5a | 10.0b | 8.0c |
| Interquartile range (n) | 21.5–24.0 (817) | 9.5–10.5 (860) | 7.5–8.5 (791) |
| Female wing length (mm) | 3.40a | 3.14b | 2.91c |
| Interquartile range (n) | 3.29–3.51 (347) | 3.00–3.26 (150) | 2.80–3.01 (207) |
| Male wing length (mm) | 2.83a | 2.61b | 2.44c |
| Interquartile range (n) | 2.77–2.90 (745) | 2.55–2.66 (810) | 2.37–2.51 (740) |
Medians with different superscripted letters are significantly different with a measured variable.
Chikungunya infection and dissemination
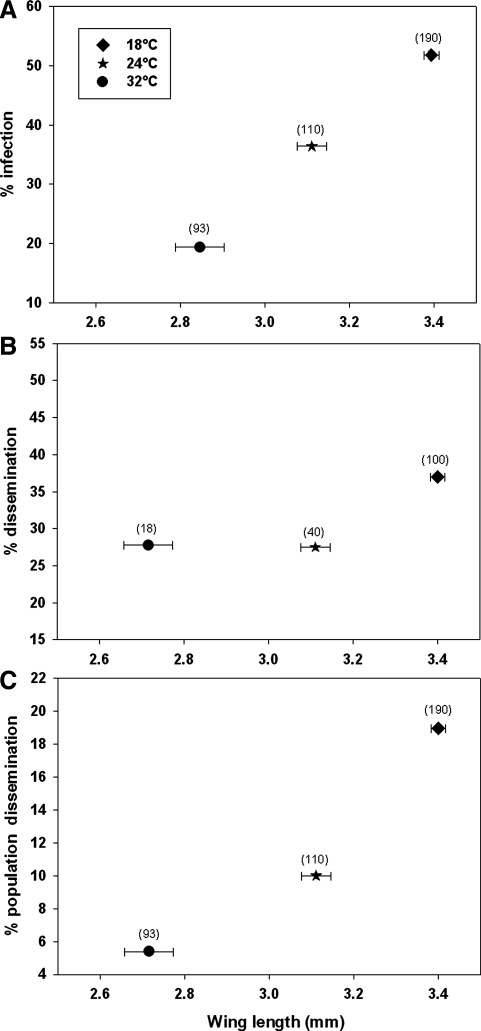
There was a significant temperature treatment effect on the percentage of females that developed CHIKV infections (F = 16.92, df = 2, p < 0.0001) (Fig. 1). Infection was six times more likely in adult females reared at 18°C than at 32°C (OR = 6.052; 95% CI 3.22–11.373), and females reared at 24°C were 2.7 times more likely to be infected than those reared at 32°C (OR = 2.722; 95% CI 1.385–5.351). Females reared at 18°C were 2.2 times more likely to be infected than those reared at 24°C (OR = 2.223; 95% CI 1.328–3.722). Among the infected individuals the number of females that developed disseminated infections did not vary significantly among larval rearing temperatures (F = 0.85, df = 2, p > 0.4293); however, there was a significant temperature effect on population dissemination (F = 6.20, df = 2, p < 0.0022) (Fig. 1). Population dissemination was approximately five times more likely in adult females reared at 18°C than at 32°C (OR = 4.905; 95% CI 1.814–13.271) and 2.3 times more likely in females reared at 18°C than at 24°C (OR = 2.291; 95% CI 1.088–4.827). There was no difference in population dissemination between the 24°C and 32°C treatments.
FIG. 1.
Bivariate plots of mean wing lengths (±standard error) and (A) percent infection, (B) percent dissemination, and (C) percent population dissemination grouped by treatment. Numbers within parentheses above symbols represent the number of mosquitoes tested.
After the 10-day EIP, no significant variation in body titer of virus-positive mosquitoes was observed among the temperature treatment groups (F = 1.14, df = 2, p > 0.4300). Ae. albopictus females that were positive for CHIKV infection in all three temperature treatments were significantly larger than uninfected females (t = − 3.59, df = 327, p = 0.0004) as measured by mean wing length. There was no significant difference in mean wing lengths of females with disseminated and nondisseminated infections (t = −0.41, df = 94, p = 0.6830).
Discussion
Due to the impact of climate on vector ecology, mosquito-borne diseases will be sensitive to projected changes in global temperatures. In this laboratory study we demonstrated that larval rearing temperature can influence survival, development time, and wing length, and may directly impact disease transmission by influencing the likelihood of infection with CHIKV. Although the proportion of infected females that developed disseminated infections did not differ significantly among the three larval rearing temperatures, the population dissemination rates were significantly higher at 18°C, when compared to the two higher temperatures of 24°C and 32°C. Disseminated infection is generally accepted as a measure of a mosquito's ability to transmit a virus through biting (Turell et al. 1984). The rate of dissemination, when expressed as a percentage of the number of mosquitoes infected, may provide information about the effect of a “midgut escape barrier” moderating whether gut infections are able to disseminate into the hemolymph. In this study, individuals reared at 32°C had significantly lower infection rates, but no significant difference was found in dissemination rate. Thus, it can be speculated that there may be a reduced midgut escape barrier in mosquitoes derived from the higher rearing temperatures. On the other hand, the population dissemination rate, expressed as a percentage of the total number of mosquitoes tested, was greater for mosquitoes from 18°C, and is epidemiologically more important in that it gives an estimate of the vector competence or the transmission potential of a population. Body titers did not differ among temperature treatments for mosquitoes with disseminated infections. Although this result was somewhat surprising, it is possible that after the 10-day EIP virus titers stabilized to the extent that treatment effects on titer were diminished. Additionally, only a limited number of mosquitoes developed disseminated infections, and although the mean titer of disseminated mosquitoes from 18°C was higher, it was not significantly different from the other treatments.
The higher temperature of 32°C decreased survivorship, when compared with 18°C and 24°C. This was unexpected because results from preliminary experiments showed no difference in survivorship between the three temperature treatments. The increased mortality could have been due to the stress of high temperature or the interaction of high temperature and excess nutritional resources resulting in the proliferation of detrimental microorganisms in the larval environment. Larvae took longer to develop at cooler temperatures, which produced larger adults. Mean wing length differences between successive temperature treatments were approximately 0.2 mm, confirming for Ae. albopictus that adult body size, within limits, exhibits an inverse relationship with larval rearing temperature (Briegel and Timmermann 2001). Upper and lower thermal limits for the growth of Ae. albopictus larvae are approximately 11°C and 35°C, at which temperatures larval development is inhibited, eventually resulting in death (Hawley 1988, Monteiro et al. 2007).
Our data indicate that at lower larval rearing temperatures there is an increased likelihood of an adult female becoming infected with CHIKV virus. This may have had a positive effect on CHIKV infection rates in locations such as highlands in La Réunion, where entomological surveys recovered Ae. albopictus at elevations of 1200 m and temperatures as low as 12.6°C (Delatte et al. 2008). However, 12.6°C is a lower larval environmental temperature than what was investigated in this study and therefore it is uncertain whether the relationship between reduced temperature and higher infection rates would hold true at this temperature. The ability of Ae. albopictus to tolerate low temperatures and adapt to diverse ecological environments combined with their vector competence for currently circulating CHIKV isolates may help to explain the 2007 northern Italy CHIKV outbreak and increases the potential for future epidemics in other temperate areas where Ae. albopictus is abundant.
This study focused only on the influence of larval temperature, and adults were maintained at a common temperature of 24°C. How combinations of different adult and larval temperatures may affect vector competence was not addressed. It is likely that adults maintained at the lower temperature will have decreased virogenesis and a longer EIP resulting in a decreased probability of transmission. Therefore, the maintenance of low adult temperatures may result in a reduction or elimination of any benefit low rearing temperature may have on increasing vector competence.
Results from this study are consistent with other systems where arboviral vector competence was reduced in female mosquitoes that were reared at higher compared to lower temperatures (Kay et al. 1989a, Hardy et al. 1990, Turell 1993). Unfortunately, none of the previous studies that explored larval temperature effects on adult arboviral susceptibility reared mosquitoes individually to separate temperature and density effects, nor did they measure variables such as wing or body size and survivorship, as we have done in this study.
In our work lower rearing temperature produced mosquitoes that were not only more susceptible to viral infection but also significantly larger. Blood consumption by females is a function of size, and large females are known to imbibe more than twice as much blood as smaller females (Briegel 1990). Thus, in smaller mosquitoes reared at the higher temperature, the imbibing of a lower blood volume would decrease the initial viral dose and, in combination with a low CHIKV titer in the blood meal, limit the establishment of infection. Low initial exposure to virus may not affect dissemination, which process requires postinfection virus replication. If this explains why large mosquitoes derived from cooler temperatures have higher infection rates than smaller mosquitoes, then we would expect that titers of freshly engorged mosquitoes will increase with body size, which will be tested in future studies. We also predict that different-sized mosquitoes derived from different temperatures and fed an infectious blood meal with a high virus titer would overcome a threshold infectious dose and may result in similar infection rates.
Because the variation in size was achieved through different temperature treatments, it is difficult to separate the effect of temperature from the response variable body size. There may be other temperature-dependent phenotypic traits that vary in a way so as to cause an increase in infection when adults are subjected to a lower temperature larval environment that we did not measure. Previous studies show a lack of consistency in relationships between vector size and pathogen transmission. Large adult Ae. aegypti females from low-density larval conditions showed higher rates of oral infection with dengue virus (DENV) compared to two other size classes from higher density larval conditions (Sumanochitrapon et al. 1998), and similar findings were reported for Ae. aegypti and Ross River virus (Nasci and Mitchell 1994). In contrast, Ae. albopictus adults reared in competitive larval environments were smaller and had higher rates of infection and dissemination for Sindbis virus and DENV, while within the same studies a competitive larval environment did not have a significant effect on vector competence in Ae. aegypti for the two viruses (Alto et al. 2005, 2008a). In Ae. aegypti, when size was examined independent of rearing conditions small adults were more susceptible to DENV; however, the size range of individual measures was extremely narrow (Alto et al. 2008b). In contrast, larger Aedes triseriatus adults produced through variation in competitive treatments had higher infection and dissemination rates for La Crosse virus (LACV) (Bevins 2008). In nutritional deprivation studies with Culex tritaeniorhynchus and Ae. triseriatus, smaller mosquitoes derived from nutrient-deprived larvae were more susceptible than their well-fed, larger counterparts, for West Nile Virus (Baqar et al. 1980) and better transmitters of LACV to suckling mice (Grimstad and Haramis 1984, Grimstad and Walker 1991). Smaller Ae. triseriatus adults generated from field-collected pupae were more likely to transmit LACV to suckling mice (Paulson and Hawley 1991). However, nutritional deprivation that led to small mosquitoes had no effect on vector competence in Culex annulirostris for Murray Valley encephalitis virus (Kay et al. 1989c) and Ae. vigilax for Ross River virus (Jennings and Kay 1999). These contradictions in the effect of size on vector competence could be based on the intrinsic differences between vector–viral systems; however, it is also possible that larval conditions, such as high temperature and low nutrients or competition, produce small mosquitoes by different mechanisms that differently affect their competence as vectors.
In summary, cooler rearing temperatures produced mosquitoes that were larger, had higher survival, and were more likely to become infected with CHIKV, emphasizing the importance of the mosquito larval environment in determining adult vector–virus interactions. Future studies should explore the connection between larval rearing temperature-infection patterns observed in the laboratory and patterns in the field, and how climate and climate change may continue to impact the mosquito larval environment and the epidemiology of CHIKV.
Acknowledgments
We thank Naoya Nishimura for rearing and measuring mosquitoes and Drs. Stephanie Richards and George O'Meara for their critical reviews of earlier versions of this article. This research was supported by NIH Grant R01 AI-044793.
Disclosure Statement
No competing financial interests exist.
References
- Alto BW. Lounibos LP. Higgs S. Juliano SA. Larval competition differentially affects arbovirus infection in Aedes mosquitoes. Ecology. 2005;86:3279–3288. doi: 10.1890/05-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW. Lounibos LP. Mores CN. Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc R Soc Lond B Biol Sci. 2008a;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW. Reiskind MH. Lounibos LP. Size alters susceptibility of vectors to dengue virus infection and dissemination. Am J Trop Med Hyg. 2008b;79:688–695. [PMC free article] [PubMed] [Google Scholar]
- Arankalle VA. Shrivastava S. Cherian S. Gunjikar RS, et al. Genetic divergence of chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic. J Gen Virol. 2007;88(Pt 7):1967–1976. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- Baqar S. Hayes CG. Ahmed T. The effect of larval rearing conditions and adult age on the susceptibility of Culex tritaeniorhynchus to infection with West Nile virus. Mosq News. 1980;40:165–171. [Google Scholar]
- Bevins SN. Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae) Biol Invasions. 2008;10:1109–1117. [Google Scholar]
- Blackmore MS. Lord CC. The relationship between size and fecundity in Aedes albopictus. J Vector Ecol. 2000;25:212–217. [PubMed] [Google Scholar]
- Briegel H. Fecundity, metabolism, and body size in Anopheles (Diptera: Culicidae), vectors of malaria. J Med Entomol. 1990;27:839–850. doi: 10.1093/jmedent/27.5.839. [DOI] [PubMed] [Google Scholar]
- Briegel H. Timmermann SE. Aedes albopictus (Diptera: Culicidae): physiological aspects of development and reproduction. J Med Entomol. 2001;38:566–571. doi: 10.1603/0022-2585-38.4.566. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Chamberlain RW. Sudia WD. The effects of temperature upon the extrinsic incubation of eastern equine encephalitis in mosquitoes. Am J Hyg. 1955;62:295–305. doi: 10.1093/oxfordjournals.aje.a119780. [DOI] [PubMed] [Google Scholar]
- Davis NC. The effect of various temperatures in modifying the extrinsic incubation period of the yellow fever virus in Aedes aegypti. Am J Hyg. 1932;16:163–176. [Google Scholar]
- Delatte H. Dehecq JS. Thiria J. Domerg C, et al. Geographic distribution and developmental sites of Aedes albopictus (Diptera: Culicidae) during a chikungunya epidemic event. Vector Borne Zoonot Dis. 2008;8:25–34. doi: 10.1089/vbz.2007.0649. [DOI] [PubMed] [Google Scholar]
- Diallo M. Thonnon J. Traore-Lamizana M. Fontenille D. Vectors of chikungunya virus in Senegal: current data and transmission cycles. Am J Trop Med Hyg. 1999;60:281–286. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- Dohm DJ. O'Guinn ML. Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- Grimstad PR. Haramis LD. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. III. Enhanced oral transmission by nutrition-deprived mosquitoes. J Med Entomol. 1984;21:249–256. doi: 10.1093/jmedent/21.3.249. [DOI] [PubMed] [Google Scholar]
- Grimstad PR. Walker ED. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. IV. Nutritional deprivation of larvae affects the adult barriers to infection and transmission. J Med Entomol. 1991;28:378–386. doi: 10.1093/jmedent/28.3.378. [DOI] [PubMed] [Google Scholar]
- Hardy JL. Meyer RP. Presser SB. Milby MM. Temporal variations in the susceptibility of a semi-isolated population of Culex tarsalis to peroral infection with western equine encephalomyelitis and St. Louis encephalitis viruses. Am J Trop Med Hyg. 1990;42:500–511. doi: 10.4269/ajtmh.1990.42.500. [DOI] [PubMed] [Google Scholar]
- Hawley WA. Biology of Aedes albopictus. J Am Mosq Control Assoc. 1988;4(Suppl 1):1–39. [PubMed] [Google Scholar]
- International Society for Infectious Diseases. [Mar 30;2009 ]. ProMED mail archive numbers 20081217.3963, 20081211.3895, 20080923.3010 2008, 20090302.9854.
- Jennings CD. Kay BH. Dissemination barriers to Ross River virus in Aedes vigilax and the effects of larval nutrition on their expression. Med Vet Entomol. 1999;13:431–438. doi: 10.1046/j.1365-2915.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp PG. McIntosh BM. Aedes furcifer and other mosquitoes as vectors of chikungunya virus at Mica, northeastern Transvaal, South Africa. J Am Mosq Control Assoc. 1990;6:415–420. [PubMed] [Google Scholar]
- Kay BH. Edman JD. Fanning ID. Mottram P. Larval diet and the vector competence of Culex annulirostris (Diptera: Culicidae) for Murray Valley encephalitis virus. J Med Entomol. 1989b;26:487–488. doi: 10.1093/jmedent/26.5.487. [DOI] [PubMed] [Google Scholar]
- Kay BH. Fanning ID. Mottram P. The vector competence of Culex annulirostris, Aedes sagax and Aedes alboannulatus for Murray Valley encephalitis virus at different temperatures. Med Vet Entomol. 1989a;3:107–112. doi: 10.1111/j.1365-2915.1989.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Kay BH. Fanning ID. Mottram P. Rearing temperature influences flavivirus vector competence of mosquitoes. Med Vet Entomol. 1989c;3:415–422. doi: 10.1111/j.1365-2915.1989.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS. Kerst AJ. Nasci RS. Godsey MS, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz L. Beaty BJ. Aitken TH. Wallis GP. Tabachnick WJ. The effect of colonization upon Aedes aegypti susceptibility to oral infection with yellow fever virus. Am J Trop Med Hyg. 1984;33:690–694. doi: 10.4269/ajtmh.1984.33.690. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. The mosquito commuity of treeholes in subtropical Florida. In: Frank JH, editor; Lounibos LP, editor. Phytotelemata: Terrestrial Plants as Hosts for Aquatic Insect Communities. Medford, NJ: Plexus Publishing Inc.; 1983. pp. 223–246. [Google Scholar]
- Madder DJ. Surgeoner GA. Helson BV. Number of generations, egg production, and developmental time of Culex pipiens and Culex restuans (Diptera: Culicidae) in southern Ontario. J Med Entomol. 1983;20:275–287. doi: 10.1093/jmedent/20.3.275. [DOI] [PubMed] [Google Scholar]
- Mercado-Curiel RF. Black WC. de L Munoz M. A dengue receptor as possible genetic marker of vector competence in Aedes aegypti. BMC Microbiol. 2008;15:118–133. doi: 10.1186/1471-2180-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro LC. de Souza JR. de Albuquerque CM. Eclosion rate, development and survivorship of Aedes albopictus (Skuse) (Diptera: Culicidae) under different water temperatures. Neotrop Entomol. 2007;36:966–971. doi: 10.1590/s1519-566x2007000600021. [DOI] [PubMed] [Google Scholar]
- Nasci RS. Mitchell CJ. Larval diet, adult size, and susceptibility of Aedes aegypti (Diptera, Culicidae) to infection with Ross River virus. J Med Entomol. 1994;31:123–126. doi: 10.1093/jmedent/31.1.123. [DOI] [PubMed] [Google Scholar]
- Paquet C. Quatresous I. Solet JL. Sissoko D, et al. Chikungunya outbreak in reunion: epidemiology and surveillance, 2005 to early January 2006. Eur Surveill. 2006;11 doi: 10.2807/esw.11.05.02891-en. E0602023. [DOI] [PubMed] [Google Scholar]
- Parola P. de Lamballerie X. Jourdan J. Rovery C, et al. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA. Epstein PR. Burke TA. Balbus JM. Global climate change and emerging infectious diseases. J Am Mosq Control Assoc. 1996;275:217–223. [PubMed] [Google Scholar]
- Paulson SL. Hawley WA. Effect of body size on the vector competence of field and laboratory populations of Aedes triseriatus for La Crosse virus. J Am Mosq Control Assoc. 1991;7:170–175. [PubMed] [Google Scholar]
- Purse BV. Mellor PS. Rogers DJ. Samuel AR. Climate change and the recent emergence of bluetongue in Europe. Nat Rev. 2005;3:171–181. doi: 10.1038/nrmicro1090. [DOI] [PubMed] [Google Scholar]
- Reeves WC. Hardy JL. Reisen WK. Milby MM. Potential effect of global warming on mosquito-borne arboviruses. J Med Entomol. 1994;31:323–332. doi: 10.1093/jmedent/31.3.323. [DOI] [PubMed] [Google Scholar]
- Reiskind MH. Pesko K. Westbrook CJ. Mores CN. Susceptibility of Florida mosquitoes to infection with chikungunya virus. Am J Trop Med Hyg. 2008;78:422–425. [PMC free article] [PubMed] [Google Scholar]
- Rezza G. Nicoletti L. Angelini R. Romi R, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- Rueda LM. Patel KJ. Axtell RC. Stinner RE. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1990;27:892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- Rutledge LC. Ward RA. Gould DJ. Studies on the feeding response of mosquitoes to nutritive solutions in a new membrane feeder. Mosq News. 1964;24:407–419. [Google Scholar]
- Scott TW. Chow E. Strickman D. Kittayapong P, et al. Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J Med Entomol. 1993;30:922–927. doi: 10.1093/jmedent/30.5.922. [DOI] [PubMed] [Google Scholar]
- Seneviratne SL. Gurugama P. Perera J. Chikungunya viral infections: an emerging problem. J Travel Med. 2007;14:320–325. doi: 10.1111/j.1708-8305.2007.00135.x. [DOI] [PubMed] [Google Scholar]
- Sumanochitrapon W. Strickman D. Sithiprasasna R. Kittayapong P, et al. Effect of size and geographic origin of Aedes aegypti on oral infection with dengue-2 virus. Am J Trop Med Hyg. 1998;58:283–286. doi: 10.4269/ajtmh.1998.58.283. [DOI] [PubMed] [Google Scholar]
- Takahashi M. The effects of environmental and physiological conditions of Culex tritaeniorhynchus on the pattern of transmission of Japanese encephalitis virus. J Med Entomol. 1976;13:275–284. doi: 10.1093/jmedent/13.3.275. [DOI] [PubMed] [Google Scholar]
- Tsetsarkin KA. Vanlandingham DL. McGee CE. Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ. Effect of environmental temperature on the vector competence of Aedes taeniorhynchus for Rift Valley fever and Venezuelan equine encephalitis viruses. Am J Trop Med Hyg. 1993;49:672–676. doi: 10.4269/ajtmh.1993.49.672. [DOI] [PubMed] [Google Scholar]
- Turell MJ. Beaman JR. Tammariello RF. Susceptibility of selected strains of Aedes aegypti and Aedes albopictus (Diptera, Culicidae) to chikungunya virus. J Med Entomol. 1992;29:49–53. doi: 10.1093/jmedent/29.1.49. [DOI] [PubMed] [Google Scholar]
- Turell MJ. Gargan TP. Bailey CL. Replication and dissemination of Rift Valley fever virus in Culex pipiens. Am J Trop Med Hyg. 1984;33:176–181. doi: 10.4269/ajtmh.1984.33.176. [DOI] [PubMed] [Google Scholar]
- van Lieshout M. Kovats RS. Livermore MTJ. Martens P. Climate change and malaria: analysis of the SRES climate and socio-economic scenarios. Global Environ Change. 2004;14:87–99. [Google Scholar]