Abstract
West Nile virus (WNV) has caused at least 1150 cases of encephalitis, 100 deaths, and an estimated 30,000–80,000 illnesses in 6 of the last 7 years. Recent evidence from several regions has implicated American robins (Turdus migratorius) as an important host for feeding by Culex mosquitoes, and, when integrated with their host competence for WNV, demonstrates that they are a key WNV amplification host. We evaluated the efficacy of a DNA plasmid vaccine at reducing the viremia and infectiousness of hatch-year American robins. We found that a single dose of vaccine injected intramuscularly resulted in more than a 400-fold (102.6) decrease in average viremia. Although sample sizes were small, these results suggest that vaccinated robins exhibit viremias that are likely to be mostly noninfectious to biting Culex mosquitoes. More broadly, if an orally effective formulation of this vaccine could be developed, new control strategies based on wildlife vaccination may be possible.
Key Words: Culex pipiens, Disease control, Experimental infection, Herd immunity, Infectiousness, Vaccine, Vector borne
Introduction
West Nile virus (WNV) was introduced into the Western hemisphere in 1999 near New York City and subsequently spread throughout North, Central, and South America (Kilpatrick et al. 2007). From 1999 to 2008, WNV caused 33,541 reported human cases in the United States and Canada, including 11,886 cases of encephalitis, and 1162 deaths (Centers for Disease Control and Prevention 2009, Health Canada 2009), with at least 1150 cases of encephalitis in 6 of the last 7 years from 2002 to 2008. It remains an important public health concern.
Recent research has resulted in the development of several livestock and human vaccines for WNV (Kramer et al. 2007). One of these vaccines, first licensed for horses, is based on recombinant DNA plasmid technology and has been shown to be efficacious and safe in mice and horses (Davis et al. 2001) as well as four species of wild birds, American crows (Corvus brachyrhynchos), fish crows (Corvus ossifragus), California condors (Gymnogyps californianus), and Andean condors (Vultur gryphus) (Turell et al. 2003, Bunning et al. 2007, Chang et al. 2007), and has been used to vaccinate two others, Sandhill cranes (Grus canadensis) and Whooping cranes (Grus americana) without ill effects (Stehn 2002). The cells that uptake the plasmid DNA in the vaccine express the premembrane and envelope proteins of WNV, and the host immune system mounts a response to these expressed antigens (Davis et al. 2001). In the studies where vaccination was followed by experimental challenge with WNV, vaccinated birds had increased survival and reduced viremias compared to the nonvaccinated group (Turell et al. 2003, Bunning et al. 2007).
Although over 300 species of birds have been infected with WNV (Kilpatrick et al. 2007), several recent studies of mosquito feeding behavior have shown that a single species, American robins (Turdus migratorius), often accounts for a substantial portion of mosquito blood meals. In six states in the United States (MD, DC, CT, TN, IL, and NJ), robins were the most frequently fed on host by the dominant WNV vectors (Turell et al. 2002, Kilpatrick et al. 2005), making up 25–71% of Culex pipiens, Cx. restuans, and Cx. quinquefasciatus blood meals (Apperson et al. 2004, Kilpatrick et al. 2006a, 2006b, Molaei et al. 2006, Savage et al. 2007, Hamer et al. 2008). Robins were also the most frequently fed on competent host by Culex tarsalis in the summer in Colorado (Kent et al. 2009). In one of these studies on Cx. pipiens, mosquito feeding data were integrated with host competence data to determine the relative importance of each bird species in WNV amplification (Kilpatrick et al. 2006a). Robins were the 9th most competent host of the 53 species that have been studied by experimental infection (Kilpatrick et al. 2007), and, using a conservative set of assumptions, were estimated to be responsible for 35–88% of the WNV-infectious mosquitoes at five urban and residential sites in the mid-Atlantic United States (Kilpatrick et al. 2006a). If a substantial fraction of American robins could be immunized by vaccination against WNV, this could have an important impact on WNV amplification. Clearly, this would require an efficacious WNV vaccine. Thus, our goal was to determine whether the DNA plasmid vaccine described above would reduce the viremia and infectiousness of American robins.
Materials and Methods
Study animals
We caught six hatch-year American robins near Albany, NY, using mist nets in July 2007 under NYS Fish and Wildlife license no. 336 and U.S. Fish and Wildlife permit MB035731-0. The birds were 3–5 weeks old when caught, based on feather development. Birds were taken into Griffin Laboratory, Wadsworth Center, and treated for lice. Birds were held individually in wire cages and maintained on an ad libitum diet of high-protein dog food supplemented with meal worms and fruit. In addition, they were given a unique color band combination for identification. Birds were placed in quarantine for 4 weeks, after which a 0.05 mL blood sample was taken from the ulnar vein by pricking the vein and collecting blood in a capillary tube. Blood was expelled into 0.45 mL of BA-1 (M199 with Hanks' salts and l-glutamine, 0.05 M TRIS, 1% bovine serum albumin, 0.35 g/L sodium bicarbonate, 100 units/ml penicillin, 100 units/ml streptomycin, and 1 μg/ml fungizone, pH 7.4) and placed at 4°C for at least 30 min to allow clotting. Samples were then stored at −70°C until being assayed for the presence of antibodies against WNV and St. Louis encephalitis virus from previous exposure or the presence of maternal antibodies (Nemeth et al. 2008) using an indirect enzyme-linked immunosorbent assay (ELISA) (Ebel et al. 2002). All birds were seronegative.
Vaccination and challenge
Birds were then moved into the BSL-3 laboratory and held for 1 week of acclimation, at which time they were 8–10 weeks old. Three of the six birds were vaccinated with 0.175 mg of the DNA vaccine by intramuscular injection of 0.350 mL of a 0.5 mg/mL phosphate-buffered saline (PBS) solution split into two injections, one in each pectoral muscle. The other three birds were mock-vaccinated by injection of PBS. After 2 weeks, five birds (including all three vaccinated birds) were challenged by intramuscular injection with 104 PFU WNV (strain 030019856 isolated from an American crow in 2003, and belonging to the WN02 clade that has higher replication in Culex mosquitoes than the NY99 clade) (Moudy et al. 2007, Kilpatrick et al. 2008), and one was inoculated with diluent (PBS w/1% fetal bovine serum) alone. Blood samples were taken, as described previously from birds on days 1–5 postinoculation, and viremia was measured by plaque assay on Vero cells (Payne et al. 2006) with a limit of detection of 101.7 PFU/mL. Fourteen days postinoculation, a 0.1 mL blood sample was again taken from the ulnar vein, and birds were euthanized by overdose of pentobarbital (15 mg/kg). Blood samples from days 1 to 14 postinoculation were tested for WNV antibodies by ELISA and plaque reduction neutralization (PRNT) assay at a 1:10 dilution with a 90% neutralization cutoff (PRNT90) (Calisher et al. 1989). The day 1 postinoculation samples were used to test for antibody response to the vaccine, and the day 14 PI samples were used to test for antibody response to WNV infection.
Results
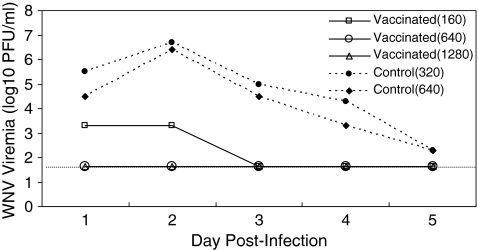
All six birds survived to the end of the experiment. All of the birds were negative for WNV antibodies by ELISA and PRNT90 at 2 weeks postvaccination, just before innoculation with WNV. After infection with WNV, one of the vaccinated birds and both mock-vaccinated birds were viremic, but two of the vaccinated birds never had detectable virus in the blood (Fig. 1). The 5-day average viremia (in PFU/mL) of vaccinated robins (101.91 ± 0.22) was significantly lower than nonvaccinated robins (104.48 ± 0.27) by repeated measures analysis of variance using the limit of detection of 101.7 PFU/mL for undetectable viremias (F1,3 = 55.2; p = 0.005). The difference in mean viremia between vaccinated birds and control birds was thus at least 102.6 PFU/mL, or 407-fold higher, and peak viremia titers of vaccinated birds were on average 104.35 or 22,000-fold lower than unvaccinated birds. Two weeks postinfection, all five WNV-challenged birds were ELISA positive, and PRNT90 positive for WNV antibodies with titers ranging from 1:160 to 1:1280 (Fig. 1).
FIG. 1.
Daily measures of viremia of three vaccinated and two control hatch-year American robins challenged with West Nile virus by injection. The horizontal dotted line shows the limit of detectability, 101.7 PFU/mL. The numbers in parentheses in the legend give the PRNT90 antibody titers 2 weeks postinoculation.
The unvaccinated robins exhibited viremias that were likely to be partially infectious to mosquitoes for 1–3 days, assuming that the threshold titer of virus needed to result in transmitting (infectious) Cx. pipiens is approximately 104.6 PFU and increases according to the expression: 0.1349 ×log10(viremia) − 0.6235 (Turell et al. 2000, Tiawsirisup et al. 2005, Kilpatrick et al. 2007). The average host competence index (Komar et al. 2003, Kilpatrick et al. 2007) of unvaccinated birds in this study based on this equation is 0.34, suggesting that averaged across days 1–5 postinfection, an average of 6.8% of mosquitoes feeding on these robins would be able to transmit WNV subsequently. In contrast, none of the vaccinated birds had viremias on any day that were likely to be infectious (0% of feeding mosquitoes would likely transmit virus after the extrinsic incubation period).
Discussion
In this pilot study, the DNA vaccine was efficacious in terms of reducing robins' WNV viremia and thus infectiousness to biting Cx. pipiens. Two vaccinated birds never had detectable virus (but were ELISA and PRNT90 positive for WNV antibodies at 14 days postinfection), and the third vaccinated bird's peak WNV viremia (103.3 PFU/mL) was 20-fold below the level that is likely to result in any transmitting mosquitoes (104.6 PFU/mL) (Kilpatrick et al. 2007). Although the sample sizes of vaccinated and control groups were very small, these results suggest that the vaccine appears promising as a means to reduce the infectiousness of American robins.
The viremia profiles observed in our study for unvaccinated robins were not significantly different from those observed in a previous experimental infection of American robins that was done by mosquito bite with an NY99 strain of WNV (Komar et al. 2003) [repeated measures analysis of variance using the limit of detection 101.7 PFU/mL for the one undetectable viremia in Komar et al. (2003); F1,2 = 6.7; p = 0.123]. If we assume the impact of the vaccine we observed in reducing daily viremias (a reduction of 2.6, 4.1, 2.8, 1.8, and 0 log PFU/mL on days 1–5 PI, respectively) would be similar on the birds studied by Komar et al. (2003), then the two robins in that study would have a host competence index of 0.04 (reduced from 1.04), and, on average, only 0.8% (reduced from 21%) of Cx. pipiens mosquitoes feeding on them on days 1–5 PI would become infectious.
None of the three vaccinated birds were ELISA or PRNT90 positive for WNV antibodies 2 weeks postvaccination. Other studies with this DNA vaccine have also observed weak and variable antibody responses at 2 weeks postinfection (e.g., only five of nine fish crows were PRNT positive at an 80% neutralization level at a 1:10 dilution) (Turell et al. 2003). Thus, while the vaccine has been shown to be effective at decreasing WNV viremias in four other species of birds, a neutralizing response is not always measurable at 2 weeks postvaccination, and 6 to 9 weeks are often required postvaccination before peak serological responses are apparent (Davis et al. 2001, Turell et al. 2003, Bunning et al. 2007, Chang et al. 2007).
These results suggest that vaccination of American robins in the field would reduce their infectiousness, and, given their importance as hosts for Cx. pipiens, Cx. restuans, Cx. tarsalis, and Cx. quinquefasciatus mosquitoes, may reduce the prevalence of WNV in mosquitoes. However, cost-effective vaccination of robins as a disease control strategy would require determining the necessary vaccine coverage for a desired efficacy, and a cheap delivery method for the vaccine such as an oral bait. Unfortunately, the DNA plasmid vaccine we used was ineffective in previous studies when orally administered (Turell et al. 2003, Bunning et al. 2007), suggesting that additional work formulating an orally effective vaccine would be required before a broad-scale field trial could be contemplated. In addition, the effects of vaccination on evolution of the transmissibility and virulence of the virus must be assessed to avoid selection for more virulent strains (Gandon et al. 2001).
There have been a few previous efforts to control zoonotic disease through vaccination of wildlife. These efforts include vaccinating raccoons (Russell et al. 2005, Slate et al. 2005) and foxes for rabies virus (MacInnes et al. 2001), ungulates for Rinderpest virus (Mukhopadhyay et al. 1999), badgers for bovine tuberculosis, Mycobacterium bovis (More 2005), and white-footed mice for the causative agent of Lyme disease, Borrelia burgdorferi (Tsao et al. 2004). In each case, vaccination was successful in reducing the prevalence of the pathogen in the arthropod vector or target host population. In the most successful effort to date, a rabies virus variant was eliminated from foxes in a large area (30,000 km2) of southern Ontario, Canada, by aerial deployment of baits containing rabies virus vaccine at a density of 20 baits/km2 (MacInnes et al. 2001). Unfortunately, invasion of rabid raccoons from New York in 1999, 6 years after the last rabid fox was found, frustrated eradication efforts.
Clearly, substantial challenges remain before the goal of controlling or eradicating a pathogen through vaccination of wildlife can be accomplished. These include developing efficacious and easily deliverable vaccines for a sufficient fraction of the host species (Russell et al. 2005). Underlying these challenges are uncertainties as to whether the pathogen can be sustained in the unvaccinated fraction of the host population. Nonetheless, wildlife vaccination offers four important advantages over human vaccination for zoonotic diseases: (1) local and even broad-scale control of a wildlife disease may be possible; (2) control efforts impact the entire local human population; (3) animal vaccines can be developed on much faster time scales than human vaccines and at much lower costs; and (4) health risks associated with human vaccination are eliminated. We believe that wildlife vaccination should be added to the tool box of disease control strategies that is regularly considered for the growing burden of emerging zoonotic diseases.
Acknowledgments
We thank the field crew who helped catch the robins for this study (R. Peters, W. Janousek, B. Evans, and J. Dawson). This study was funded by NIAID contract #NO1-AI-25490, Centers for Disease Control and Prevention Grant 1RO1AI069217-01, and NSF Grant EF-0622391 as part of the joint NSF-NIH Ecology of Infectious Disease program.
Disclosure Statement
No competing financial interests exist.
References
- Apperson CS. Hassan HK. Harrison BA. Savage HM, et al. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonot Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunning ML. Fox PE. Bowen RA. Komar N, et al. DNA vaccination of the American crow (Corvus brachyrhynchos) provides partial protection against lethal challenge with West Nile virus. Avian Dis. 2007;51:573–577. doi: 10.1637/0005-2086(2007)51[573:DVOTAC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Calisher CH. Karabatsos N. Dalrymple JM. Shope RE, et al. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol. 1989;70:37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. West Nile virus. 2009. www.cdc.gov/ncidod/dvbid/westnile/index.htm. [Jan 16;2009 ]. www.cdc.gov/ncidod/dvbid/westnile/index.htm
- Chang GJJ. Davis BS. Stringfield C. Lutz C. Prospective immunization of the endangered California condors (Gymnogyps californianus) protects this species from lethal West Nile virus infection. Vaccine. 2007;25:2325–2330. doi: 10.1016/j.vaccine.2006.11.056. [DOI] [PubMed] [Google Scholar]
- Davis BS. Chang GJJ. Cropp B. Roehrig JT, et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75:4040–4047. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel GD. Dupuis Alan P., II Nicholas D, et al. Detection by enzyme-linked immunosorbent assay of antibodies to West Nile virus in birds. Emerg Infect Dis. 2002;8:979–982. doi: 10.3201/eid0809.020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandon S. Mackinnon MJ. Nee S. Read AF. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414:751–756. doi: 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- Hamer GL. Kitron UD. Brawn JD. Loss SR, et al. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J Med Entomol. 2008;45:125–128. doi: 10.1603/0022-2585(2008)45[125:cpdcab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Health Canada. West Nile virus. 2009. www.phac-aspc.gc.ca/wnv-vwn/mon_e.html. [Jan 16;2009 ]. www.phac-aspc.gc.ca/wnv-vwn/mon_e.html
- Kent R. Juliusson L. Weissmann M. Evans S. Komar N. Seasonal blood feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J Med Entomol. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM. Daszak P. Jones MJ. Marra PP. Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proc R Soc B Biol Sci. 2006a;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM. Kramer LD. Campbell S. Alleyne EO, et al. West Nile virus risk assessment and the bridge vector paradigm. Emerg Infect Dis. 2005;11:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM. Kramer LD. Jones MJ. Marra PP. Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006b;4:606–610. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM. LaDeau SL. Marra PP. Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk. 2007;124:1121–1136. [Google Scholar]
- Kilpatrick AM. Meola MA. Moudy RM. Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathogens. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N. Langevin S. Hinten S. Nemeth N, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LD. Li J. Shi PY. West Nile virus. Lancet Neurol. 2007;6:171–181. doi: 10.1016/S1474-4422(07)70030-3. [DOI] [PubMed] [Google Scholar]
- MacInnes CD. Smith SM. Tinline RR. Ayers NR, et al. Elimination of rabies from red foxes in eastern Ontario. J Wildl Dis. 2001;37:119–132. doi: 10.7589/0090-3558-37.1.119. [DOI] [PubMed] [Google Scholar]
- Molaei G. Andreadis T. Armstrong P. Anderson J. Vossbrinck C. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More SJ. Towards eradication of bovine tuberculosis in Ireland: a critical review of progress. Cattle Pract. 2005;13:313–318. [Google Scholar]
- Moudy RM. Meola MA. Morin LL. Ebel GD. Kramer LD. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am J Trop Med Hyg. 2007;77:365–370. [PubMed] [Google Scholar]
- Mukhopadhyay AK. Taylor WP. Roeder PL. Rinderpest: a case study of animal health emergency management. Rev Sci Tech Off Int Epizoot. 1999;18:164–178. doi: 10.20506/rst.18.1.1156. [DOI] [PubMed] [Google Scholar]
- Nemeth NM. Oesterle PT. Bowen RA. Passive immunity to West Nile virus provides limited protection in a common passerine species. Am J Trop Med Hyg. 2008;79:283–290. [PubMed] [Google Scholar]
- Payne AF. Binduga-Gajewska I. Kauffman EB. Kramer LD. Quantitation of flaviviruses by fluorescent focus assay. J Virol Methods. 2006;134:183–189. doi: 10.1016/j.jviromet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Russell CA. Smith DL. Childs JE. Real LA. Predictive spatial dynamics and strategic planning for raccoon rabies emergence in Ohio. PLoS Biol. 2005;3:382–388. doi: 10.1371/journal.pbio.0030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HM. Aggarwal D. Apperson CS. Katholi CR, et al. Host choice and West Nile virus infection rates in blood fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee 2002–2003. Vector Borne Zoonot Dis. 2007;7:365–386. doi: 10.1089/vbz.2006.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slate D. Rupprecht CE. Rooney JA. Donovan D, et al. Status of oral rabies vaccination in wild carnivores in the United States. Virus Res. 2005;111:68–76. doi: 10.1016/j.virusres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Stehn T. Whooping Crane recovery activities. 2002. www.ccbirding.com/twc/2002/2002_Mar-Aug.doc. [Jan 20;2009 ]. www.ccbirding.com/twc/2002/2002_Mar-Aug.doc
- Tiawsirisup S. Platt KB. Evans RB. Rowley WA. A comparison of West Nile virus transmission by Ochlerotatus trivittatus (COQ.), Culex pipiens (L.), and Aedes albopictus (Skuse) Vector Borne Zoonot Dis. 2005;5:40–47. doi: 10.1089/vbz.2005.5.40. [DOI] [PubMed] [Google Scholar]
- Tsao JI. Wootton JT. Bunikis J. Luna MG, et al. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci USA. 2004;101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell M. Bunning M. Ludwig G. Ortman B, et al. DNA vaccine for West Nile virus infection in fish crows (Corvus ossifragus) Emerg Infect Dis. 2003;9:1077–1081. doi: 10.3201/eid0909.030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ. O'Guinn M. Oliver J. Potential for New York mosquitoes to transmit West Nile Virus. Am J Trop Med Hyg. 2000;62:413–414. doi: 10.4269/ajtmh.2000.62.413. [DOI] [PubMed] [Google Scholar]
- Turell MJ. Sardelis MR. O'Guinn ML. Dohm DJ. Potential vectors of West Nile virus in North America. In: Mackenzie J, editor; Barrett A, editor; Deubel V, editor. Japanese Encephalitis and West Nile Viruses Vol. 267 Current Topics in Microbiology and Immunology. Berlin: Springer-Verlag; 2002. pp. 241–252. [DOI] [PubMed] [Google Scholar]